Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silver-catalysed double decarboxylative addition–cyclisation–elimination cascade sequence for the synthesis of quinolin-2-ones†
C. Munashe
Mazodze
 and
Wade F.
Petersen
and
Wade F.
Petersen
 *
*
Department of Chemistry, University of Cape Town, Rondebosch, Cape Town, 7700, South Africa. E-mail: wade.petersen@uct.ac.za
First published on 11th April 2022
Abstract
An atom-efficient silver-catalysed double carboxylative strategy for the one-step synthesis of quinolin-2-ones via an addition–cyclisation–elimination cascade sequence of oxamic acids to acrylic acids, mediated either thermally or photochemically, is reported. The reaction was applicable to the synthesis of a broad range of quinolin-2-ones and featured a double-disconnection approach that constructed the quinolin-2-one core via the formal and direct addition of a C(sp2)–H/C(sp2)–H olefin moiety to a phenylformamide precursor.
Introduction
Quinolin-2-ones represent an important class of privileged nitrogen-containing heterocycles as they feature impressive biological profiles and utility in chemical biology (Fig. 1, 1–4).1–4 For example, tipifarnib (1)2 is used as an experimental agent in the treatment of cancer (FPT: IC50 = 0.86 nm [lamin B] and 7.9 nm [K-RasB]), while NI-42 (2) is a high affinity BRPF inhibitor (BRPF1: IC50 = 7.9 nm).3 Trifluoromethylated analogues (4) are known androgen receptor antagonists and are particularly useful fluorescence tracers in chemical biology.4 Furthermore, quinoline-2-ones serve as important synthetic building blocks for accessing their bioactive 3,4-dihydroquinolin-2-one relatives (such as 5 and 6)1—particularly toward their enantioselective synthesis.5In broad terms, the construction of quinolin-2-ones are largely envisaged via two main disconnections; either between the aryl group and the double bond (Fig. 2A-left)—via arylation processes (of the type I)6 or between the carbonyl group and the double bond (Fig. 2A-right)—via insertion into double/triple bonds (of the type II).7 Other approaches include oxidation of quinoline salts,8a ring expansion of isatins,8b,c and aldol-type ring closures.8d By contrast, we were intrigued by the notion of simultaneously disconnecting at both ends of the double bond for a more modular synthesis—formally enabling the direct installation of a C(sp2)–H/C(sp2)–H olefin moiety into a phenylformamide precursor (Fig. 2B; of the type III).9
To the best of our knowledge, only two reports of this type of approach toward the synthesis of quinolin-2-ones have been described in the literature (Fig. 2B).10 Donald and co-workers reported a photoredox catalysed addition–cyclisation sequence to produce 3,4-dihydroquinolin-2-ones, that spontaneously eliminated HCl to afford quinolin-2-ones when using chloroacrylate derived Michael acceptors.10a This however featured only 3 examples (with modest yields), and the use of the N-phthalimido esters is somewhat of a disadvantage in terms of the overall atom efficiency. Additionally, substituted chloroacrylates and chloroacrylonitriles are not easily obtained. The second report is by Feng and co-workers who instead described the use of oxamic acids and vinyl sulfones in a related transformation.10b While the use of oxamic acids is more atom efficient, the method was limited to producing only unsubstituted quinolin-2-ones at C3 and C4. In context, the work by Jiao must be highlighted.7a Here, they reported an impressive rhodium catalysed three-component reaction involving an aniline, carbon monoxide, and an internal alkyne, that could additionally assemble the amide moiety of the quinolin-2-one. Some drawbacks however involved the use of toxic CO gas, a stoichiometric amount of a transition-metal oxidant, and the need for high reaction temperatures—with minor limitations on the internal alkyne used. Inspired by these reports, we herein describe a silver-catalysed addition–cyclisation–elimination cascade sequence for the synthesis of quinolin-2-ones utilising readily available oxamic and acrylic acids via a double radical decarboxylation mediated either thermally or photochemically (Fig. 2C).
Results and discussion
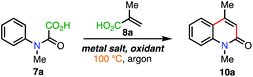
In our work toward to the synthesis of bisoxindoles, we recently reported that oxindole-derived quaternary acids of the type 9 could generate their corresponding methine radical under oxidative conditions,11 and hypothesised that when applied to 6-membered systems (9), a relatively fast H-abstraction would produce the desired quinolin-2-ones 10—rather than a 3,4-dihydroquinolin-2-one dimer as per our previous work11—due to the stability of the extended π-system of 10. This led us to identify readily available and/or easily synthesised acrylic acids (8) and oxamic acids (7) as key starting materials to facilitate a more atom efficient oxidative cascade sequence through the loss of CO2 (Fig. 2C). Indeed, radical decarboxylation strategies12 and related deoxygenations13 have emerged as powerful tools toward enabling sustainable and atom efficient transformations.In search of a suitable set of oxidative conditions for our envisaged cascade, our optimisation studies began using oxamic 7a and acrylic acid 8a as model substrates (Table 1, see the ESI† for full data). In accordance with our prior work,11 we first investigated the use of stoichiometric of Mn(OAc)3 as the oxidant for the reaction—the use of 3 equiv. accounting for each of the 3 independent oxidation steps (Table 1, entry 1). Gratifyingly, the desired quinolin-2-one 10a was produced in 52% yield. Although pleasing, we were acutely aware of the significant drawbacks associated with the use of super stoichiometric transition-metal metals in the context of sustainability. To this end, we opted for 50 mol% Mn(OAc)3·2H2O (∼17 mol% effective loading over the 3 steps) in the presence of K2S2O8—a low-cost and readily available oxidant14—which produced 10a in a poor 16% yield (Table 1, entry 2). The use of AgNO3/K2S2O8 is a well-known system for net oxidative transformations, particularly in the context of decarboxylation chemistry.6a,14 Pleasingly, an improved 34% yield was obtained in the presence of 20 mol% AgNO3 (Table 1, entry 3), while 50 mol% AgNO3 produced the best set of conditions; affording 10a in 58% yield (Table 1, entry 4). It is worth noting that 50 mol% AgNO3 (17 mol% effective loading per oxidation step) was found to be optimum as higher loadings (such as 80 and 100 mol%) did not affect the yield and 30 mol% afforded 10a in 40% yield (see ESI†). Carrying out the reaction at room temperature was ineffective for the reaction (Table 1, entry 5, while performing the reaction in the absence of a metal salt afforded 10a in 35% yield (Table 1, entry 6). Various other metal salts, solvent systems, and persulfates where also investigated, but these were all found to produce inferior results (see the ESI†).
| Entry | Metal salt (mol%) | Oxidant (equiv.) | Solvent | Yielda (%) |
|---|---|---|---|---|
| a Determined by 1H NMR (1,3,5-trimethoxybenzene standard). b As the dihydrate. c Reaction carried out at room temperature. d Starting material recovered. | ||||
| 1 | — | Mn(OAc)3b (3) | PhMe | 52 |
| 2 | Mn(OAc)3b (50) | K2S2O8 (3) | ACN/H2O | 16 |
| 3 | AgNO3 (20) | K2S2O8 (3) | ACN/H2O | 34 |
| 4 | AgNO 3 (50) | K 2 S 2 O 8 (3) | ACN/H 2 O | 58 |
| 5c,d | AgNO3 (50) | K2S2O8 (3) | ACN/H2O | Trace |
| 6 | None | K2S2O8 (3) | ACN/H2O | 35 |
Reactions that require significant heating are very energy intensive and therefore raises sustainability concerns in the context of global energy security. Furthermore, accessing these chemistries are particularly challenging in regions with fragile or severely constrained national energy grids—especially when extended heating periods are required.15 Indeed, high temperatures preclude thermally sensitive starting materials and products. For this reason, there is notable value in developing room temperature alternatives for these processes. In this light, and with the chemistry of the cascade sequence established, we set out to develop a comparable photoredox-mediated room temperature alternative. As mentioned, the room temperature reaction as per Table 1, entry 5 was found to be ineffective and this is due to the high activation barrier associated with disproportionation of K2S2O8 to afford its highly oxidising SO4˙− radical anion.16 On the other hand, photoexcited ruthenium complexes are known to efficiently reduce persulfate anions (S2O82−) to their corresponding sulfate radical anions (SO4˙−) at room temperature,14c,17 and we therefore envisaged that employing this strategy would enable our cascade sequence to proceed efficiently at room temperature. In the event, modifying our reaction conditions accordingly produced 10a in a comparable 54% yield when using [Ru(Bpz)3](PF6)2 in the presence of visible-light (Table 2, entry 1). Switching to the organic photocatalyst 4-CzIPN18 provided the optimum photoredox conditions; affording 10a in 58% yield (Table 2, entry 2), while acridium and iridium photocatalysts produced 10a, in 43% and 38% yields, respectively (Table 2, entries 3 and 4). In the absence of a photocatalyst, 10a was obtained in 22% yield, indicating that light activated homolysis of S2O82− is facilitated to some extent. (Table 2, entry 5). Full data is available in the ESI.† With the optimised conditions at hand, the substrate scope of the addition–cyclisation–elimination cascade sequence was investigated, paying attention to any differences between the thermal and photochemical strategies in a randomised sample set (Scheme 1). Indeed, reaction time is an obvious advantage for the thermal reaction (18 h vs. 48 h).
