 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Epoxidation of olefins using diaryltellurium dicarboxylates†
Yuga
Shibuya
a,
Shiori
Ohmura
a,
Akane
Ito
a,
Makoto
Oba
b and
Shinichi
Koguchi
 *a
*a
aDepartment of Chemistry, Tokai University, 4-1-1 Kitakaname, Hiratsuka-shi, Kanagawa, 259-1292 Japan. E-mail: koguchi@tokai-u.jp
bDepartment of Bioengineering, Tokai University, 4-1-1 Kitakaname, Hiratsuka-shi, Kanagawa, 259-1292 Japan
First published on 6th July 2022
Abstract
This paper reports an efficient method for the epoxidation of a variety of functionalized olefins using diaryltellurium dicarboxylates as hypervalent tellurium compounds. This method is able to efficiently convert olefins into epoxides using catalytic amounts of tellurium and urea hydrogen peroxide. Furthermore, we propose that this reaction proceeds via the formation of peroxides of phenol, carboxylic acid, and tellurium peroxide when diaryltellurium dicarboxylates and hydrogen peroxide react. This is the first example of an epoxidation reaction using hypervalent tellurium compounds.
Organotellurium compounds are very interesting because they can adopt a variety of structures, including a hypervalent state, and various oxidation states. Our group is particularly interested in the synthesis and reactivity of organic tellurium compounds, which are relatively unexplored and rarely reported. So far, we have reported the cross-coupling of diarylditellurides with arylboronic acids using copper thiophene-2-carbosylate under mild conditions1 and an oxidation reaction using ionic-liquid-supported telluride.2 Furthermore, we have recently reported a new, concise, and efficient one-pot synthesis of a variety of functionalized diaryltellurium dicarboxylates, which are hypervalent tellurium compounds. The molecular structures of these diaryltellurium dicarboxylates were determined unambiguously using single-crystal X-ray diffraction analysis. A detailed examination of the structure of the diaryltellurium dicarboxylates suggested that they might be effective for various oxidation reactions (Scheme 1).3 In the present paper, we report the epoxidation of olefins using such diaryltellurium dicarboxylates.
 | ||
| Scheme 1 Synthesis of diaryltellurium dicarboxylates.3 | ||
Only a few studies have been reported so far on epoxidation reactions that use organotellurium compounds. To the best of our knowledge, the only relevant report in this area is the epoxidation of an olefin using polymer-supported telluric acid. However, in that paper, the structure of telluric acid was not determined unequivocally, and the reaction mechanism remains unclear. Furthermore, the authors stated that the epoxidation does not proceed via telluric acid that is not supported on the polymer.4
On the other hand, there are some reports on epoxidation reactions using selenium, which, like tellurium, is a chalcogen. Sharpless and Sheldon have independently reported epoxidation reactions that use a catalytic amount of selenic acid.5 However, these reactions are limited due to the requirement of e.g. a fluorous solvent and high-concentrations of hydrogen peroxide. Goodman has reported an epoxidation reaction using selenoxide and hydrogen peroxide.6 Although this reaction proceeds under mild conditions, it is not an effective generic epoxidation reaction. Trost has reported the epoxidation of olefins using stoichiometric amounts of selenic acid and hydrogen peroxide.7
In the present study, we focused on tellurium, which is more likely to adopt a hypervalent state compared to selenium, and investigated the epoxidation of olefins using diaryltellurium dicarboxylates.
Synthesis
Initially, we investigated the epoxidation of olefins using diaryltellurium dicarboxylates under a variety of reaction conditions. The diaryltellurium dicarboxylates used in the reaction can be easily obtained using our previously developed method, which involves the photooxidation of telluride and carboxylic acid under an oxygen atmosphere (Scheme 1).3For that purpose, a solution of citronellyl acetate (1a), the catalyst (10 mol%), and the oxidant [urea hydrogen peroxide (UHP)] was stirred under reflux conditions. The product yields are summarized in Table 1. We found that bis(2,4,6-triisopropylphenyl) tellurium diacetate (Tip2Te(OAc)2) and dimesityl tellurium diacetate (Mes2Te(OAc)2) (Table 1, entries 3 and 4), i.e., diaryltellurium dicarboxylates with substituents at the ortho position, act as effective catalysts for this reaction, whereas diphenyl tellurium diacetate (Ph2Te(OAc)2), bis(4-methoxyphenyl) tellurium diacetate (An2Te(OAc)2), and dimesityl tellurium bis(2,2,2-trifluoroacetate) (Mes2Te(OTFA)2) (Table 1, entries 1, 2, and 5) are ineffective. We also discovered that the reaction temperature is important for the progress of the reaction. The yield of the product decreased when low-boiling-point solvents such as methylene chloride or THF were used (Table 1, entries 7 and 9). When ethyl acetate, which has a boiling point that is close to that of chloroform, was used in the reaction, the product was obtained in 75% yield. On the other hand, when methanol was used in this reaction, the yield of the product decreased, probably due to the limited solubility of dimesityl tellurium diacetate in methanol (Table 1, entries 8 and 11). Moreover, when toluene was used, the reaction did not proceed, most likely because the boiling point of toluene is too high, which probably leads to the premature decomposition of the organotellurium compounds and/or UHP (Table 1, entry 10). Furthermore, when a hydrogen-peroxide solution was used as the oxidizing agent for this reaction, almost no product was formed. However, the epoxide-ring-opened product 6,7-dihydroxy-3,7-dimethyloctyl acetate was obtained in 45% yield (Table 1, entry 13). Previous studies have shown that telloxide is effective in a variety of oxidative reactions.2 However, when telloxide was used in this reaction, virtually no product was obtained. The best results for this model reaction were obtained using a solution of Mes2Te(OAc)2 as the catalyst and UHP as the oxidant in refluxing chloroform (Table 1, entry 4).
