Open Access Article
Open Access ArticleCyclopenta-fused polyaromatic hydrocarbons: synthesis and characterisation of a stable, carbon-centred helical radical†
Stefan
Herzog
 a,
Alexander
Hinz
a,
Alexander
Hinz
 *b,
Frank
Breher
*b,
Frank
Breher
 *b and
Joachim
Podlech
*b and
Joachim
Podlech
 *a
*a
aInstitut für Organische Chemie, Karlsruher Institut für Technologie (KIT), 76131 Karlsruhe, Fritz-Haber-Weg 6, Germany
bInstitut für Anorganische Chemie, Karlsruher Institut für Technologie (KIT), 76131 Karlsruhe, Engesserstraße 15, Germany. E-mail: breher@kit.edu; alexander.hinz@kit.edu
First published on 15th March 2022
Abstract
An air- and moisture-stable helical radical with seven six- and five-membered rings arranged alternately was synthesized by cyclizations in a suitably ortho,ortho′-substituted terphenyl and re-establishment of its conjugation. Mesityl groups at the five-membered rings prevent radical reactions. This cyclopenta-fused polyaromatic hydrocarbon (CP-PAH) was characterized by X-ray crystallographic analysis, EPR and UV/Vis spectroscopy, and by cyclic voltammetry. Further properties and spectra were determined by quantum chemical calculation (spin densities, orbital energies, UV/Vis/NIR and ECD spectra). It turned out that this radical is best described with its radical centre being in the outer five-membered rings, which allows for the largest number of fully intact benzene rings. Its triradical character is rather small and can be neglected. The five-membered rings show significant antiaromatic character, which is highest in the central ring.
Introduction
π-Conjugated polycyclic hydrocarbons (PHs),1 in which six- and five-membered rings are arranged alternately, have been referred to as cyclopenta-fused polyaromatic hydrocarbons (CP-PAHs),2 as non-alternant conjugated polycyclic hydrocarbons (CPHs),3 or as compounds with quinoidal conjugated units.4 Substrates with five or nine rings are occasionally only termed with their rational names as indenofluorenes (IFs)5 and indacenodifluorenes (IDFs),1f,2a,6 respectively. A selection of representatives (1–3) with roughly linear structure is given in Fig. 1. Depending on the specific fusion, the number of rings, and the maximum number of fully intact benzene rings (Clar's rule7) in the respective resonance structures, these compounds show either a closed shell behaviour (e.g., 1A), di- or tetraradical character (e.g., 3B), or are radicals (e.g.2A). Decomposition and side reactions8 based on possibly emerging radical properties are typically avoided by attachment of bulky aryl groups at specific positions (e.g., of mesityl groups, Mes), usually at the five-membered rings.9 A number of unique electronic (e.g., small HOMO–LUMO gaps), optical, and magnetic properties have been associated with these compounds, which could possibly lead to applications in organic electronics,1g,10 in spintronics, and non-linear optics,11 and might be used for energy storage.1f | ||
| Fig. 1 A selection of CP-PAHs containing two, three, or four five-membered rings. Fully benzoid rings are highlighted in bold. | ||
While CP-PAHs with two or four five-membered rings can either be given with closed shell resonance structures or with di- or tetraradical structures (e.g., 1, 3),1f,12 the CP-PAHs with three five-membered rings (e.g., 2) are necessarily radicals and thus need to possess an open-shell configuration. Neither of the 21 supposable unbranched compounds with seven rings (e.g., 2; all possible structures are given in the ESI†) seems to have been synthesized yet, but a branched truxene derivative 4 with a presumed triradical structure has been synthesized and investigated.13 These and similar persistent organic radicals and diradicals9b,d,14 are of significant interest due to their redox properties, their possible application as electrode-active materials,15 and their magnetic behavior.16
CP-PAHs with two helical arms (5 and 6, which are given in Fig. 1 with their closed shell configuration) have similarly been synthesized and investigated.6,12d These compounds turned out to be achiral and adopt meso structures. According to the IUPAC definition, helicenes are ortho-fused polycyclic aromatic or heteroaromatic compounds in which all rings (minimum five) are angularly arranged so as to give helically shaped molecules, which are thus chiral,17 while according to a more recent statement of the IUPAC, the term helicene might only be applicable to systems with at least six ortho-fused rings.18 While non-conjugated19 or cationic20 helicenes containing five-membered rings have already been synthesized, no report came to our attention dealing with chiral CP-PAH helicenes (e.g., 7). Herein we present the synthesis of helical CP-PAHs, for which the above-mentioned properties might be supplemented by chiroptic properties.