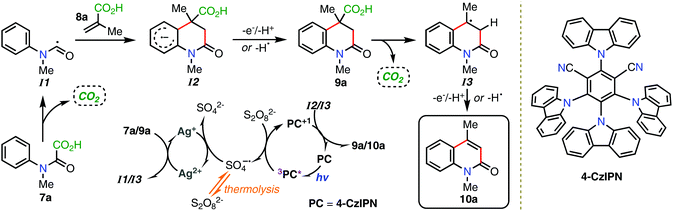
Repeating the reaction with the model system afforded 10a in 60% and 58% isolated yields for the thermal and photochemical reactions, respectively, while the mono-halogenated oxamic acids afforded 10b–10d in 55–82% yields. The 4-methoxy-substituted product 10e was produced in comparable isolated yields of 76% and 75% for the thermal and photochemical reactions, respectively. On the other hand, 10f, the 3-substituted variant was obtained in only 16% isolated yield, which improved to 57% yield under photoredox catalysis. Electron-withdrawing substituents were well tolerated, affording 10g–10i in 60–84% isolated yields. The difluorinated oxamic acid analogue 10j was obtained in 45% yield, while the dichlorinated variant 10k was produced in 42% yield, that could be improved to 53% when performed under photochemical conditions. Trisubstituted oxamic acids were also suitable, affording 10l in 43% yield, and improved to 54% yield using photoredox catalysis. As mentioned, 4-trifluoromethylated quinolin-2-ones have particularly utility in chemical biology. Pleasingly in context, 10m was produced in 54% yield using 2-(trifluoromethyl)acrylic acid, which was improved to an excellent 84% under the room temperature photochemical conditions. Modification of the nitrogen protecting group was also well tolerated affording 10n–10r in up to 82% yield, with notable increases in yield when performed under photoredox conditions (i.e.10p and 10r). Variation of the acrylic acid was also possible producing the 3-substituted quinolin-2-one 10s in 22% yield that could be significantly improved to 52% yield photochemically. Disubstituted acyclic and cyclic acrylic acids were also suitable; producing 3,4-disubstituted quinolin-2-ones 10t–10v in 52–59% isolated yields, with no obvious advantage photochemically (as per 10v). It is worth noting that compounds 10m and 10r were produced using recycled 4-CzIPN obtained in sequence during the substrate scope explorations. Specifically, 10m was obtained using the photocatalyst recovered during the synthesis of 10l, and then 10r was obtained using the photocatalyst recovered from 10l. Clear limitations to the cascade sequence, though, were apparent; both thermally and photochemically. For example, aryl substituted products (such as 10ua and 10ub) could not be produced, while 2-substituted oxamic acids were also found to be incompatible (producing 10uc). Unprotected oxamic acids were not suitable (producing 10ud) however this, in principle, could be overcome by debenzylation of 10n. Across the sample set, the photoredox-mediated reaction was either found to be comparable to the thermal reaction, or significantly outperform it. All things considered, we would deem the photoredox-mediated cascade sequence a superior strategy, particularly in the context of sustainable and energy efficient synthesis. The proposed mechanism of the reaction, using the model system as a representative example is shown in Scheme 2. Following visible-light excitation, and in accordance with previous literature,14c,17 the excited state catalyst (3PC*) reduces the persulfate anion (S2O82−) to the sulfate radical anion (SO4˙−), generating its PC+1 state. The highly oxidising SO4˙−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 then oxidises Ag+ to the active Ag2+ species16 that subsequently generates carbamoyl radical I1via radical decarboxylation of 7a, with concomitant return to its Ag+ state. It should be noted that stoichiometric AgNO3 on its own did not promote the reaction (see the ESI†), supporting Ag2+ as the active oxidizing species.16 Then, an addition–cyclisation sequence produces cyclohexadienyl radical anion I2, which is transformed into dihydroquinolin-2-one 9a following SET oxidation by PC+1 and the loss of a proton, while returning the catalyst to its ground state (PC). This overall transformation affording 9a is consistent with related literature reports.10,20 Elimination toward product 10a is envisaged to follow a similar pathway. Namely, radical decarboxylation of 9a by Ag2+ to afford methine radical I3, oxidation by PC+1 to the benzylic tertiary carbocation and finally the loss of a proton. As for the thermal reaction, SO4˙− (×2) is instead generated via thermolysis, following which the reaction mechanism proceeds as described. But, rather than SET oxidation by PC+1 and loss of a proton to afford 9a and 10a, H-abstraction by the second SO4˙− generated during thermolysis is proposed. Mechanistic studies supported the existence of carbamoyl radical I1 as well as carboxylic acid 9a as transient intermediates (Scheme 3).
