Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A convenient synthetic route to (2S,4S)-methylproline and its exploration for protein engineering of thioredoxin†
Andrea
Caporale‡
,
Jennie
O′ Loughlin
,
Yannick
Ortin
and
Marina
Rubini
 *
*
School of Chemistry, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: marina.rubini@ucd.ie
First published on 14th July 2022
Abstract
4-Substituted prolines, especially 4-fluoroprolines, have been widely used in protein engineering and design. Here, we report a robust and stereoselective approach for the synthesis of (2S,4S)-methylproline starting from (2S)-pyroglutamic acid. Incorporation studies with both (2S,4R)- and (2S,4S)-methylproline into the Trx1P variant of the model protein thioredoxin of E. coli show that the stereochemistry of the 4-methyl group might be a key determinator for successful incorporation during ribosomal synthesis of this protein.
Proline (Pro) presents unique features among the proteinogenic amino acids and plays crucial structural and functional roles in peptides and proteins.1 Two of its backbone atoms are constrained in the pyrrolidine ring, thus increasing the local structural rigidity. In peptides and proteins, the likelihood for the adoption of a cis-peptide bond increases from 0.04% for non-prolyl peptide bonds up to 6% for Xaa–Pro bonds (Xaa = any amino acid).2 The trans-to-cis-isomerisation of prolyl–peptide bonds is a source of slow folding phases both in vivo and in vitro for proteins containing a cis-Pro in the native state.3 The pyrrolidine ring of Pro adopts mainly the Cγ-endo (Cγ and Pro carbonyl group are on the same side of the plane) or the Cγ-exo (Cγ and Pro carbonyl group are on opposite sides of the plane) pucker. Both the conformational preference of the ring and the cis/trans equilibrium of the peptide bond can be modulated by installing electron-withdrawing or sterically demanding substituents on the Cγ atom.4 In fact, the endo pucker conformation enhances the stability of the cis peptide bond, while the exo conformation enhances the stability of the trans peptide bond.5 Therefore, 4-substituted Pro analogues (especially 4-fluoroproline analogues) have been widely used in molecular design e.g. for tuning the conformational stability of peptides and proteins,6 for investigations of protein folding kinetics,7 for studying structure–activity relationships,8 and for developing novel peptide-based materials.9 However, 4-methylprolines (4-Mep), that could explore steric instead of electronic effects, have been by far less exploited in peptide/protein engineering in comparison to 4-fluoroproline analogues. A convenient access to 4-Mep could enable such complementary studies to those performed with 4-fluoroproline.
Furthermore, the non-proteinogenic amino acid (2S,4S)-methylproline (4S-Mep) is present in several secondary metabolites, predominantly from marine organisms, endowed with antibiotic, anticancer, cytotoxic, and anti-proteolytic activities.10 Therefore, a simple and highly diastereoselective synthetic route for 4S-Mep would also be advantageous for the synthesis of various natural products and drugs. The 4-Mep commercially available diastereomers, especially (2S,4S)-Mep, are more expensive than the corresponding 4-fluoroprolines analogues. Although approaches for the synthesis of 4-Mep are reported in the literature, most protocols do not offer a unique synthetic route to each diastereomer. Moreover, difficult separations, long synthetic routes and the use of unusual and/or expensive reagents are required in order to obtain both 4-Mep isomers with high enantioselectivity.11 The original protocol for the synthesis of 4-Mep reported by Del Valle and Goodman relied on stereodivergent hydrogenations of a 4-exomethylene-proline.11a A more recent procedure involving the use of Crabtree's catalyst provided a succinct synthesis of 4-methylprolines with an improved stereoselectivity for the 4R-Mep derivate.11b An alternative approach to catalytic hydrogenation based on the use of sterically bulky Evans’ chiral auxiliaries was also recently reported for the synthesis of the four diastereoisomers of Boc-protected 4-methylproline.11c Finally, a novel synthetic route that enables the control of the stereochemistry of the substituents at the Cγ position by employing phase transfer catalysis was later reported by Hulme and co-workers.11g
Most synthetic routes towards the synthesis of 4-Mep involve the hydrogenation of a 4-methylenepyrrolydine intermediate, sometimes entailing expensive and toxic catalysts (Table 1).