| Entry | Catalyst | Oxidant | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Conditions: citronellyl acetate (1 mmol), catalyst (0.1 mmol), oxidant (4 mmol), solvent (2 mL), reflux, 12 h. b Isolated yield. | ||||
| 1 | Ph2Te(OAc)2 | UHP | CHCl3 | 21 |
| 2 | An2Te(OAc)2 | UHP | CHCl3 | 16 |
| 3 | Tip2Te(OAc)2 | UHP | CHCl3 | 53 |
| 4 | Mes2Te(OAc)2 | UHP | CHCl3 | 98 |
| 5 | Mes2Te(OTFA)2 | UHP | CHCl3 | 33 |
| 6 | Mes2TeO | UHP | CHCl3 | 20 |
| 7 | Mes2Te(OAc)2 | UHP | CH2Cl2 | 28 |
| 8 | Mes2Te(OAc)2 | UHP | EtOAc | 75 |
| 9 | Mes2Te(OAc)2 | UHP | THF | 30 |
| 10 | Mes2Te(OAc)2 | UHP | Toluene | 28 |
| 11 | Mes2Te(OAc)2 | UHP | MeOH | 53 |
| 12 | Mes2Te(OAc)2 | UHP (2 eq.) | CHCl3 | 63 |
| 13 | Mes2Te(OAc)2 | 30% H2O2 aq. | CHCl3 | Trace |
| 14 | Mes2Te(OAc)2 (5 mol%) | UHP | CHCl3 | 27 |
| 15 | None | UHP | CHCl3 | n.r. |
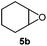
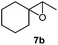
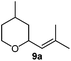
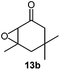
Next, we used the optimal reaction conditions to investigate the substrate scope of the reaction (Table 2). When racemic citronellic acetate (1a) or (−)-β-citronellol (2a) was treated with Mes2Te(OAc)2 and UHP, the corresponding epoxides (Table 2, entries 1 and 2) were obtained in good yields. 1b and 2b are enantiomeric/diastereomeric mixtures and diastereomeric mixtures, respectively, although it was difficult to spectroscopically analyze these diastereomers by e.g. NMR. These were chosen based on the report by Davis et al.8 Furthermore, alkyl olefins and cyclic olefins also furnished the corresponding epoxides (Table 2, entries 3–7) in high yields. Styrene (8a) was less suitable, resulting in a lower product yield (Table 2, entry 8). When racemic rose oxide (9a) was used under otherwise identical reaction conditions, the corresponding product was obtained in a moderate yield; it should also be noted here that 9b is a diastereomeric mixture that was difficult to analyze spectroscopically by e.g. NMR analysis (Table 1, entry 10). The epoxidation of (R)-(+)-limonene (11a), in which there are two olefins, i.e., bi- and tri-substituted olefin moieties, produced the corresponding mono-epoxides (11b) and di-epoxides (11c) (Table 1, entry 11). Since only the tri-substituted olefin is epoxidized in the mono-epoxide products, it seems feasible to conclude that under the applied reaction conditions, the epoxidation of the tri-substituted olefin occurs preferentially. Furthermore, when this reaction was conducted using an excess of UHP (8 equivalents), only the di-epoxides (11c) were obtained in a good yield (81%); it is worth noting here that 11b has two isomers, while 11c has four isomers. It was possible to identify 11b and 11c based on literature values,9 although it was difficult to separately analyze each isomer. (R)-(−)-Carvone (12a) and isophorone (13a), which contain an olefinic moiety conjugated to a carbonyl group, did not react under these conditions (Table 2, entries 12 and 13), which suggests that this reaction involves an electrophilic addition to an olefin with an electron-deficient oxygen atom. Epoxidation of cholesterol derivatives (14a) under these reaction conditions produces two isomers (α and β). As evident from entry 10, epoxidation from the direction with less steric hindrance is prioritized (Table 2, entry 14). The ratio of these two diastereomers (α and β) was calculated based on the integral 1H NMR peak ratio according to Carvalho et al.10
Additional experiments were conducted to elucidate the mechanism of this reaction in more detail (Scheme 2). Mes2Te(OPh)2 (0.25 mmol) and UHP (1.25 mmol) were stirred in refluxing chloroform for 2 hours, and the resulting products were analysed. The substrate Mes2Te(OPh)2 disappeared, and the components of the reaction mixture were separated by silica gel column chromatography on silica gel to isolate 2,4,6-trimethylphenol (0.31 mmol) and benzoic acid (0.48 mmol). During further analysis of the reaction mixture using MALDI-TOF mass spectrometry, we observed a peak derived from tellurium peroxide (for details, see the ESI†). However, this tellurium peroxide could not be isolated, and no peak derived from tellurium peroxide was observed in the NMR spectrum as the corresponding NMR peak was very broad. We think that this compound, which we have at present tentatively assigned to be tellurium peroxide, may not exist stably as a monomer and may oligomerize.