Synthesis of helical CP-PAHs
We recently developed a method in which the fusion of ortho,ortho′-substituted terphenyls yielded [5]helicene-type compounds21 and we considered this strategy suitable for the construction of even larger helicenes by using similarly substituted quaterphenyls or even higher oligophenyls. We envisioned the synthesis of helical CP-PAHs with this method and at first aimed for a helicene with five rings (containing two five-membered rings). These compounds have already been prepared, albeit via differing routes.1k,6,22 We started this endeavour with purchasable dimethyl 2-aminoterephthalate 8, which was brominated with N-bromosuccinimide (NBS) and then subjected to a Sandmeyer reaction yielding the dihalogenated diester 10 (Scheme 1). Double Suzuki coupling with 2-(4-methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 1123 and double intramolecular Friedel–Crafts-type reaction using Eaton's reagent (a solution of phosphorus pentoxide in methanesulfonic acid)24 furnished the [5]helicene-type compound 13. Double addition of mesityllithium yielded diol 14 as a mixture of stereoisomers. 14 was not isolated but immediately reduced with tin(II) chloride in trifluoroacetic acid (TFA)22 to the fully conjugated CP-PAH [5]helicene 15. The methoxy groups were used to facilitate the detection during chromatographic purification of the intermediates, to simplify analysis of the 1H NMR spectra, and to promote the intramolecular SEAr reactions. Later on (vide infra) it turned out that the methoxy groups are not necessary and were thus not used in further investigations. Since CP-PAH [5]helicenes very similar to 15 have already been synthesized via different routes and have been investigated thoroughly (including the measuring of UV/Vis spectra and cyclic voltammograms),1k,6,22 we did not aim for further representatives but turned to higher helicenes of this type.Helicene 7 was synthesized using a similar strategy starting with 1,3-dibromobenzene (16), which was converted to tetrabromobiphenyl 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and subjected to a halogen-metal exchange in the presence of methyl chlorocarbonate to yield fluorenone 18 (Scheme 2). Addition of mesitylmagnesium bromide and subsequent reduction of the alcohol group with triethylsilane furnished dibromide 20, which could be coupled with ortho-formylated phenylboronic acid pinacol ester 2126 in a double Suzuki reaction. Both formyl groups are again reacted with a mesityl Grignard reagent, treated with boron trifluoride, and oxidized with tetrachloro-p-benzoquinone yielding [7]helicene-type CP-PAH 7. Its synthesis was completed starting with 1,3-dibromobenzene (16) in 8 steps with a total yield of 2.3%.
25 and subjected to a halogen-metal exchange in the presence of methyl chlorocarbonate to yield fluorenone 18 (Scheme 2). Addition of mesitylmagnesium bromide and subsequent reduction of the alcohol group with triethylsilane furnished dibromide 20, which could be coupled with ortho-formylated phenylboronic acid pinacol ester 2126 in a double Suzuki reaction. Both formyl groups are again reacted with a mesityl Grignard reagent, treated with boron trifluoride, and oxidized with tetrachloro-p-benzoquinone yielding [7]helicene-type CP-PAH 7. Its synthesis was completed starting with 1,3-dibromobenzene (16) in 8 steps with a total yield of 2.3%.