| Substrate | Reaction conditions | dra | Ref. |
|---|---|---|---|
| a Diastereomeric ratio. | |||

|
Crabtree's catalyst, CHCl3, H2, rt, 5 days | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 1 4R 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
11b |

|
Pd/C, CH2Cl2, H2, rt, 16 h | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 70 4R 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
11b |

|
PtO2, MeOH, 1 atm, 20 h | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 80 4R 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
12 |

|
Pd/C 5%, H2O, 2.96 atm, 8 h | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 90 4R 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
13 |
|
Pd/C 10%, 2-propanol, 1 atm, 18 h | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 16 4R 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 |

|
PtO2, MeOH, 1 atm, 12 h | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 50 4R 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
15 |

|
Crabtree's catalyst, CH2Cl2, H2, 1 atm, 18 h | 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 1 4R 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
11a |

|
(1) Pd/C, MeOH, 1 bar | (1) 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 25 4R 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
11a |
| (2) RANEY®-Ni, MeOH, 1 bar | (2) 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 30 4R 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
||
All reported procedures strive to increase the poor control of the stereochemistry of the 4-methyl group during hydrogenation using sterically hindered catalysts or substituents on the modified proline. In this work, we report on a different strategy that yields a robust and highly stereoselective approach for the synthesis of 4S-Mep, based on cheap and commercially available (2S)-pyroglutamic acid.
Initially, we reproduced the hydrogenation protocol reported by Munro and coworkers using (2S,4R/S)-hydroxyproline as starting material (Scheme 1). Briefly, the carboxylic group was protected by esterification with thionyl chloride in methanol followed by N-tert-butyl-oxycarbonylation (Boc protection) using published protocols.16 Compound 1 was oxidized with trichloroisocyanuric acid (TCCA) and catalytic TEMPO to afford 2 in 90% yield after flash chromatography purification.11a Wittig olefination of the resulting oxopyrrolidine was undertaken according to literature procedures to deliver 3 with a 35% overall yield after purification.17 Hydrogenation of compound 3 with 10% Pd/C gave isomers 4 and 5 (ratio 4S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4R 65
4R 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35), consistent with literature data. The ratio between the two diastereoisomers was determined using a chiral HPLC column packed with cellulose tris (4-methylbenzoate) coated on silica-gel. NMR analysis on the Cα peak areas confirmed the ratio calculated using analytical HPLC (see ESI†). The stereoselectivity for the synthesis of (4S)-Mep achieved in this route was not satisfactory for our purpose of incorporating this non-proteinogenic amino acid into a model protein. However, this route can be exploited for the synthesis of further modified proline analogues. In fact, we used intermediate 2 to synthesize 4,4-difluoroproline in good yields using DAST (diethylaminosulfur trifluoride) for nucleophilic fluorination.18
35), consistent with literature data. The ratio between the two diastereoisomers was determined using a chiral HPLC column packed with cellulose tris (4-methylbenzoate) coated on silica-gel. NMR analysis on the Cα peak areas confirmed the ratio calculated using analytical HPLC (see ESI†). The stereoselectivity for the synthesis of (4S)-Mep achieved in this route was not satisfactory for our purpose of incorporating this non-proteinogenic amino acid into a model protein. However, this route can be exploited for the synthesis of further modified proline analogues. In fact, we used intermediate 2 to synthesize 4,4-difluoroproline in good yields using DAST (diethylaminosulfur trifluoride) for nucleophilic fluorination.18
 | ||
| Scheme 1 Synthetic route to (2S,4S)-methylproline and (2S,4R)-methylproline starting from protected (2S,4R/S)-hydroxyproline: (a) TCCA, TEMPO, CH2Cl2; (b) KOtBu, CH3P(Ph)3Br, THF; (c) EtOAc, Pd/C. | ||
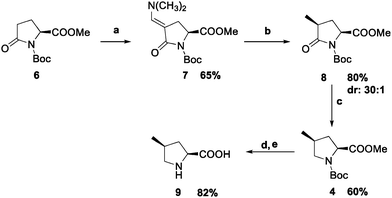
We then established an alternative synthetic route to (4S)-Mep to enhance stereoselectivity during hydrogenation using the Pd/C catalyst (Scheme 2). (2S)-Pyroglutamic acid was subjected to esterification with thionyl chloride in methanol followed by protection of the amino group with tert-butyloxycarbonyl (Boc) according to literature procedures to give compound 6.16b
Compound 6 was reacted with Bredereck's reagent in toluene at 100 °C to give the enaminone 7 in 65% yield.
