Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An enantio- and diastereoselective approach to indoloquinolizidines in continuous flow†
Moreshwar B.
Chaudhari
 *,
Prachi
Gupta
*,
Prachi
Gupta
 ,
Patricia
Llanes
,
Leijie
Zhou
,
Nicola
Zanda
and
Miquel A.
Pericàs
,
Patricia
Llanes
,
Leijie
Zhou
,
Nicola
Zanda
and
Miquel A.
Pericàs
 *
*
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans 16, 43007, Tarragona, Spain. E-mail: mapericas@gmail.com; mchaudhari@iciq.es
First published on 4th October 2022
Abstract
Merging polymer-supported asymmetric organocatalysis with continuous flow in a packed bed reactor has been used as the key, enantiodetermining step in a short synthesis of indoloquinolizidines. Using this approach, a highly enantioselective, solvent-free and rapid conjugate addition of dimethyl malonate to a diverse family of cinnamaldehydes in continuous flow, allowing the preparation of relevant oxodiesters in multigram amounts has been developed. The obtained Michael adducts have been used to complete an expedient diastereoselective synthesis of indoloquinolizidine via cascade Pictet–Splengler cyclisation-lactamisation in continuous flow. The conversion of enantiopure Michael adducts into δ-lactones via telescoped reduction/cyclisation in continuous flow has also been explored.
Introduction
The indoloquinolizidine skeleton is present in the structures of a variety of bioactive alkaloids, such as yohimibine, reserpine, corynantheidol and hirustine, among many others (Fig. 1).1 Structurally diverse indoloquinolizidines like 1 thus represent an important synthetic goal in medicinal chemistry, but their systematic study and their use are hampered by the inherent complexity of the synthetic approaches leading to them. | ||
| Fig. 1 Naturally occurring alkaloids of the indoloquinolizidine type and the targets of this study (1). | ||
A variety of strategies towards the preparation of these interesting compounds in enantiomerically pure form have been developed; they include multistep processes starting from materials available in the chiral pool,2 organocatalytic strategies for the creation of the key stereocenters in these structures,3 and, most interestingly, cascade approaches for the fast assembly of the tetracyclic indoloquinolizidine system.4 Overall, the main limitations of the previous asymmetric Michael addition approaches are use of non-recyclable homogeneous catalyst and prolonged reaction times. In terms of previous approaches towards indoloquinolizidine, the reaction scalability and extended reaction times are notable drawbacks.
The execution of complex synthetic transformations in a way that aligns with the principles of green chemistry, and thus involves low environmental footprint, is not only highly desirable, but a must in view of the increasingly accelerated climate change. This endeavour, however, remains challenging owing to the still common step-by-step approach to the construction of molecular skeletons and the almost unavoidable use of harmful reagents and large volumes of solvents in the most reliable tools in the synthetic chemist handbook.5
With no doubt, the formulation of the principles of green chemistry has boosted the interest on continuous-flow chemistry and has contributed to the paradigm change from batch to flow in academic laboratories as well as in industrial chemical manufacturing, since work in continuous flow brings “intrinsic greenness” to overall processes.6,7 The encouragement and recommendations from various regulatory agencies in favour of the implementation of continuous manufacturing at industrial level unlocks the preferential choice of this alternative over conventional batch reactions,8 and the reason for that can be found in the fact that flow processes, when implementable, offer clear advantages over batch processing from both the economic and the technical points of view. Thus, flow processes can be easily engineered to provide improved heat and mass transfer, by-product minimization, rapid reactions, scalability, superheating of solvents, real-time analysis, safety control, and the possibility of a 24/7 working regime.9
In a parallel manner, the requisite for enantiopure forms over racemates in approved drugs is also rising due to pharmaceutical regulations;10 therefore, the search for catalytic enantioselective transformations with large scale applicability is becoming more evident.11
Amid the different possible approaches to simultaneously fulfil the requirements of sustainability and enantioselectivity, one of the most promising alternatives is the merging of asymmetric catalysis and flow processing, using polymer-supported (PS) organocatalysts in packed bed reactors (PBR). The advantages of this approach for the fast production of enantiomerically pure compounds in a simple and readily scalable manner have already been demonstrated.12 The organocatalyst immobilisation strategy offers facile recovery and reuse after each reaction, cost minimisation and robustness which can be utilised to produce from milligrams to multigram scale of enantioenriched entities, while avoiding by design any metal contamination in the reaction products.