Characterization of helical radical 7
The carbon-centred helical radical 7 is a moisture- and air-stable solid that appears green in solution. It was subjected to a liquid/liquid diffusion in a chloroform/ethanol solvent system and furnished very dark, virtually black crystals suitable for X-ray crystallographic analysis (Fig. 2).† The two independent molecules in the unit cell show slightly differing geometries (what is relevant for determination of the HOMA values, vide infra). The most relevant difference between both molecules lies in the angles between the planes of terminal rings A and G, which differ by 11.0° and thus highlight some flexibility of the helix. The mesityl groups deviate from the planes perpendicular to the respective cyclopentane planes by 9 to 25°. These distortions are in line with the findings from the vibrational analysis (see ESI†), showing that a twisting of the mesityl groups is found at frequencies around 10–15 cm−1, i.e. at very low energies. 7 crystallizes in the space group P21/c and the racemic crystal is composed of both helical enantiomers. Details to the crystallographic analysis are given in the ESI.† The thus obtained crystals show a melting behaviour at 514 K. At this point the crystals seem to lose solvent and keep their solid character. No further phase transition was observed until about 620 K.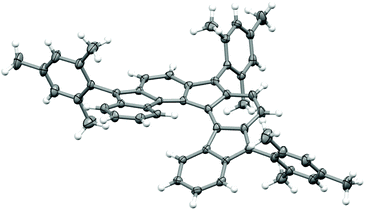 | ||
| Fig. 2 A projection of the molecular structure of compound 7. Only one of the crystallographically independent molecules is shown. (Thermal ellipsoids are depicted at the 30% probability level.).† | ||
Clean NMR spectra could be measured from [5]helicene 15, while it shows no EPR signal. This clearly confirms its closed-shell configuration, what has already been noted previously.22
[7]Helicene 7, on the other hand, gave no NMR spectra and a singlet resonance with no discernible hyperfine structure at g = 2.0013 was detected by X-band EPR spectroscopy (Fig. 3).27 According to computed hyperfine coupling constants (see ESI†) nearly all protons contribute in the same order of magnitude which corroborates the experimentally observed singlet resonance with no resolved multiplet but a broadened line instead (ω1/2 = 0.8 mT). No decay of the EPR resonance of a sample in oxygen-saturated toluene could be observed over time.
The aromaticity of a CP-PAH (i.e. its aromatic or antiaromatic character) is correlated with its HOMO–LUMO gap being of great importance for its electronic properties. Standard procedures for its evaluation are determination of HOMA values (harmonic oscillator model of aromaticity),28 which give the mean deviation from an ideally aromatic non-alternating bond length, and of NICS values (nucleus-independent chemical shift),29 which give the absolute magnetic shielding, usually calculated at 1 Å distance from the respective ring centre [NICSzz(1.0) values]. Both values are typically determined for each individual ring of a polycyclic compound. HOMA values can be extracted from crystallographic or from calculated data, while NICS values are only obtained from calculations. HOMA values for compound 7 are given in Fig. 4 (left: black numbers from crystallographic data; blue numbers from calculated data); they clearly account for an aromatic character in the outer benzene rings A and G, for a considerably reduced aromaticity in the inner benzene rings C and E, and for a significant antiaromatic character in the five-membered rings. The lowest HOMA values (lowest aromaticity) were determined for the central ring; they are 0.04 and 0.24, respectively. The approach to these values and some values for comparison are given in the ESI.† These findings were supported by the calculated NICSzz(1.0) values (Fig. 4, right): since we suspected some of the values to be obscured by the mesityl groups, we additionally determined all values for the parent compound 25 (R = H). It turned out that the values for both compounds are virtually identical; they are fully in line with the information deduced from the HOMA values: The outer rings A and G show aromatic behaviour (negative NICS values), the central benzene rings C and E are slightly antiaromatic, while a significant antiaromatic character can be determined for the five-membered rings (the highest positive value again for the central ring D).
The aromatic vs. antiaromatic character can furthermore be estimated from 1H NMR spectra since protons at the outer rim are shielded in antiaromatic compounds.30 While NMR spectra of 7 could not easily31 be measured due to its paramagnetic behaviour, we were able to calculate magnetic shielding tensors for this compound, which are easily converted into chemical shifts (δ values). Since we suspected the mesityl groups to obscure the shielding, we additionally calculated these values for the parent compound 25, which furthermore allowed the determination of the NMR-spectroscopic properties of protons at the five-membered rings (see ESI†). Protons 3-H and 4-H (for numbering, see Fig. 1) in 25 have calculated shifts of 7.04 and 7.16 ppm, which is expected for the aromatic ring. However, the protons 5-H, 6-H, 7-H, and 8-H, which are bound to rings with antiaromatic character according to the calculated NICS values, show only slightly upfield-shifted resonances at δ = 6.66, 6.77, 6.62, and 6.43 ppm, respectively. The shifts for protons 1-H and 2-H (6.55, 6.60 ppm) are similarly shifted upfield, possibly due to interactions with the opposite ring G.
A spin density map for 7 is given in Fig. 5. Positions 5 and 11 show Mulliken atomic spin densities of 0.34 each, while the central position 8 carries a spin density of only −0.24 (see ESI†). Since the SOMO shows a nodal plane through the centre of the molecule (cf.Fig. 6) and thus through atom C-8, the significant spin density observed at this position is most likely due to negative spin polarization. These findings give evidence for the predominance of the degenerate resonance formulas 7A (with the radical centres at the peripheral cyclopentane rings) over resonance formula 7B (where the radical is located in the central ring). This goes in line with Clar's rule, expressing that the most contributing resonance formulas are those with the largest number of fully intact benzene rings.7 SOMOs of radical 7 for both the α and the β electron are given in Fig. 6. More frontier orbitals together with their energies are given in the ESI.† It could be noted that the SOMO and at least the first two HOMOs and LUMOs are MOs essentially build up by the helicene core; the mesityl groups are hardly involved herein.
 | ||
| Fig. 5 Calculated spin density of 7. Blue and green surfaces represent α and β spin density, respectively (isovalue: 0.004 electrons per bohr3). | ||
 | ||
| Fig. 6 Calculated SOMOs of α and β electrons, respectively, for radical 7 (isovalue: 0.02 electrons1/2·bohr−3/2). | ||
The electrochemical properties of 7 were determined by cyclic voltammetry (CV, potentials vs. the ferrocene/ferrocenium couple, Fc/Fc+); the voltammograms are given in Fig. 7. Four quasi-reversible redox processes were observed in tetrahydrofuran at E01/2(THF) values of −2.73, −2.15, −1.36, and −0.05 V, while five quasi-reversible redox processes at E01/2(CH2Cl2) values of −2.14, −1.37, −0.16, +1.11, and +1.55 V were observed in methylene chloride (for further details, see ESI†). The values obtained in CH2Cl2 suggest that three reductions (1–3) and two oxidations (4, 5) are possible for 7. In addition, an electrochemical SOMO–LUMO gap can be deduced from these values:32ESOMO: −(4.8 + E01/2(4, CH2Cl2)) eV = −(4.8 + 1.11) eV = −5.91 eV; ELUMO: −(4.8 + E01/2(3, CH2Cl2)) eV = −(4.8 − 0.16) eV = −4.64 eV. The thus obtained gap of 1.27 eV is reasonably close to the optical gap obtained from TD calculations (1.17 eV, vide infra). The electrochemical HOMO–SOMO gap might analogously be estimated from the difference of E01/2(3, CH2Cl2) = −0.16 and E01/2(2, CH2Cl2) = −1.37 to be 1.21 eV. Nevertheless, it should be noted that electrochemical gaps and even more optical gaps were reported to underestimate the actual gaps to some extent.32c
The triradical character of 7 (which would be exemplified by resonance formula 7C) was determined by natural orbital occupation number (NOON)33 calculations at the spin unrestricted B3LYP/6-31 g(d,p) level. According to Yamaguchi's scheme12a,34 the triradical character y (see ESI†) was calculated to be only 0.003 and thus to be significantly smaller than the diradical character in, e.g., polycycle 6 (y = 0.12).12d Calculation at the uCAM-B3LYP/6-31 g(d,p) level (considering long-range correction) resulted in a somewhat higher triradical character (y = 0.03). This leads once more to the conclusion that the degenerate resonance formulas 7A are most significant; the triradical resonance formula 7C showing three fully intact benzene rings is of minor relevance. The rather small contribution of resonance formula 7B has already been deduced from the spin densities (vide supra).
The singlet/triplet gap is important for closed shell compounds and this might lead analogously to a contingent importance of doublet/quartet gaps in radicals like 7. The energy difference of these states in helicene 7 was calculated at the uM06/6-311++g(d,p) level to be 83.5 kJ mol−1 (0.87 eV), i.e., the state in which three unpaired electrons have the same spin is significantly less stable und might thus be of minor relevance.
UV/Vis/NIR and electronic circular dichroism (ECD) spectra were calculated with a time-dependent (TD) calculation at the uM06/6-311 g(d,p) level applying a modelled dichloromethane solvent field (Fig. 8, bottom). The functional M06 was reported to be well suited for TD calculations.35 The calculated UV/Vis spectrum of 7 in CH2Cl2 is in good agreement with a measured spectrum (Fig. 8, top; spectra with identical und thus comparable sections and scalings are given in the ESI†). It shows a very weak absorption band at 1056 nm (1.17 eV; oscillator strength f = 0.002), induced by the transition from the α-spin SOMO to the LUMO and from the β-spin HOMO–1 to the respective SOMO (see ESI†). This absorption is not visible in the measured UV/Vis/NIR spectrum (see ESI†). A sharp absorption band at 812 nm (f = 0.172) is essentially due to α-spin SOMO → LUMO and β-spin HOMO−1 → SOMO transitions. The next significant absorption band (here plotted as a shoulder) at 552 nm (f = 0.073) represents mainly α-HOMO−1 → LUMO, β-HOMO−2 → SOMO, and β-HOMO−1 → LUMO transitions. All these transitions are between orbitals fully spread over the helicene's π-system (see ESI†). The dominant transition at 812 nm is also the most significant band in the ECD spectrum.
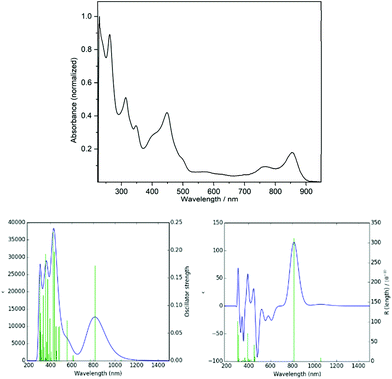 | ||
| Fig. 8 Measured UV/Vis spectrum of 7 (top) in CH2Cl2. Calculated UV/Vis/NIR (bottom left) and ECD spectra (bottom right). | ||
Conclusion
In summary we synthesized a helical CP-PAH consisting of four benzene and three cyclopentadiene rings which is a further example of a stable carbon-centred radical. It was obtained by cyclization of an ortho,ortho′-substituted terphenyl and re-establishment of the conjugation. The radical centre is predominantly situated at the outer cyclopentadienes, most probably to retain a maximum number of fully intact benzene rings. It shows a very small triradical character and a small SOMO–LUMO gap.Experimental
Detailed experimental procedures and spectroscopic data for all new compounds are given in the ESI.†2,2′-[9-(2,4,6-Trimethylphenyl)-9H-fluorene-4,5-diyl]dibenzaldehyde (22)
PdCl2(PPh3)2 (18 mg, 26 μmol) was added with positive argon pressure to a degassed (ultrasonication) solution of fluorene 20 (200 mg, 452 μmol), 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde26 (21, 262 mg, 1.13 mmol), and Na2CO3 (220 mg, 2.06 mmol) in THF/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 9 mL), placed in a Schlenk tube. The flask was closed and the mixture was heated to 80 °C for 15 h. The mixture was cooled to rt, half-concentrated brine (5 mL) was added, and the mixture was extracted with EtOAc (3 × 15 mL). The combined organic layers were dried (Na2SO4), concentrated at reduced pressure, and purified by column chromatography (silica gel, hexane/EtOAc, 10
4, 9 mL), placed in a Schlenk tube. The flask was closed and the mixture was heated to 80 °C for 15 h. The mixture was cooled to rt, half-concentrated brine (5 mL) was added, and the mixture was extracted with EtOAc (3 × 15 mL). The combined organic layers were dried (Na2SO4), concentrated at reduced pressure, and purified by column chromatography (silica gel, hexane/EtOAc, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 22 as a colourless solid (115 mg, 233 μmol, 52%). The product was obtained as a mixture of atropisomers. Rf = 0.38 (hexane/EtOAc 4
1) to yield 22 as a colourless solid (115 mg, 233 μmol, 52%). The product was obtained as a mixture of atropisomers. Rf = 0.38 (hexane/EtOAc 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (ATR):
1); IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2842 (vw), 2749 (vw), 1689 (m), 1594 (w), 1447 (w), 1392 (w), 1247 (w), 1194 (w), 1159 (vw), 854 (vw), 829 (w), 757 (m), 715 (w), 644 (w), 578 (vw), 449 (vw) cm−1; MS (FAB): m/z (%): 493.3 (16) [M + 1]+, 492.3 (15) [M]+, 475.3 (28), 474.3 (23); HRMS (FAB): m/z calcd for C36H28O2: 492.2084 [M+]; found: 492.2085.
= 2842 (vw), 2749 (vw), 1689 (m), 1594 (w), 1447 (w), 1392 (w), 1247 (w), 1194 (w), 1159 (vw), 854 (vw), 829 (w), 757 (m), 715 (w), 644 (w), 578 (vw), 449 (vw) cm−1; MS (FAB): m/z (%): 493.3 (16) [M + 1]+, 492.3 (15) [M]+, 475.3 (28), 474.3 (23); HRMS (FAB): m/z calcd for C36H28O2: 492.2084 [M+]; found: 492.2085.
4,8,11-Tris(2,4,6-trimethylphenyl)-8,11-dihydro-4H-cyclopenta[1,2-c:4,3-c′]difluorene (24)
Following a published protocol6 MesMgBr (1 M in THF; 1.15 mL, 1.15 mmol) was added dropwise within 5 min under argon atmosphere to a cooled (0 °C) solution of fluorene 22 (115 mg, 233 μmol) in anhydrous THF (6 mL). The cooling bath was removed and the mixture was stirred for 15 min at rt. Saturated aqueous NH4Cl solution (20 mL) was added, stirring was continued for 10 min, and the mixture was extracted with CH2Cl2 (3 × 25 mL). The combined organic layers were dried (MgSO4) and concentrated at reduced pressure. The residue (23) was dissolved in anhydrous CH2Cl2 (25 mL), cooled to 0 °C, and BF3·OEt2 (0.15 mL, 173 mg, 1.22 mmol) was added within 5 min. Stirring was continued for 1 h at rt and saturated aqueous NH4Cl solution (25 mL) was added. The mixture was stirred for 10 min and extracted with CH2Cl2 (2 × 20 mL). The organic layers were dried (MgSO4), concentrated at reduced pressure, and purified by column chromatography (silica gel, hexane/CH2Cl2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) furnishing an oil, which was digested in CH2Cl2 (2 mL) and precipitated with MeOH to yield 24 as a white powder (117 mg, 167 μmol, 63%). The product was obtained as a mixture of isomers; reasonable NMR spectra could thus not be measured. Rf = 0.17 (hexane/CH2Cl2 4
1) furnishing an oil, which was digested in CH2Cl2 (2 mL) and precipitated with MeOH to yield 24 as a white powder (117 mg, 167 μmol, 63%). The product was obtained as a mixture of isomers; reasonable NMR spectra could thus not be measured. Rf = 0.17 (hexane/CH2Cl2 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (ATR):
1); IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2914 (vw), 1611 (vw), 1447 (w), 1401 (vw), 1377 (vw), 1342 (vw), 1016 (vw), 849 (w), 814 (vw), 774 (w), 732 (m), 709 (w), 661 (vw), 632 (vw), 551 (vw), 439 (vw) cm−1; MS (FAB): m/z (%): 698.4 (32) [M + 2]+, 697.4 (85) [M + 1]+, 696.4 (100) [M]+, 695.4 (34) [M – 1]+, 578.3 (34), 577.3 (75) [M – Mes]+, 576.3 (30), 458.2 (15) [M – 2Mes]+; HRMS (FAB): m/z calcd for C54H48: 696.3751 [M]+; found: 696.3752.
= 2914 (vw), 1611 (vw), 1447 (w), 1401 (vw), 1377 (vw), 1342 (vw), 1016 (vw), 849 (w), 814 (vw), 774 (w), 732 (m), 709 (w), 661 (vw), 632 (vw), 551 (vw), 439 (vw) cm−1; MS (FAB): m/z (%): 698.4 (32) [M + 2]+, 697.4 (85) [M + 1]+, 696.4 (100) [M]+, 695.4 (34) [M – 1]+, 578.3 (34), 577.3 (75) [M – Mes]+, 576.3 (30), 458.2 (15) [M – 2Mes]+; HRMS (FAB): m/z calcd for C54H48: 696.3751 [M]+; found: 696.3752.