19 then oxidises Ag+ to the active Ag2+ species16 that subsequently generates carbamoyl radical I1via radical decarboxylation of 7a, with concomitant return to its Ag+ state. It should be noted that stoichiometric AgNO3 on its own did not promote the reaction (see the ESI†), supporting Ag2+ as the active oxidizing species.16 Then, an addition–cyclisation sequence produces cyclohexadienyl radical anion I2, which is transformed into dihydroquinolin-2-one 9a following SET oxidation by PC+1 and the loss of a proton, while returning the catalyst to its ground state (PC). This overall transformation affording 9a is consistent with related literature reports.10,20 Elimination toward product 10a is envisaged to follow a similar pathway. Namely, radical decarboxylation of 9a by Ag2+ to afford methine radical I3, oxidation by PC+1 to the benzylic tertiary carbocation and finally the loss of a proton. As for the thermal reaction, SO4˙− (×2) is instead generated via thermolysis, following which the reaction mechanism proceeds as described. But, rather than SET oxidation by PC+1 and loss of a proton to afford 9a and 10a, H-abstraction by the second SO4˙− generated during thermolysis is proposed. Mechanistic studies supported the existence of carbamoyl radical I1 as well as carboxylic acid 9a as transient intermediates (Scheme 3).
Carrying out the reaction in the presence of TEMPO but without the acrylic acid (8a), produced TEMPO-carbamate 12a in 64% yield; supporting the formation of carbamoyl radical I1 (Scheme 3A). It should be noted that in the presence of the acrylic acid (8a), TEMPO adduct 12a was obtained as the major product in 83% yield, together with a 16% yield of the quinolin-2-one product 10a. Committing the independently synthesised quaternary 3,4-dihydroquinolin-2-one 9a to the reaction conditions using 1.2 equiv. K2S2O8 (accounting for only one decarboxylation) successfully afforded 10a in 41% yield; demonstrating that 9a is a likely intermediate in the reaction (Scheme 3B), while quaternary TEMPO intermediate—in support of methine radical I3—could not be detected in the corresponding TEMPO trapping experiment. Instead, similar conversion to 10a was observed.