Bredereck's reagent is used for this step as it has been reported that direct methylation of pyroglutamate ester analogs using methyl iodide in the presence of a strong base leads to a mixture of monoalkylated and dialkylated products without cis-stereospecificity.19 The enaminone 7 was hydrogenated with Pd/C (200 psi) in isopropanol/ethyl acetate to give the 4S-methylpyroglutamate 8 as the main product by a process involving reduction, elimination and reduction. In fact, the less hindered Cγ-endo side was almost quantitatively preferred during the hydrogenation with Pd/C resulting in high cis-stereospecificity (dr = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which is consistent with literature reports for similar reactions16b,20 and represents the highest selectivity for the installation of the 4S-methyl group into the proline scaffold reported to date. The carbonyl group on Cδ was reduced chemoselectively using borane dimethylsulfide (BH3Me2S) to give compound 4. Using NMR and mass spectroscopy analysis, we identified the Boc protected (2S,4S)-methylprolinol as the main by-product of this reduction reaction, which became the major product when the reaction time was increased above 16 h or a large excess (>2.5 eq.) of BH3Me2S was used. Methylprolinol can be recovered and the hydroxyl group can be oxidized with Jones reagent to minimise loss of material.11a,21 The following steps were the hydrolysis of the methyl ester using LiOH and finally the cleavage of the Boc protecting group under mild conditions (TFA/CH2Cl2) to give the (2S,4S)-Mep 9.22
1), which is consistent with literature reports for similar reactions16b,20 and represents the highest selectivity for the installation of the 4S-methyl group into the proline scaffold reported to date. The carbonyl group on Cδ was reduced chemoselectively using borane dimethylsulfide (BH3Me2S) to give compound 4. Using NMR and mass spectroscopy analysis, we identified the Boc protected (2S,4S)-methylprolinol as the main by-product of this reduction reaction, which became the major product when the reaction time was increased above 16 h or a large excess (>2.5 eq.) of BH3Me2S was used. Methylprolinol can be recovered and the hydroxyl group can be oxidized with Jones reagent to minimise loss of material.11a,21 The following steps were the hydrolysis of the methyl ester using LiOH and finally the cleavage of the Boc protecting group under mild conditions (TFA/CH2Cl2) to give the (2S,4S)-Mep 9.22
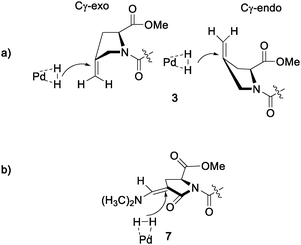
All procedures reported so far for the hydrogenation of a methylene pyrrolidine substrate showed that the crucial step for stereoselectivity is the heterogeneous hydrogenation.10b,11a,b,15,16b,20d During the hydrogenation catalyzed by Pd/C, the alkene is absorbed on the surface of the catalyst before the hydrogen shifts from the metal to the carbon sp2 orbitals. The resulting saturated hydrocarbon leaves the catalyst surface. During this process, the preferential face of hydrogenation of the substrate depends on the puckering of the molecule. If no steric constraint is present, the hydrogenation results in a low stereoselectivity because of the presence of different puckering isomers.23 The predominant ring pucker of substituted proline can be biased by hydrogen bonding or by the functionalization of Cγ with either hindered functional groups or electronegative substituents.4 Hydrogenation of 3 (Scheme 3a) with Pd/C showed a slight preference for the formation of the 4S product. This preference can be explained by the favouring of its endo ring pucker in solution. H2 will be added across the C–C double bond at the less hindered face, resulting in a preference for the 4S product when 3 adopts the endo pucker. Nevertheless, a significant amount of the 4R product is also formed as 3 also significantly populates the exo pucker. In contrast, the Boc-protected oxopyrrolidine ester 7 used in our synthesis adopts a single, pseudo planar conformation (Scheme 3b) due to the presence of the carboxy group on the Cδ carbon. For this reason, the hydrogenation of 7 occurred almost exclusively on the less hindered face of the pyroglutamate ring, resulting in the formation of the 4S diastereomer. In this case, the stereoselectivity is elicited by the steric bulk of the substituents around the double bond, as previously observed.16b
Next, we investigated the suitability of both 4-Mep isomers as substrates for ribosomal translation. We attempted the incorporation of 4S-Mep and commercially available 4R-Mep into the thioredoxin variant Trx1P of E. coli, which contains cisPro76 as a single proline residue.6c
Briefly, expression of Trx1P with a His6 tag at the C-terminus (Trx1P-His6) in the presence of 2.5 mM 4S- or 4R-Mep was performed in M9 minimal medium as previously described,7b using the proline auxotrophic cell strain CAG18515 transformed with an additional plasmid for overexpression of an engineered E. coli prolyl-tRNA-synthetase, ProRS (C443G) to increase the incorporation of the non-natural proline analogue.24 After purification by Ni-NTA affinity chromatography, the identity of the purified protein was assessed by mass spectrometry.
In the presence of 4S-Mep only wild type Trx1P was detected. In-gel trypsin digest was performed to exclude the accumulation of the modified protein in inclusion bodies due to solubility issues. However, the peptide fragments also only showed natural Pro at position 76, indicating that either 4S-Mep had not been incorporated into the protein (e.g. due to poor recognition by E. coli prolyl-tRNA-synthetase) or that the protein containing 4S-Mep at position 76 was intracellularly degraded due to destabilization or misfolding. Conversely, 4R-Mep could be incorporated into Trx1P under the same conditions in place of cisPro76 with more than 60% incorporation yield (Fig. 1). These results could also potentially be explained by the pucker preferences of the 4-Mep isomers. In fact, 4S-Mep favours the exo pucker of the pyrrolydine ring, while 4R-Mep favours the adoption of an endo pucker.6i In the thioredoxin fold there is an almost unique preference for the endo pucker of the conserved Pro, which is in cis conformation, in the context of the tertiary structure.6c Therefore, the partial incorporation of 4R-Mep, could potentially be attributed to the preferred adoption of the endo pucker in the protein's tertiary structure. In fact, we have previously reported that the pucker preferences of proline analogues can play a substantial role in the success of incorporation experiments.7c
Conclusions
(2S,4S)-4-Methylproline was successfully synthesized in 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr from (2S)-pyroglutamic acid as the starting material. This synthetic route is based on the use of reagents and equipment readily available also in resource-limited settings. We have reported for the first time the attempt to incorporate 4-Mep isomers into a globular protein. The results from the incorporation experiments with both 4-Mep isomers into the model protein Trx1P showed the suitability of (4R)-Mep for ribosomal protein translation. Further studies will be needed to fully explore the reasons behind the lack of incorporation of (4S)-Mep into this protein. These and similar studies will be enabled by the straightforward synthesis method established here.
1 dr from (2S)-pyroglutamic acid as the starting material. This synthetic route is based on the use of reagents and equipment readily available also in resource-limited settings. We have reported for the first time the attempt to incorporate 4-Mep isomers into a globular protein. The results from the incorporation experiments with both 4-Mep isomers into the model protein Trx1P showed the suitability of (4R)-Mep for ribosomal protein translation. Further studies will be needed to fully explore the reasons behind the lack of incorporation of (4S)-Mep into this protein. These and similar studies will be enabled by the straightforward synthesis method established here.