5,8,11-Tris(2,4,6-trimethylphenyl)cyclopenta[1,2-c:4,3-c′]difluoren-5-yl (7)
Following a published protocol13 heptacycle 24 (100 mg, 144 μmol) and tBuOK (229 mg, 2.04 mmol), placed in a Schlenk tube, were dissolved under an argon atmosphere in anhydrous THF (7 mL) and the mixture was heated to 60 °C for 16 h. The mixture was cooled to rt, chloranil (160 mg, 649 μg) was added, and after a short stirring (4 min) the mixture was concentrated at reduced pressure. The residue was purified by column chromatography (silica gel, hexane/CH2Cl2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to furnish 7 as a black solid (84 mg, 121 μmol, 84%). It was digested in degassed CHCl3 (2 mL) and covered with degassed MeOH (3 mL). Slow, undisturbed diffusion yielded black, rod-shaped crystals, suitable for X-ray crystallography. Rf = 0.35 (hexane/CH2Cl2 4
1) to furnish 7 as a black solid (84 mg, 121 μmol, 84%). It was digested in degassed CHCl3 (2 mL) and covered with degassed MeOH (3 mL). Slow, undisturbed diffusion yielded black, rod-shaped crystals, suitable for X-ray crystallography. Rf = 0.35 (hexane/CH2Cl2 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); m.p. 241 °C (CHCl3/EtOH); IR (ATR):
1); m.p. 241 °C (CHCl3/EtOH); IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 2962 (w), 2914 (w), 1737 (vw), 1610 (w), 1561 (vw), 1459 (w), 1442 (w), 1376 (w), 1347 (w), 1260 (w), 1189 (w), 1140 (w), 1090 (w), 1014 (w), 941 (w), 911 (vw), 847 (w), 807 (m), 793 (m), 745 (m), 692 (m), 576 (vw), 549 (w), 438 (vw) cm−1; UV/Vis (CH2Cl2): λmax (ε) = 262 (54
= 2962 (w), 2914 (w), 1737 (vw), 1610 (w), 1561 (vw), 1459 (w), 1442 (w), 1376 (w), 1347 (w), 1260 (w), 1189 (w), 1140 (w), 1090 (w), 1014 (w), 941 (w), 911 (vw), 847 (w), 807 (m), 793 (m), 745 (m), 692 (m), 576 (vw), 549 (w), 438 (vw) cm−1; UV/Vis (CH2Cl2): λmax (ε) = 262 (54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 315 (33
000), 315 (33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 348 (22
000), 348 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 449 (27
000), 449 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 769 (6000), 855 nm (12
000), 769 (6000), 855 nm (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mol−1 dm3 cm−1); MS (FAB): m/z (%): 694.4 (20) [M + 1]+, 693.4 (19) [M]+; HRMS (FAB): m/z calcd for C54H45: 693.3516 [M]+; found: 693.3518.
000 mol−1 dm3 cm−1); MS (FAB): m/z (%): 694.4 (20) [M + 1]+, 693.4 (19) [M]+; HRMS (FAB): m/z calcd for C54H45: 693.3516 [M]+; found: 693.3518.
Author contributions
S. H. and J. P. designed the project, S. H. performed the syntheses and measured NMR, IR, and MS spectra. A. H. performed the XRD analysis, J. P. did the quantum chemical calculations, A. H. and F. B. measured and analysed EPR spectra and the cyclic voltammograms. All authors contributed to the interpretation of the results. S. H. and J. P. prepared the manuscript with input from all contributing authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are deeply indebted to Sebastian T. Jung for developing a script to facilitate NICS calculations and to Sebastian Höfener and Burkhard Luy for helpful discussions. We further thank Bernhard Birenheide, Fabian Weick, Simon Petrick, and Alexander Colsmann for their help in measuring spectra.Notes and references
