Yu Fang, Shangfeng Ren, Chen He, Huiqi Han, Jin-Biao Liu, Fumin Liao and Min Yang
Org. Biomol. Chem., 2022,20, 6550-6553
DOI:
10.1039/D2OB01165D,
Communication
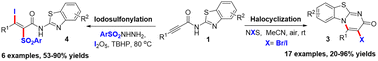
The selective halocyclization and iodosulfonylation of N-benzothiazol-2-yl alkynamides under mild conditions is described. An effective synthetic strategy to pyrimidobenzothiazoles via a 6-endo-dig halocyclization of N-benzothiazol-2-yl alkynamides was developed at room temperature with a broad substrate scope. Furthermore, several multisubstituted α,β-enones were synthesized using the same starting materials.