Author contributions
Andrea Caporale: investigation, data curation, methodology, conceptualization, writing first draft. Jennie O'Loughlin: methodology, investigation, writing first draft. Yannick Ortin: NMR data analysis. Marina Rubini: conceptualization, supervision, funding acquisition, finalisation of manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MR and AC would like to acknowledge the Synthesis and Solid State Pharmaceutical Centre (SSPC), funded by Science Foundation Ireland (grant number 12/RC/2275_P2), for financial support. JOL and MR are grateful to the UCD School of Chemistry and to the Seed Funding Scheme (SF1888) for financial support. MR would like to thank Vincent Conticello for the pWK2 plasmid containing the E. coli ProRS (C443G) gene and Stephan Hacker for fruitful discussion.Notes and references
- (a) M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS PubMed; (b) K. P. Lu, G. Finn, T. H. Lee and L. K. Nicholson, Nat. Chem. Biol., 2007, 3, 619–629 CrossRef CAS PubMed; (c) G. Fischer, Chem. Soc. Rev., 2000, 29, 119–127 RSC.
- A. Jabs, M. S. Weiss and R. Hilgenfeld, J. Mol. Biol., 1999, 286, 291–304 CrossRef CAS PubMed.
- U. Reimer, G. Scherer, M. Drewello, S. Kruber, M. Schutkowski and G. Fischer, J. Mol. Biol., 1998, 279, 449–460 CrossRef CAS PubMed.
- H. K. Ganguly and G. Basu, Biophys. Rev., 2020, 12, 25–39 CrossRef CAS PubMed.
- (a) M. L. DeRider, S. J. Wilkens, M. J. Waddell, L. E. Bretscher, F. Weinhold, R. T. Raines and J. L. Markley, J. Am. Chem. Soc., 2002, 124, 2497–2505 CrossRef CAS PubMed; (b) N. V. Costantini, H. K. Ganguly, M. I. Martin, N. A. Wenzell, G. P. A. Yap and N. J. Zondlo, Chem. – Eur. J., 2019, 25, 11356–11364 CrossRef CAS PubMed.
- (a) T. Steiner, P. Hess, J. H. Bae, B. Wiltschi, L. Moroder and N. Budisa, PLoS One, 2008, 3, e1680 CrossRef PubMed; (b) C. Renner, S. Alefelder, J. H. Bae, N. Budisa, R. Huber and L. Moroder, Angew. Chem., Int. Ed. Engl., 2001, 40, 923–925 CrossRef CAS; (c) M. Rubini, M. A. Scharer, G. Capitani and R. Glockshuber, ChemBioChem, 2013, 14, 1053–1057 CrossRef CAS PubMed; (d) D. Naduthambi and N. J. Zondlo, J. Am. Chem. Soc., 2006, 128, 12430–12431 CrossRef CAS PubMed; (e) T. Y. Zheng, Y. J. Lin and J. C. Horng, Biochemistry, 2010, 49, 4255–4263 CrossRef CAS PubMed; (f) K. Deepankumar, S. P. Nadarajan, N. Ayyadurai and H. Yun, Biochem. Biophys. Res. Commun., 2013, 440, 509–514 CrossRef CAS PubMed; (g) U. Arnold and R. T. Raines, Org. Biomol. Chem., 2016, 14, 6780–6785 RSC; (h) H. C. Tang, Y. J. Lin and J. C. Horng, Proteins, 2014, 82, 67–76 CrossRef CAS PubMed; (i) M. D. Shoulders, J. A. Hodges and R. T. Raines, J. Am. Chem. Soc., 2006, 128, 8112–8113 CrossRef CAS PubMed; (j) M. D. Shoulders, K. A. Satyshur, K. T. Forest and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 559–564 CrossRef CAS; (k) S. K. Holmgren, L. E. Bretscher, K. M. Taylor and R. T. Raines, Chem. Biol., 1999, 6, 63–70 CrossRef CAS; (l) M. D. Shoulders and R. T. Raines, Adv. Exp. Med. Biol., 2009, 611, 251–252 CrossRef CAS PubMed; (m) D. Dietz, V. Kubyshkin and N. Budisa, ChemBioChem, 2015, 16, 403–406 CrossRef CAS PubMed.
- (a) D. Roderer, R. Glockshuber and M. Rubini, ChemBioChem, 2015, 16, 2162–2166 CrossRef CAS PubMed; (b) J. O′ Loughlin, S. Napolitano and M. Rubini, ChemBioChem, 2021, 22, 3326–3332 CrossRef PubMed; (c) M. D. Crespo and M. Rubini, PLoS One, 2011, 6(5), e19425 CrossRef CAS PubMed.
- C. Held, H. Hubner, R. Kling, Y. A. Nagel, H. Wennemers and P. Gmeiner, ChemMedChem, 2013, 8, 772–778 CrossRef CAS PubMed.
- (a) R. S. Erdmann and H. Wennemers, J. Am. Chem. Soc., 2012, 134, 17117–17124 CrossRef CAS PubMed; (b) M. R. Aronoff, J. Egli, M. Menichelli and H. Wennemers, Angew. Chem., Int. Ed., 2019, 58, 3143–3146 CrossRef CAS PubMed; (c) J. Egli, C. Siebler, B. Maryasin, R. S. Erdmann, C. Bergande, C. Ochsenfeld and H. Wennemers, Chem. – Eur. J., 2017, 23, 7938–7944 CrossRef CAS PubMed; (d) R. S. Erdmann and H. Wennemers, Angew. Chem., Int. Ed., 2011, 50, 6835–6838 CrossRef CAS PubMed.
- (a) L. Liu, J. Jokela, L. Herfindal, M. Wahlsten, J. Sinkkonen, P. Permi, D. P. Fewer, S. O. Doskeland and K. Sivonen, ACS Chem. Biol., 2014, 9, 2646–2655 CrossRef CAS PubMed; (b) K. Fukuhara, K. Takada, S. Okada and S. Matsunaga, J. Nat. Prod., 2016, 79, 1694–1697 CrossRef CAS PubMed; (c) J. C. Kehr, D. G. Picchi and E. Dittmann, Beilstein J. Org. Chem., 2011, 7, 1622–1635 CrossRef CAS PubMed; (d) T. Teruya, H. Sasaki, H. Fukazawa and K. Suenaga, Org. Lett., 2009, 11, 5062–5065 CrossRef CAS PubMed.
- (a) J. R. Del Valle and M. Goodman, J. Org. Chem., 2003, 68, 3923–3931 CrossRef CAS PubMed; (b) A. C. Murphy, M. I. Mitova, J. W. Blunt and M. H. Munro, J. Nat. Prod., 2008, 71, 806–809 CrossRef CAS PubMed; (c) K. Sun, C. Tao, B. Long, X. Zeng, Z. Wu and R. Zhang, RSC Adv., 2019, 9, 32017–32020 RSC; (d) A. M. P. Koskinen and H. Rapoport, J. Org. Chem., 1989, 54, 1859–1866 CrossRef CAS; (e) C. Heindl, H. Hübner and P. Gmeiner, Tetrahedron: Asymmetry, 2003, 14, 3153–3172 CrossRef CAS; (f) S. R. Crosby, R. B. Sessions and C. L. Willis, Synlett, 2010, 539–542 CAS; (g) H. J. Johnston, F. S. McWhinnie, F. Landi and A. N. Hulme, Org. Lett., 2014, 16, 4778–4781 CrossRef CAS PubMed.
- S. K. Das, Y. L. Bennani, B. Liu, C. Cadilhac, J. Henderson, R. Denis, C. Poisson, M. A. Morris, L. C. C. Kong, T. J. Reddy, C. Yannopoulos, S. Giroux, L. Vaillancourt, O. Z. Pereira, G. Falardeau and J. Maxwell, WO2011/119853, Vertex Pharmaceuticals Incorporated, 2011.