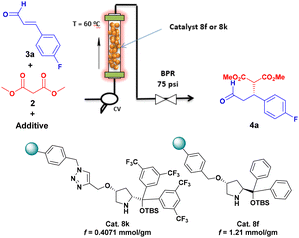
For the efficient synthesis of indoloquinolizidines (1) we considered translating from batch to flow one of the most efficient approaches developed to date; that is, combining the enantioselective Michael addition of a dialkyl malonate (2) onto a variety of substituted cinnamaldehydes (3), leading to the enantioenriched oxodiester (4) with a diastereoselective Pictet–Spengler/lactamization cascade with tryptamine derivatives (5).4e (Scheme 1). As an additional bonus, we also planned to use 4 for the synthesis of enantio- and diastereomerically pure δ-lactones (6) using a telescoped continuous flow process.
 | ||
| Scheme 1 Retrosynthetic analysis for chiral lactones and indoloquinolizidines from enantiopure Michael adducts. | ||
Jørgensen–Hayashi catalysts (7)13 are probably the most used organocatalysts for Michael-type additions.14 Very efficient immobilized versions of them (8) have been developed starting from readily available, enantiopure trans-4-hydroxyproline (Fig. 2).15 Some of these immobilized species have shown their suitability for use in continuous flow and, among them 8g, formally derived from cis-4-hydroxyproline,16 offers the important sustainability advantage of allowing work in continuous flow under solventless conditions. With the use of this catalyst, a variety of Michael-type adducts en route to enantiomerically pure important drugs (Paroxetine, Baclofen, Phenibut, Fluorophenibut, Rolipram) having been prepared.17
According to these precedents, we decided to explore the possibility of developing a continuous flow approach to indoloquinolizidines 1, where the key chiral center at C2 was created through a solvent-free organocatalytic Michael addition mediated by an immobilized Jørgensen–Hayashi catalyst (8), while the configuration at the C3 and C12b would result from the interplay of kinetic and thermodynamic factors in a subsequent domino Pictet–Splengler cyclization/lactamization sequence.
For our purposes, it was very important to identify immobilized catalysts of the 8-type able to provide high enantioselectivity in the Michael addition of malonates 2 to substituted cinnamaldehydes 3. After a preliminary screening, we concentrated our efforts on the catalyst 8f, which has been previously used with success in the Michael initiated cyclopropanation of enals with bromomalonates,15h and 8k, which has been newly prepared for the purpose of this study.18
We first studied the addition of dimethyl malonate (2) to 4-fluorocinnamaldehyde (3a) in batch in the presence of these two polymer-supported organocatalysts (see Table S1 in the ESI for these optimization studies†). In this manner, we could establish that the hard Lewis acid Ca(OTf)2 is required as an additive with 8k, while with 8f the reactions can be efficiently promoted with the simple addition of acetic acid. Enantioselectivities of 95–96% were achieved in these conditions. A library of oxodiesters 4′ was subsequently prepared in batch using catalyst 8k with moderate to good yield and excellent enantioselectivity (Scheme S2†). Once the suitability of 8k and 8f for the target additions were established in batch, the experimental conditions (reaction temperature, concentration, and flow rate) for the corresponding addition in flow were optimized (Table 1).
| No. | Cat. | Flow rate [μL min−1] | Additive | Yieldb [%] | Solv. | t R [min] | eec [%] |
|---|---|---|---|---|---|---|---|
a The reactions were performed in packed bed reactors containing 0.5 g (0.203 mmol) of 8k or 1.0 g (1.21 mmol) of 8f.
b Isolated yield.
c Determined by chiral HPLC using OJ-H column.
d 0.05M DCM solution (2/3a molar ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
e 0.05M DCM solution (2/3a molar ratio was 15 1).
e 0.05M DCM solution (2/3a molar ratio was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
f 0.2M DCM solution (ratio of 2 & 3 was 15 1).
f 0.2M DCM solution (ratio of 2 & 3 was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
g 0.2M DCM solution (ratio of 2 & 3 was 15 1).
g 0.2M DCM solution (ratio of 2 & 3 was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In the case of neat reactions the (2/3a molar ratio was 2 1). In the case of neat reactions the (2/3a molar ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In all experiments 1 equiv. of Ca(OTf)2, or 0.6 equiv. of AcOH were used. [CV = check valve, BPR = back pressure regulator]. 1). In all experiments 1 equiv. of Ca(OTf)2, or 0.6 equiv. of AcOH were used. [CV = check valve, BPR = back pressure regulator].
|
|||||||
| 1d | 8k | 100 or 50 | AcOH | Trace | DCM | — | — |
| 2d | 8k | 20 | Ca(OTf)2 | 35 | DCM | 330 | −91 |
| 3e | 8k | 20 | Ca(OTf)2 | <5 | DCM | — | — |
| 4 | 8k | 20 | AcOH | 15 | Neat | 125 | −96 |
| 5f | 8k | 20 | Ca(OTf)2 | <5 | Neat | 125 | — |
| 6g | 8f | 100 | AcOH | 60 | DCM | 63 | 96 |
| 7 | 8f | 100 | AcOH | 32 | Neat | 25 | 98 |
| 8 | 8f | 50 | AcOH | 68 | Neat | 50 | 98 |
| 9 | 8f | 20 | AcOH | 73 | Neat | 125 | 98 |
Only traces of product were observed when the reaction was carried out in flow in the presence of catalyst 8k with acetic acid as an additive at 60 °C and flow rates of 50 or 100 μL min−1 (entry 1). Even after a substantial decrease in flow rate under solventless conditions (entry 4), conversion remained low. On the other hand, although 8k worked well with Ca(OTf)2 as an additive in batch, poor solubility of Ca(OTf)2 in DCM had a negative impact on the use of this additive in continuous flow. Moreover, since Ca(OTf)2 is soluble in dimethyl malonate, poor yields are recorded in the presence of this additive even at long residence times, probably due to leaching (entries 2 and 3). Again, working under solventless conditions did not improve the situation (entry 5).
Gratifyingly, the use of catalyst 8f with a large excess of dimethyl malonate at a flow rate of 100 μL min−1 provided 4a in 60% isolated yield and 96% ee (entry 6). Importantly, the reaction mediated by 8f in the presence of AcOH tolerated well the use of solventless conditions, with 4a being obtained with improved enantioselectivity (98% ee) in 32% yield (entry 7). Further decreases in flow rate (entries 8 and 9) showed 50 μL min−1 to be the optimal value, leading to 4a in 68% yield and 98% ee, with a reasonable 50 min residence time (entry 8).
With these optimized conditions we explored the substrate scope in the solvent free conjugate addition reaction (Scheme 2). A continuous flow assembly was set up by placing 1 g of catalyst 8f (f = 1.21) in an Omnifit© column (10 mm bore size and 70 mm maximum adjustable height) with outer jacket having oil circulation for heating (60 °C), with the reactants being flowed by a Vapourtec SF-10 pump (Fig. S3, ESI†). The height of the catalyst bed was ca. 3.2 cm after swelling with dimethyl malonate. This simple device could be used for the gram-scale preparation of a family of enantiopure oxodiesters 4 in a sequential manner. For instance, compound 4b was prepared from a homogeneous mixture of dimethyl malonate 2 (2 equiv., 98.48 mmol), trans-cinnamaldehyde 3b (1 equiv., 49.24 mmol), and acetic acid (0.6 equiv., 25.97 mmol) circulated at a flow rate of 50 μL min−1 at 60 °C for 4.5 h. A back pressure regulator (BPR) loaded at 75 psi was used to prevent the formation of bubbles inside the catalyst gel. To our delight, 19.94 mmol of dimethyl (R)-2-(3-oxo-1-phenylpropyl)malonate 4b with 97% ee were obtained in the experiment, corresponding to a TOF of 3.66 mmol4b h−1 mmol8f−1. Before performing the next preparative experiment, the packed bed of catalyst 8f was simply washed with acetic acid (200 μL min−1 for 30 min) and ethyl acetate (200 μL min−1 for 30 min). In this manner, we explored the scope of the asymmetric Michael reaction with up to seven β-(hetero)aryl substituted α,β-unsaturated aldehydes.
The reaction worked well with 4-methoxycinnamaldehyde (3c), bearing an electron-donating substituent on the aryl group, to afford 11.47 mmol of dimethyl (R)-2-(1-(4-methoxyphenyl)-3-oxopropyl)malonate (4c) with 96% ee in 4 hours. Substrates bearing weak electron withdrawing groups such 4-fluorocinnamaldehyde (3a); 4-chlorocinnamaldehyde (3d) and 4-bromocinnamaldehyde (3e) also afforded product 4a (18.39 mmol, 3.38 mmol h−1), 4d (8.10 mmol, 2.68 mmol h−1) and 4e (4.74 mmol, 1.12 mmol h−1) with excellent enantioselectivity of 98, 97 and 97% respectively. Noteworthy, compounds 4a and 4b are key intermediate for the synthesis of antidepressant drugs (−)-Paroxetine and (−)-Femoxetine, while 4d is a precursor of the peptidomimetic inhibitor Roche-1.19 In the case of compound 4e, a larger excess (6 equiv.) of dimethyl malonate was used to ensure the solubility of the starting aldehyde. Likewise, due to poor solubility of 4-nitrocinnamaldehyde (3f), 10.4 equiv. of dimethyl malonate was used to obtain 1.65 mmol of compound 4f in 3.18 hours at a 100 μL min−1 flow rate. Additionally, the reaction of (E)-3-(furan-2-yl)acrylaldehyde (3g) with dimethyl malonate afforded 6.02 mmol (1.58 mmol h−1) of oxodiester 4g with 92% ee. It is remarkable that the reaction exhibits an important dependence on the nature of the dialkyl malonate partner. Thus, when the use of diethyl malonate was tested in front of trans-cinnamaldehyde, poor reaction performance was observed. Catalyst 8k was also tested in the reaction, for the formation of ent-4b′. Much lower conversion was observed, and flow rate was accordingly reduced to 20 μL min−1. In this manner, 1.00 g of ent-4b′ with 92% ee could be obtained in 4.5 h operation.
At this point, it is interesting to realize the operational advantages associated to the use of the immobilized catalyst 8f for the preparation of 4 in continuous flow: besides the suppression of solvent use (excess of dimethyl malonate can be easily removed by evaporative distillation), reuse of the PBR containing the catalyst only requires intermediate washing, and scale-up to the decimol scale can be simply achieved by reasonable extension of the operation time. For instance, the preparation of 100 mmol of enantiopure 4a would require some 22 h operation under the standard operation conditions.
Next, the study of the continuous-flow synthesis of indoloquinolizidines 1 from enantiopure oxodiesters 4 was undertaken. The desired Pictet–Spengler cyclization with subsequent lactamization process was first studied by reacting dimethyl (R)-2-(1-(4-fluorophenyl)-3-oxopropyl)malonate (4a) and tryptamine (5) in presence of trifluoroacetic acid (TFA), as summarized in Table 2. The continuous flow set-up consisted of two syringe or peristaltic pumps to deliver the reagents, T-mixer, check valve to prevent back flow, coil reactor with acid resistant PTFE tube (5 mL volume), a heating source from Vapourtec R-series and a BPR loaded at 40 psi (Fig. S4†). A solution of 4a and 5 in dichloromethane (DCM) was flown by one of the pumps, and a solution of TFA in DCM was flown by the other pump. At the outset, equimolar solutions of 4c + 5 and TFA in DCM were circulated at 50 μL min−1 each at 20 °C, which resulted in no lactamization (Table 2, entry 1). By increasing the temperature to 55 °C, compound 1a was formed in 35% yield with 92% ee (entry 2). We attributed this rather low yield to the incomplete cyclization of the unstable, epimerizable intermediates Int. a and Int. b, since we observed that these intermediates slowly undergo non-diastereoseletive lactamization in CDCl3 or CD2Cl2 in NMR tubes on standing. Due to the unstable nature of these intermediates, we are not able to isolate them. We hypothesized that higher temperature and the presence of additional acid promoter would accelerate the reaction and, to our delight (entry 5), when 2.5 equiv. of TFA were used at 55 °C and flow rate was reduced to 50 μL min−1 for each reactant, the cascade process took place to completion, affording 1c in 76% isolated yield with 93% ee with residence time of 50 min (entry 5).
| Entry | TFA equiv. | Flow rate [μL min−1] | Temp. | Yieldb | t R | eec |
|---|---|---|---|---|---|---|
| a Compound 4a with 96% ee (0.1 mmol, 1 equiv., 10 mM solution in 10 mL solvent) + 1.5 equiv. of 5, trifluoroacetic acid (2.5 equiv., 25 mM solution in 10 mL DCM). b Isolated yields. c Determined by chiral HPLC using AD-H column. d Performed with 23 mM concentration of 4a. >20/1 dr was observed. Flow rate = 50 μL min−1 for each syringe. | ||||||
| 1 | 1 | 50 | 20 | — | 50 | — |
| 2 | 1 | 100 | 55 | 35 | 25 | 92 |
| 3 | 2 | 100 | 55 | 40 | 25 | 91 |
| 4 | 2 | 50 | 55 | 56 | 50 | 93 |
| 5 | 2.5 | 50 | 55 | 76 | 50 | 93 |
| 6d | 2.5 | 50 | 55 | 49 | 50 | 92 |
| 7 | 10 | 100 | 80 | Traces | 25 | — |
| 8 | 2.5 | 50 | 55 | 40 | 50 | 90 |
| 9 | 2.5 | 50 | 55 | — | 50 | — |
| 10 | 2.5 | 50 | 55 | Traces | 50 | — |
| 11 | 2.5 | 50 | 55 | — | 50 | — |
When the concentration of the solution of 4a and 5 in DCM was increased to 23 mM with the purpose of increasing productivity, the yield of 1a dropped significantly (entry 6). In the same manner, the use of a ten-fold excess of TFA had a deleterious effect (entry 7). Likewise, a drastic decrease in yield was observed when THF was used as a solvent (entry 8). Other solvents like ethanol, DMF, DMSO were ineffective (entries 9–11).
The optimized conditions of entry 5 were next applied for the synthesis of a family of indoloquinolizidines (ent-1′) in continuous-flow (Scheme 3). In all cases moderate to very good yield and excellent diastereselectivity were observed, with preservation of the enantiopurity of the oxodiester precursors. The samples of the starting oxodiesters used in this study had been prepared in batch by using catalyst 8k, and thus belonged to the S-enantiomeric series (see ESI, Scheme S2†). It is to be mentioned that the same catalyst sample 8k was used throughout the synthesis campaign of library of 4′ series, the re-conditioning between successive preparations simply involving washings with ethyl acetate and DCM and drying at 40 °C.
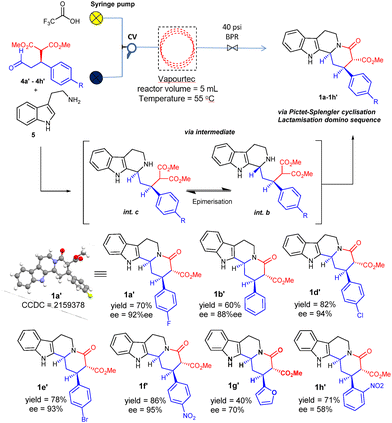 | ||
| Scheme 3 Scope of diastereoselective Picetet–Splengler-lactamisation domino reaction in continuous flow. Reactor volume 5 mL, flow rate = 50 μL min−1 for each, residence time (tR) = 50 min. >20/1 dr. | ||
Since it is well established that the cascade Pictet–Spengler plus lactamization sequence performed in batch requires working at low concentration for success,20 this being confirmed by our own observations when the process is performed in continuous flow (Table 2, entry 6), any desired increase in production should be tackled from the perspective of an increased throughput, using either a sequential or a parallel numbering up strategy. With this purpose, we combined three coiled reactors (5 + 5 + 10 mL volume) to give a total reactor volume of 20 mL, and the flow rates of the 10 mM solutions of 4 + 5 and 25 mM TFA in DCM were increased to 200 μL min−1 each, which corresponds to a theoretical fourfold increase in production, keeping a constant residence time of 50 min (Scheme 4; see ESI, Fig. S5† for the actual reactor image). Enantiopure compounds prepared using catalyst 8f (Scheme 2) were used to perform the large-scale synthesis of indoloquinolizidines (Scheme 4).
In the initial experiment, a 10 mM solution of dimethyl (R)-2-(3-oxo-1-phenylpropyl)malonate 4b in 100 mL DCM and a 25 mM TFA solution in DCM (100 mL) were flown at a flow rate of 200 μL min−1 each at 55 °C to afford 1b in 48% yield. However, lowering the flow rate of each of the solutions to 100 μL min−1 resulted in a remarkable increase in yield of 1b to 65%, with 97% enantioselectivity. Likewise, the oxodiester 4c, bearing an electron donating methoxy group in the para position, also worked well to afford compound 1c in 55% yield with 96% ee (dr = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Subsequently, the reaction of 4a, 4d and 4e were also performed in flow to efficiently produce the tetracyclic products 1a, 1d and 1e in good yield and excellent enantioselectivity (Scheme 4). Compound 4f bearing a para nitro group also worked to provide 1f in 73% yield with 91% ee. Finally, the reaction of dimethyl (R)-2-(1-(furan-2-yl)-3-oxopropyl)malonate (4g) also worked well to afford 1g in 53% yield and 92% ee.
10). Subsequently, the reaction of 4a, 4d and 4e were also performed in flow to efficiently produce the tetracyclic products 1a, 1d and 1e in good yield and excellent enantioselectivity (Scheme 4). Compound 4f bearing a para nitro group also worked to provide 1f in 73% yield with 91% ee. Finally, the reaction of dimethyl (R)-2-(1-(furan-2-yl)-3-oxopropyl)malonate (4g) also worked well to afford 1g in 53% yield and 92% ee.
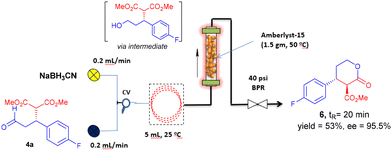
As an additional bonus of these studies, we decided to explore the conversion of oxodiesters 4 into enantiopure, trans-3,4-disubstituted δ-lactones 6. Chiral lactones are an important class of compounds which shows interesting biological properties.21 In particular, batch synthesis of δ-lactones involves the chemoselective reduction of oxodiesters using NaBH3CN at 0 °C, followed by basic workup and silica-gel mediated cyclisation.19 To avoid the additional work-up step, possibility of exotherms, and prolonged reaction time we have established a continuous flow protocol for the chemoselective reduction of oxodiester 4a at room temperature, and we have telescoped this step with a cyclisation induced by the acidic resin Amberlyst-15. In this manner, the whole reduction-cyclisation sequence can be performed in continuous flow, in a very short time and without isolating the intermediate alcohol (Scheme 5). To perform the telescoped process, a 5 mL coiled reactor was combined with an Omnifit© PBR (6.6 mm bore size × 70 mm height) filled with 1.5 g of Amberlyst-15 (see ESI, Fig. S6†). Then, a 35 mM solution of compound 4a (0.35 mmol + 0.42 mL acetic acid in THF) and 53 mM solution of NaBH3CN in THF were circulated at 200 μL min−1 through the coiled reactor at 25 °C and the Amberlyst-15 packed bed reactor (Temp = 50 °C) to afford lactone 6 in 53% isolated yield and 95.5% ee, with >20/1 dr in only 20 min of residence time. Although this is beyond the goals of the present study, the extension of this methodology to the preparation of diversely substituted enantiopure lactones 6 looks straightforward.
Conclusions
In summary, we have developed a rapid and solvent-free, continuous flow protocol for the preparation of enantiopure oxodiesters 4 using polymer-supported Jørgensen–Hayashi catalysts to perform enantioselective Michael additions at the multi-gram scale through safe and operationally simple procedures. We have also demonstrated the successful use of these enantiopure Michael adducts in the expedient diastereoselective synthesis of complex indoloquinolizidines 1 through a domino Pictet–Spengler plus lactamization sequence performed in continuous flow. Moreover, we have also shown the possibility of using enantiopure Michael adducts 4 as starting materials for a telescoped, diastereoselective synthesis of trans-3,4-disubstituted δ-lactones in continuous flow through a safe process taking place at near ambient temperature with short residence time. In comparison with previous reports, we are able to achieve a rapid, solvent-free, scalable synthesis of Michael adducts and also an expedient and scalable continuous flow synthesis of diverse indoloquinolizidines. We believe that the use of highly recyclable, robust and enantioselective organocatalysts working in solventless conditions will pave the way for the development of further applications of enantiopure indoloquinolizidine alkaloid derivatives.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by MINECO/FEDER (grant PID2019-109236RB-I00), MICINN through Severo Ochoa Excellence Accreditation 2020-2023 (CEX2019-000925-S, MIC/AEI), and the CERCA Program/Generalitat de Catalunya. This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 890452.References
-
(a)
The Alkaloids: Chemistry and Biology, ed. G. A. Cordell, Academic Press, New York, 1998, vol. 50 Search PubMed
; (b) C. Szántay and K. Honty, in The Chemistry of Heterocyclic Compounds, ed. J. E. Saxton, Wiley, New York, 1994, vol. 25, pp. 161–216 Search PubMed
.
- For some examples, see:
(a) M. Amat, M. Pérez, A. T. Managlia, N. Casamitjana and J. Bosch, Org. Lett., 2005, 7, 3653–3656 CrossRef CAS PubMed
; (b) G. Stork, P. C. Tang, M. Casey, B. Goodman and M. Toyota, J. Am. Chem. Soc., 2005, 127, 16255–16262 CrossRef CAS PubMed
; (c) M. Amat, M. M. M. Santos, O. Bassas, N. Llor, C. Escolano, A. Gómez-Esqué, E. Molins, S. M. Allin, V. McKee and J. Bosch, J. Org. Chem., 2007, 72, 5193–5201 CrossRef CAS
; (d) M. Amat, A. Gómez-Esqué, C. Escolano, M. M. M. Santos, E. Molin and J. Bosch, J. Org. Chem., 2009, 74, 1205–1211 CrossRef CAS PubMed
.
- For some recent approaches to the indoloquinolizidine skeleton involving either metal-catalyzed or organocatalytic processes, see:
(a) A. Olmos, B. Louis and P. Pale, Chem. – Eur. J., 2012, 18, 4894–4901 CrossRef CAS
; (b) M. P. Lalonde, M. A. McGowan, N. S. Rajapaksa and E. N. Jacobsen, J. Am. Chem. Soc., 2013, 135, 1891–1894 CrossRef CAS PubMed
; (c) M. Amat, F. Arioli, M. Pérez, E. Molins and J. Bosch, Org. Lett., 2013, 15, 2470–2473 CrossRef CAS
; (d) Y. Liu, Q. Wang, Y. Zhang, J. Huang, L. Nie, J. Chen, W. Cao and X. Wu, J. Org. Chem., 2013, 78, 12009–12017 CrossRef CAS
; (e) V. Gobe and X. Guinchard, Org. Lett., 2014, 16, 1924–1927 CrossRef CAS PubMed
; (f) A. Younai, B.-S. Zeng, H. Y. Meltzer and K. A. Scheidt, Angew. Chem., Int. Ed., 2015, 54, 6900–6904 CrossRef CAS
; (g) X. chen, L. Yu, H. Wang, W. Zhang, P. Tang and F. Chen, Chem. Commun., 2021, 57, 11669–11672 RSC
.
-
(a) J. Franzén and A. Fisher, Angew. Chem., Int. Ed., 2009, 48, 787 CrossRef PubMed
; (b) W. Zhang and J. Franzén, Adv. Synth. Catal., 2010, 352, 499 CrossRef CAS
; (c) X. Y. Wu, X. Y. Dai, L. L. Nie, H. H. Fang, J. Chen, Z. J. Ren, W. G. Cao and G. Zhao, Chem. Commun., 2010, 46, 2733–2735 RSC
; (d) H. Fang, X. Wu, L. Nie, X. Dai, J. Chen, W. Cao and G. Zhao, Org. Lett., 2010, 12, 5366–5369 CrossRef CAS PubMed
; (e) M. Rueping, C. M. R. Volla, M. Bolte and G. Raabe, Adv. Synth. Catal., 2011, 353, 2853–2859 CrossRef CAS
; (f) X.-Y. Wu, X.-Y. Dai, H.-H. Fang, L.-L. Nie, J. Chen, W. G. Cao and G. Zhao, Chem. – Eur. J., 2011, 17, 10510–10514 CrossRef CAS PubMed
; (g) H.-L. Zhu, J.-B. Ling and P.-F. Xu, J. Org. Chem., 2012, 77, 7737–7743 CrossRef CAS
; (h) G. He, B. List and M. Christmann, Angew. Chem., Int. Ed., 2021, 60, 13591–13596 CrossRef CAS
.
-
(a)
P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 2000 Search PubMed
; (b) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC
; (c) R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC
; (d) H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC
; (e) For a recent essay on sustainable chemistry, see: K. Kümmerer, Angew. Chem., Int. Ed., 2017, 56, 16420–16421 CrossRef
.
-
(a) S. G. Newman and K. F. Jensen, Green Chem., 2013, 15, 1456–1472 RSC
; (b) L. Rogers and K. F. Jensen, Green Chem., 2019, 21, 3481–3498 RSC
; (c) D. Dallinger and C. O. Kappe, Curr. Opin. Green Sustainable Chem., 2017, 7, 6–12 CrossRef
.
-
(a) R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2013, 17, 1479–1484 CrossRef CAS
; (b) S. Falß, N. Kloye, M. L. Holtkamp, A. Prokofyeva, T. Bieringer and N. Kockmann, in Handbook of Green Chemistry, Green Chemical Engineering, Wiley Online Library, 2019, vol. 12 Search PubMed
; (c) Flow Chemistry in Drug Discovery, ed. J. Alcazar, A. de la Hoz and A. Diaz-Ortiz, Topics in Medicinal Chemistry, Springer, vol. 38, 2022 Search PubMed
.
- https://www.regulations.gov/document/FDA-2019-D-0298-0002 .
- For some recent reviews on flow chemistry, see:
(a) M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796 CrossRef CAS PubMed
; (b) J. Wegner, S. Ceylan and A. Kirschning, Adv. Synth. Catal., 2012, 354, 17–57 CrossRef CAS
; (c) J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849–8869 RSC
; (d) R. Gérardy, N. Emmanuel, T. Toupy, V.-E. Kassin, N. N. Tshibalonza, M. Schmitz and J.-C. M. Monbaliu, Eur. J. Org. Chem., 2018, 2301–2351 CrossRef
; (e) R. L. Hartman, Curr. Opin. Chem. Eng., 2020, 29, 42–50 CrossRef
; (f) S. B. Ötvös and O. Kappe, Green Chem., 2021, 23, 6117–6138 RSC
; (g) A. S. Burange, S. M. Osman and R. Luque, iScience, 2022, 25, 103892 CrossRef CAS
; (h) A. Bonner, A. Loftus, A. C. Padgham and M. Baumann, Org. Biomol. Chem., 2021, 19, 7737–7753 RSC
; (i) T. Yu, Z. Ding, W. Nie, J. Jiao, H. Zhang, Q. Zhang, C. Xue, X. Duan, Y. M. A. Yamada and P. Li, Chem. – Eur. J., 2020, 26, 5729–5747 CrossRef CAS PubMed
; (j) W.-J. Yoo, H. Ishitani, B. Laroche and S. Kobayashi, J. Org. Chem., 2020, 85, 5132–5145 CrossRef CAS PubMed
.
- L. A. Nguyen, H. He and C. Pham-Huy, Int. J. Biomed. Sci., 2006, 2, 85–100 CAS
.
- For a pioneering review, see: H.-U. Blaser, B. Pugin and F. Spindler, J. Mol. Catal. A: Chem., 2005, 231, 1–20 CrossRef CAS
.
-
(a) C. Rodríguez-Escrich and M. A. Pericàs, Chem. Rec., 2019, 19, 1872–1890 CrossRef
; (b) I. Atodiresei, C. Vila and M. Rueping, ACS Catal., 2015, 5, 1972–1985 CrossRef CAS
; (c) T. Tsubogo, T. Ishiwata and S. Kobayashi, Angew. Chem., Int. Ed., 2013, 52, 6590–6604 CrossRef CAS PubMed
; (d) K. Masuda, T. Ichitsuka, N. Koumura, K. Sato and S. Kobayashi, Tetrahedron, 2018, 74, 1705–1730 CrossRef CAS
; (e) F. Herbrik, M. Sanz, A. Puglisi, S. Rossi and M. Benaglia, Chem. – Eur. J., 2022, 28 DOI:10.1002/chem.202200164
; (f) H. Ishitani, Y. Furiya and S. Kobayashi, Chem. – Asian J., 2020, 15, 1688 CrossRef CAS
; (g) S. Rossi, R. Porta, D. Brenna, A. Puglisi and M. Benaglia, Angew. Chem., Int. Ed., 2017, 56, 4290–4293 CrossRef CAS
; (h) S. Ogasawara and Y. Hayashi, Synthesis, 2017, 424 CAS
; (i) T. Tsubogo, H. Oyamada and S. Kobayashi, Nature, 2015, 520, 329 CrossRef CAS PubMed
; (j) C. De Risi, O. Bortolini, A. Brandolese, G. Di Carmine, D. Ragno and A. Massi, React. Chem. Eng., 2020, 5, 1017–1052 RSC
; (k) Catalyst Immobilization: Methods and Applications, ed. M. Benaglia and A. Puglisi, Wiley, 2020 Search PubMed
.