We postulate a mechanism for the epoxidation of olefins by diaryltellurium dicarboxylates, which is shown in Scheme 3. We propose that in the first step, the diaryltellurium dicarboxylates produce carboxylic acid and phenol via a reaction with hydrogen peroxide to form telluric acid anhydride (Scheme 3, C; this reaction is similar to the cumene process). In the next step, another molecule of hydrogen peroxide transforms the telluric acid anhydride into telluric acid peroxide (Scheme 3, D). The telluric acid peroxide then electrophilically attacks the olefin double bond to form a stereospecific epoxide (Scheme 3, E). Telluric acid (Scheme 3, F) then reacts with hydrogen peroxide and is used again for epoxidation.
Conclusions
We have developed an olefin-epoxidation reaction using diaryltellurium dicarboxylates. The optimal conditions for this reaction involve the use of Mes2Te(OAc)2 and urea hydrogen peroxide (UHP). This method is effective for the epoxidation of various olefins, and we discovered that diaryltellurium dicarboxylates react with hydrogen peroxide, phenol, and carboxylic acid to generate tellurium peroxides.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Dr. Ryoji Tanaka and Mrs. Kaori Sasaki, a former laboratory member, for helping to create the cover picture.Notes and references
- S. Koguchi, Y. Shibuya, Y. Igarashi and H. Takemura, Synlett, 2019, 30, 99 CrossRef CAS.
- (a) A. Mihoya, Y. Shibuya, A. Ito, A. Toyoda, M. Oba and S. Koguchi, Synlett, 2020, 31, 2043 CrossRef CAS; (b) A. Mihoya, S. Koguchi, Y. Shibuya, M. Mimura and M. Oba, Catalysts, 2020, 10, 398 CrossRef CAS.
- Y. Shibuya, A. Toyoda, S. Ohmura, G. Higashikawa and S. Koguchi, RSC Adv., 2021, 11, 32837 RSC.
- J. D. White, E. G. Nolen Jr. and C. H. Miller, J. Org. Chem., 1986, 51, 1150 CrossRef CAS.
- (a) T. Hori and K. B. Sharpless, J. Org. Chem., 1978, 43, 1689 CrossRef CAS; (b) G. J. ten Brink, B. C. M. Fernandes, M. C. A. van Vliet, I. W. C. E. Arends and R. A. Sheldon, J. Chem. Soc., Perkin Trans. 1, 2001, 224 RSC.
- M. A. Goodman and M. R. Detty, Synlett, 2006, 1100 CAS.
- B. M. Trost and Y. Matsumura, J. Org. Chem., 1977, 42, 2036 CrossRef CAS PubMed.
- C. E. Davis, J. L. Bailey, J. W. Lockner and R. M. Coates, J. Org. Chem., 2003, 68, 75 CrossRef CAS PubMed.
- (a) D. Steiner, L. Ivison, C. T. Goralski, R. B. Appell, J. R. Gojkovic and B. Singaram, Tetrahedron: Asymmetry, 2002, 13, 2359 CrossRef CAS; (b) L. Schutz, F. Kazemi, E. Mackenzie, J. Bergeron, E. Gagnon and J. P. Claverie, J. Polym. Sci., 2021, 59, 321 CrossRef CAS.
- (a) J. F. S. Carvalho, M. M. C. Silva and M. L. Melo, Tetrahedron, 2009, 65, 2773 CrossRef CAS; (b) J. F. S. Carvalho, M. M. C. Silva, J. N. Moreira, S. Simoes and M. L. Melo, J. Med. Chem., 2009, 52, 4007 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob01020h |
| This journal is © The Royal Society of Chemistry 2022 |


























![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:
