H–F bond insertions into α-diazo carbonyl compounds†
Abstract
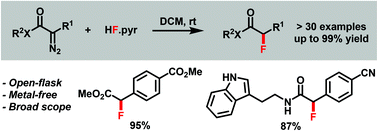
A reaction for H–F bond insertion into α-diazo carbonyl compounds is reported. The protocol describes a simple reaction setup employing commercially available HF·pyr (Olah reagent) as the fluorine source. The method is rapid and practical, and allows access to a broad range of α-fluorinated carbonyl compounds in generally good yields.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...