-
(a) J. Franzén, M. Marigo, D. Fielenbach, T. C. Wabnitz, A. Kjærsgaard and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 18296–18304 CrossRef
; (b) M. Marigo, T. C. Wabnitz, D. Fielenbach and K. A. Jørgensen, Angew. Chem., Int. Ed., 2005, 44, 794–797 CrossRef CAS PubMed
; (c) Y. Hayashi, H. Gotoh, T. Hayashi and M. Shoji, Angew. Chem., Int. Ed., 2005, 44, 4212–4215 CrossRef CAS PubMed
.
- For some reviews on the applications of Jørgensen–Hayashi catalysts, see:
(a) A. Mielgo and C. Palomo, Chem. – Asian J., 2008, 3, 922–948 CrossRef CAS
; (b) K. L. Jensen, G. Dickmeiss, H. Jiang, Ł. Albrecht and K. A. Jørgensen, Acc. Chem. Res., 2011, 45, 248–264 CrossRef PubMed
; (c) B. S. Donslund, T. K. Johansen, P. H. Poulsen, K. S. Halskov and K. A. Jørgensen, Angew. Chem., Int. Ed., 2015, 54, 13860–13874 CrossRef CAS PubMed
.
-
(a) E. Alza and M. A. Pericàs, Adv. Synth. Catal., 2009, 351, 3051–3056 CrossRef CAS
; (b) E. Alza, S. Sayalero, X. C. Cambeiro, R. Martín-Rapún, P. O. Miranda and M. A. Pericàs, Synlett, 2011, 464–468 CAS
; (c) E. Alza, S. Sayalero, P. Kasaplar, D. Almasşi and M. A. Pericàs, Chem. – Eur. J., 2011, 17, 11585–11595 CrossRef CAS
; (d) P. Riente, C. Mendoza and M. A. Pericàs, J. Mater. Chem., 2011, 21, 7350–7355 RSC
; (e) X. Fan, C. Rodríguez-Escrich, S. Sayalero and M. A. Pericàs, Chem. – Eur. J., 2013, 19, 10814–10817 CrossRef CAS PubMed
; (f) X. Fan, C. Rodríguez-Escrich, S. Wang, S. Sayalero and M. A. Pericàs, Chem. – Eur. J., 2014, 20, 13089–13093 CrossRef CAS PubMed
; (g) C. A. Mak, S. Ranjbar, P. Riente, C. Rodríguez-Escrich and M. A. Pericàs, Tetrahedron, 2014, 70, 6169–6173 CrossRef CAS
; (h) P. Llanes, C. Rodríguez-Escrich, S. Sayalero and M. A. Pericàs, Org. Lett., 2016, 18, 6292–6295 CrossRef CAS
; (i) J. Lai, S. Sayalero, A. Ferrali, L. Osorio-Planes, F. Bravo, C. Rodríguez-Escrich and M. A. Pericàs, Adv. Synth. Catal., 2018, 360, 2914–2924 CrossRef CAS
; (j) C. Lizandara-Pueyo, X. M. Fan, C. Ayats and M. A. Pericàs, J. Catal., 2021, 393, 107–115 CrossRef CAS
; (k) Y. Hayashi, S. Hattori and S. Koshino, Chem. – Asian J., 2022, 17, e202200314 CrossRef CAS PubMed
.
- I. Arenas, A. Ferrali, C. Rodríguez-escrich, F. Bravo and M. A. Pericàs, Adv. Synth. Catal., 2017, 359, 2414–2424 CrossRef CAS
.
-
(a) S. B. Ötvös, M. A. Pericàs and C. O. Kappe, Chem. Sci., 2019, 10, 11141–11146 RSC
; (b) S. B. Ötvös, P. Llanes, M. A. Pericàs and C. O. Kappe, Org. Lett., 2020, 22, 8122–8126 CrossRef PubMed
; (c) B. S. Nagy, P. Llanes, M. A. Pericàs, C. O. Kappe and S. B. Ötvös, Org. Lett., 2022, 24, 1066–1071 CrossRef CAS PubMed
.
- Details for the preparation of 8f and 8k can be found in the ESI.†.
- S. Brandau, A. Landa, J. Franzén, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2006, 45, 4305–4309 CrossRef CAS PubMed
.
-
(a) I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 13404–13405 CrossRef CAS PubMed
; (b) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796–10797 CrossRef CAS
; (c) M. L. Van Linn and J. M. Cook, J. Org. Chem., 2010, 75, 3587–3599 CrossRef CAS PubMed
.
- P. Kowalczyk, B. Gawdzik, D. Trzepizur, M. Szymczak, G. Skiba, S. Raj, K. Kramkowski, R. Lizut and R. Ostaszewski, Materials, 2021, 14, 2956 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure, characterisation data, copies of NMR spectra. CCDC 2159378. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ob01462a |
| This journal is © The Royal Society of Chemistry 2022 |