Conclusions
In summary, we have developed a silver-catalysed addition–cyclisation–elimination cascade sequence for the synthesis of biologically relevant quinolin-2-ones, mediated either thermally or photochemically, and enabled by a double radical decarboxylation strategy. The reaction was applicable to a wide substrate scope and the photochemical conditions were found to be largely superior to the thermal reaction—particularly in the context of sustainability. Mechanistic studies such as TEMPO trapping experiments were used to support the proposed reaction mechanism presented. Inspired by the utility of carboxylic acids to serve as environmentally friendly and sustainable radical precursors, this work has demonstrated a formal and direct installation of an olefin moiety to an organic molecule, and it is hoped that this strategy will pioneer interesting advances in late-stage olefination chemistry.Author contributions
All synthesis investigation work was carried out by C. M. M. Conceptualisation, supervision, validation, and writing of the manuscript was completed by W. F. P. Final approval was obtained from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Central Analytical Facilities (Stellenbosch University, South Africa) for their assistance in obtaining the HRMS data. We would also like to thank the Royal Society and the African Academy of Sciences (FLR\R1\190531), the Royal Society of Chemistry (RF21-7183233767), the National Research Foundation (W. F. P., grant no: 138082; C. M. M, grant no: MND200409512018) and the University of Cape Town for their funding contributions.References
- Selected reviews: (a) S. B. Matada, R. Pattanashettar and N. G. Ternale, Bioorg. Med. Chem., 2021, 32, 115973 CrossRef PubMed; (b) W. P. Hong, I. Shin and H. E. Lim, Molecules, 2020, 25, 5450 CrossRef CAS PubMed. See also: (c) R. Uchida, R. Imasato, K. Shiomi, H. Tomoda and S. Omura, Org. Lett., 2005, 7, 5701 CrossRef CAS PubMed.
- Selected review: (a) X. Thomas and M. Elhamri, Biologics, 2007, 1, 415 CAS. See also: (b) D. W. End, G. Smets, A. V. Todd, T. L. Applegate, C. J. Fuery, P. Angibaud, M. Venet, G. Sanz, H. Poignet, S. Skrzat, A. Devine, W. Wouters and C. Bowden, Cancer Res., 2001, 61, 131 CAS; (c) A. Marcuzzi, L. De Leo, G. Decorti, S. Crovella, A. Tommasini and A. Pontillo, Pediatr. Res., 2011, 70, 78 CrossRef CAS PubMed.
- (a) N. Igoe, E. D. Bayle, O. Fedorov, C. Tallant, P. Savitsky, C. Rogers, D. R. Owen, G. Deb, T. C. P. Somervaille, D. M. Andrews, N. Jones, A. Cheasty, H. Ryder, P. E. Brennan, S. Müller, S. Knapp and P. V. Fish, J. Med. Chem., 2017, 60, 668 CrossRef CAS PubMed.
- (a) S. Pillai, M. Kozlov, S. A. E. Marras, L. N. Krasnoperov and A. Mustaev, J. Fluoresc., 2012, 22, 1021 CrossRef CAS PubMed; (b) T. L. Micotto, A. S. Brown and J. N. Wilson, Chem. Commun., 2009, 48, 7548 RSC; (c) A. Mohamadi and L. W. Miller, Bioconjugate Chem., 2016, 27, 2540 CrossRef CAS PubMed.
- (a) T. Hu, L. Lückemeier, C. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2021, 60, 23193 CrossRef CAS PubMed; (b) Q.-K. Zhao, X. Wu, F. Yang, P.-C. Yan, J.-H. Xie and Q.-L. Zhou, Org. Lett., 2021, 23, 3593 CrossRef CAS PubMed; (c) Y. Guo and S. R. Harutyunyan, Angew. Chem., Int. Ed., 2019, 58, 12950 CrossRef CAS PubMed. See also: (d) G. Li, H. Liu, G. Lv, Y. Wang, Q. Fu and Z. Tang, Org. Lett., 2015, 17, 4125 CrossRef CAS PubMed.
- (a) W.-P. Mai, G.-C. Shun, J.-T. Wang, G. Song, P. Mao, L.-R. Yang, J.-W. Yuan, Y.-M. Xiao and L.-B. Qu, J. Org. Chem., 2014, 79, 8094 CrossRef CAS PubMed; (b) X. Liu, X. Xin, D. Xiang, R. Zhang, S. Kumar, F. Zhou and D. Dong, Org. Biomol. Chem., 2012, 10, 5643 RSC; (c) Y. Kobayashi and T. Harayama, Org. Lett., 2009, 11, 1603 CrossRef CAS PubMed; (d) C. Zaugg, G. Schmidt and S. Abele, Org. Process Res. Dev., 2017, 21, 1003 CrossRef CAS; (e) C. E. Kaslow and D. J. Cook, J. Am. Chem. Soc., 1945, 67, 1969 CrossRef CAS.