- J. A. Henderson, J. Maxwell, L. Vaillancourt, M. A. Morris, R. Grey Jr., S. Giroux, L. C. C. Kong, S. K. Das, B. Liu, C. Poisson, C. Cadilhac, M. Bubenik, T. J. Reddy, G. Falardeau, C. Yannopoulos, J. Wang, O. Z. Pereira, Y. L. Bennani, A. C. Pierce, G. R. Bhisetti, K. M. Cottrell and V. Marone, WO2011/009084A2, Vertex Pharmaceuticals Incorporated, 2011.
- R. Lavoie, J. A. Bender, Z. Yang, M. Belema, O. D. Lopez, Q. Chen, G. Wang and P. Hewawasam, WO2011/059887A1, Bristol-Myers Squibb, 2011.
- C. Gardelli, E. Nizi, E. Muraglia, B. Crescenzi, M. Ferrara, F. Orvieto, P. Pace, G. Pescatore, M. Poma, R. Ferreira Mdel, R. Scarpelli, C. F. Homnick, N. Ikemoto, A. Alfieri, M. Verdirame, F. Bonelli, O. G. Paz, M. Taliani, E. Monteagudo, S. Pesci, R. Laufer, P. Felock, K. A. Stillmock, D. Hazuda, M. Rowley and V. Summa, J. Med. Chem., 2007, 50, 4953–4975 CrossRef CAS PubMed.
- (a) S. M. Dixon, K. A. Milinkevich, J. Fujii, R. Liu, N. Yao, K. S. Lam and M. J. Kurth, J. Comb. Chem., 2007, 9, 143–157 CrossRef CAS PubMed; (b) E. Coudert, F. Acher and R. Azerad, Synthesis, 1997, 1997(8), 863–865 CrossRef.
- S. Loosli, C. Foletti, M. Papmeyer and H. Wennemers, Synlett, 2019, 30, 508–510 CrossRef CAS . As previously reported in the literature, we could confirm that the reaction yields for the Wittig olefination could be increased from 35% to 70% by replacing the methylester with a tert-butyl ester.
- J. R. Sufrin, T. M. Balasubramanian, C. M. Vora and G. R. Marshall, Int. J. Pept. Protein Res., 1982, 20, 438–442 CrossRef CAS PubMed.
- J. D. Charrier, J. E. S. Duffy, P. B. Hitchcock and D. W. Young, J. Chem. Soc., Perkin Trans. 1, 2001, 2367–2371 RSC.
- (a) R. A. August, J. A. Khan, C. M. Moody and D. W. Young, Tetrahedron Lett., 1992, 33, 4617–4620 CrossRef CAS; (b) C. M. Moody and D. W. Young, Tetrahedron Lett., 1993, 34, 4667–4670 CrossRef CAS; (c) C. M. Moody and D. W. Young, Tetrahedron Lett., 1994, 35, 7277–7280 CrossRef CAS; (d) M. Katoh, H. Mizutani and T. Honda, Heterocycles, 2006, 69, 193–216 CrossRef CAS.
- U. K. Mishra and N. G. Ramesh, Tetrahedron Lett., 2020, 61, 152081 CrossRef.
- M. Rodriquez, S. Terracciano, E. Cini, G. Settembrini, I. Bruno, G. Bifulco, M. Taddei and G.-P. Luigi, Angew. Chem., Int. Ed., 2006, 45, 423–427 CrossRef CAS PubMed.
- L. Massaro, J. Zheng, C. Margarita and P. G. Andersson, Chem. Soc. Rev., 2020, 49, 2504–2522 RSC.
- W. Kim, A. George, M. Evans and V. P. Conticello, ChemBioChem, 2004, 5, 928–936 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob01011a |
| ‡ Present address: Institute of Crystallography, C.N.R., Basovizza (TS), Italy. |
| This journal is © The Royal Society of Chemistry 2022 |