- (a) X. Li, X. Li and N. Jiao, J. Am. Chem. Soc., 2015, 137, 9246 CrossRef CAS PubMed; (b) J. Hou, A. Ee, W. Feng, J.-H. Xu, Y. Zhao and J. Wu, J. Am. Chem. Soc., 2018, 140, 5257 CrossRef CAS PubMed; (c) A. Charoenpol, J. Meesin, O. Khaikate, V. Reutrakul, M. Pohmakotr, P. Leowanawat, D. Soorukram and C. Kuhakarn, Org. Biomol. Chem., 2018, 16, 7050 RSC; (d) J. Ferguson, F. Zeng, N. Alwis and H. Alper, Org. Lett., 2013, 15, 1998 CrossRef CAS PubMed; (e) W. Wang, X. Peng, X. Qin, X. Zhao, C. Ma, C.-H. Tung and Z. Xu, J. Org. Chem., 2015, 80, 2835 CrossRef CAS PubMed; (f) B. Reichart, G. G. de la Cruz, K. Zangger, C. O. Kappe and T. Glasnov, Adv. Synth. Catal., 2016, 358, 50 CrossRef CAS; Z. Zhang, L.-L. Liao, S.-S. Yan, L. Wang, Y.-Q. He, J.-H. Ye, J. Li, Y.-G. Zhi and D.-G. Yu, Angew. Chem., Int. Ed., 2016, 55, 7068 Search PubMed; (g) T. K. Hyster and T. Rovis, Chem. Sci., 2011, 2, 1606 RSC; (h) J. W. Dankwardt and L. A. Flippen, J. Org. Chem., 1995, 60, 2312 CrossRef CAS; (i) H. Fan, P. Pan, Y. Zhang and W. Wang, Org. Lett., 2018, 20, 7929 CrossRef CAS PubMed.
- (a) A. Motaleb, A. Berat and P. Maity, Org. Biomol. Chem., 2018, 16, 5081 RSC; (b) S. Lv, Y. Sun, Y. Xu, S. Yang and L. Wang, Chin. Chem. Lett., 2020, 31, 1568 CrossRef CAS; (c) M. F. Jamali, E. Gupta, A. Kumar, R. Kant and K. Mohanan, Chem. – Asian J., 2020, 15, 757 CrossRef CAS PubMed; (d) C. Weng, J. Qiao, X. Liu, H. Song, Z. Sun and W. Chu, J. Org. Chem., 2018, 83, 1422 CrossRef PubMed.
- Strictly speaking, this could be considered as a Fujiwara–Moritani type reaction. For a selected reviews see: W. Ali, G. Prakash and D. Maiti, Chem. Sci., 2021, 12, 2735 RSC.
- (a) W. F. Petersen, R. J. K. Taylor and J. R. Donald, Org. Biomol. Chem., 2017, 15, 5831 RSC; (b) C. Jin, J.-Y. He, Q.-F. Bai and G. Feng, Synlett, 2020, 31, 1517 CrossRef CAS.
- F. Dobah, C. M. Mazodze and W. F. Petersen, Org. Lett., 2021, 23, 5466 CrossRef CAS PubMed.
- Selected reviews: (a) A. Varenikov, E. Shapiro and M. Gandelman, Chem. Rev., 2021, 121, 412 CrossRef CAS PubMed; (b) M. Font, J. M. Quibell, G. J. P. Perry and I. Larrosa, Chem. Commun., 2017, 53, 5584 RSC; (c) T. Patra and D. Maiti, Chem. – Eur. J., 2017, 23, 7382 CrossRef CAS PubMed; (d) J. Xuan, Z.-G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632 CrossRef CAS PubMed. See also: (e) L. Chu, C. Ohta, Z. Zuo and D. W. G. Macmillan, J. Am. Chem. Soc., 2014, 136, 10886 CrossRef CAS PubMed; (f) C. C. Nawrat, C. R. Jamison, Y. Slutsky, D. W. C. Macmillan and L. E. Overman, J. Am. Chem. Soc., 2015, 137, 11270 CrossRef CAS PubMed; (g) A. Bhattacherjee, M. Sneha, L. Lewis-Borrell, O. Tau, I. P. Clark and A. J. Orr-Ewing, Nat. Commun., 2019, 10, 5152 CrossRef PubMed; (h) L. Candish, M. Freitag, T. Gensch and F. Glorius, Chem. Sci., 2017, 8, 3618 RSC; (i) J. D. Griffin, M. A. Zeller and D. A. Nicewicz, J. Am. Chem. Soc., 2015, 137, 11340 CrossRef CAS PubMed; (j) P. Xu, P. López-Rojas and T. Ritter, J. Am. Chem. Soc., 2021, 143, 5349 CrossRef CAS PubMed; (k) X.-J. Wei, W. Boon, V. Hessel and T. Nöel, ACS Catal., 2017, 7, 7136 CrossRef CAS PubMed; K. S. McClymont, F.-Y. Wang, A. Minakar and P. S. Baran, J. Am. Chem. Soc., 2020, 142, 8608 Search PubMed; (l) C. Shu, R. S. Mega, B. J. Andreassen, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2018, 57, 15430 CrossRef CAS PubMed; (m) K. Donabauer, M. Maity, A. L. Berger, G. S. Huff, S. Crespi and B. König, Chem. Sci., 2019, 10, 5162 RSC.
- Selected review: (a) J. A. Rossi-Ashton, A. K. Clarke, W. P. Unsworth and R. J. K. Taylor, ACS Catal., 2020, 10, 7250 CrossRef CAS PubMed. See also: (b) E. E. Stache, A. B. Ertel, T. Rovis and A. G. Doyle, ACS Catal., 2018, 8, 11134 CrossRef CAS PubMed; (c) H.-M. Guo and X. Wu, Nat. Commun., 2021, 12, 5365 CrossRef CAS PubMed; (d) A. K. Clarke, A. Parkin, R. J. K. Taylor, W. P. Unsworth and J. A. Rossi-Ashton, ACS Catal., 2020, 10, 5814 CrossRef CAS PubMed; (e) D. Rackl, V. Kais, E. Lutsker and O. Reiser, Eur. J. Org. Chem., 2017, 2130 CrossRef CAS PubMed.
- Selected reviews: (a) S. Mandal, T. Bera, G. Dubey, J. Saha and J. K. Laha, ACS Catal., 2018, 8, 5085 CrossRef CAS; (b) J. Lee, U. von Gunten and J.-H. Kim, Environ. Sci. Techol., 2020, 54, 3064 CrossRef CAS PubMed. See also: (c) C. Dai, F. Meschini, J. M. R. Narayanam and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 4425 CrossRef CAS PubMed.
- Part of this work was completed during a series of severe rolling blackouts in South Africa. This shut of power to various areas for up to 8 hours per day for several weeks. To overcome this, we implemented a UPS backup system, but these were not compatible with heated reactions, and so was the impetus for our photoredox strategy due to the low power consumption of LEDs and room temperature reactions.
- J. M. Anderson and J. K. Knochi, J. Am. Chem. Soc., 1970, 92, 1651 CrossRef CAS.
- D. A. Fancy and T. Kodadek, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 6020 CrossRef CAS PubMed.
- Selected review: T.-Y. Shang, L.-H. Lu, Z. Cao, Y. Liu, W.-M. He and B. Yu, Chem. Commun., 2019, 55, 5408 RSC.
- R. Memming, J. Electrochem. Soc., 1969, 116, 785 CrossRef CAS.
- (a) W. F. Petersen, R. J. K. Taylor and J. R. Donald, Org. Lett., 2017, 19, 874 CrossRef CAS PubMed; (b) Q.-F. Bai, C. Jin, J.-Y. He and G. Feng, Org. Lett., 2018, 20, 2172 CrossRef CAS PubMed; (c) J. E. M. N. Klein, A. Perry, D. S. Pugh and R. J. K. Taylor, Org. Lett., 2010, 12, 3446 CrossRef CAS PubMed; (d) X.-Y. Jia and E. P. Kündig, Angew. Chem., Int. Ed., 2009, 48, 461 CrossRef PubMed; (e) S. Ghosh, S. De, B. N. Kakde, S. Bhunia, A. Adhikary and A. Bisai, Org. Lett., 2012, 14, 5864 CrossRef CAS PubMed; (f) J. R. Donald, R. J. K. Taylor and W. F. Petersen, J. Org. Chem., 2017, 82, 11288 CrossRef CAS PubMed; Q. Liu, L. Wang, J. Liu, S. Ruan and P. Li, Org. Biomol. Chem., 2021, 19, 3489 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, optimisation data, and compound characterisation. See DOI: https://doi.org/10.1039/d2ob00521b |
| This journal is © The Royal Society of Chemistry 2022 |