- (a) D. Hellwinkel and G. Reiff, Angew. Chem., 1970, 82, 516–517 ( Angew. Chem., Int. Ed. Engl. , 1970 , 9 , 527–528 ) CrossRef; (b) D. Hellwinkel, G. Reiff and V. Nykodym, Justus Liebigs Ann. Chem., 1977, 1013–1025 CrossRef CAS; (c) S. Marković, J. Đurđević, S. Jeremić and I. Gutman, J. Serb. Chem. Soc., 2010, 75, 1241–1249 CrossRef; (d) H. Hopf, Angew. Chem., 2013, 125, 12446–12449 ( Angew. Chem., Int. Ed. , 2013 , 52 , 12224–12226 ) CrossRef; (e) S. Das and J. Wu, in Polycyclic Arenes and Heteroarenes: Synthesis, Properties, and Applications, ed. Q. Miao, Weinheim-VCH, Weinheim, 2015, pp. 1–36 Search PubMed; (f) P. Hu, S. Lee, T. S. Herng, N. Aratani, T. P. Gonçalves, Q. Qi, X. Shi, H. Yamada, K.-W. Huang, J. Ding, D. Kim and J. Wu, J. Am. Chem. Soc., 2016, 138, 1065–1077 CrossRef CAS PubMed; (g) X. Yang, X. Shi, N. Aratani, T. P. Gonçalves, K.-W. Huang, H. Yamada, C. Chi and Q. Miao, Chem. Sci., 2016, 7, 6176–6181 RSC; (h) C. K. Frederickson, L. N. Zakharov and M. M. Haley, J. Am. Chem. Soc., 2016, 138, 16827–16838 CrossRef CAS PubMed; (i) S. Das and J. Wu, Phys. Sci. Rev., 2017, 2, 20160109 Search PubMed; (j) K. Yamamoto, Y. Ie, N. Tohnai, F. Kakiuchi and Y. Aso, Sci. Rep., 2018, 8, 17663 CrossRef PubMed; (k) T. Jousselin-Oba, P. E. Deal, A. G. Fix, C. K. Frederickson, C. L. Vonnegut, A. Yassar, L. N. Zakharov, M. Frigoli and M. M. Haley, Chem. – Asian J., 2019, 14, 1737–1744 CrossRef CAS PubMed; (l) H. Miyoshi, I. Hisaki and Y. Tobe, Eur. J. Org. Chem., 2021, 3528–3534 CrossRef CAS; (m) Q. Chen, M. Baumgarten, M. Wagner, Y. Hu, I. C.-Y. Hou, A. Narita and K. Müllen, Angew. Chem., 2021, 133, 11400–11404 ( Angew. Chem., Int. Ed. , 2021 , 60 , 11300–11304 ) CrossRef; (n) J. R. Dias, J. Phys. Chem. A, 2021, 125, 8482–8497 ( J. Phys. Chem. A , 2021 , 125 , 9940 ) CrossRef CAS PubMed; (o) T. Xu, Y. Han, Z. Shen, X. Hou, Q. Jiang, W. Zeng, P. W. Ng and C. Chi, J. Am. Chem. Soc., 2021, 143, 20562–20568 CrossRef CAS PubMed.
- (a) G. E. Rudebusch and M. M. Haley, in Polycyclic Arenes and Heteroarenes: Synthesis, Properties, and Applications, ed. Q. Miao, Wiley-VCH, Weinheim, 2015, pp. 37–60 Search PubMed; (b) J. Ma, J. Liu, M. Baumgarten, Y. Fu, Y.-Z. Tan, K. S. Schellhammer, F. Ortmann, G. Cuniberti, H. Komber, R. Berger, K. Müllen and X. Feng, Angew. Chem., 2017, 129, 3328–3332 ( Angew. Chem., Int. Ed. , 2017 , 56 , 3280–3284 ) CrossRef.
- (a) Y. Tobe, Chem. Rec., 2015, 15, 86–96 CrossRef CAS PubMed; (b) A. Konishi and M. Yasuda, Chem. Lett., 2021, 50, 195–212 CrossRef CAS.
- (a) A. Shimizu and Y. Tobe, Angew. Chem., 2011, 123, 7038–7042 CrossRef; Angew. Chem., Int. Ed., 2011506906–6910 Search PubMed; (b) Y. Tobe and T. Kubo, in Top. Curr. Chem, Springer, Cham, 2018 Search PubMed; (c) J. Guo, Z. Li, J. Zhang, B. Li, Y. Liang, Y. Wang, S. Xie, H. Phan, T. S. Herng, J. Ding, J. Wu, B. Z. Tang and Z. Zeng, CCS Chem., 2021, 3, 399–407 Search PubMed.
- (a) A. G. Fix, D. T. Chase and M. M. Haley, in Polyarenes I, ed. J. S. Siegel and Y.-T. Wu, Springer, Berlin, Heidelberg, 2014, pp. 159–195 Search PubMed; (b) C. K. Frederickson, B. D. Rose and M. M. Haley, Acc. Chem. Res., 2017, 50, 977–987 CrossRef CAS PubMed.
- H. Sharma, P. K. Sharma and S. Das, Chem. Commun., 2020, 56, 11319–11322 RSC.
- (a) E. Clar, in Polycyclic Hydrocarbons, Academic Press, London, Springer-Verlag, Berlin, 1964, vol. 1, pp. 32–39 Search PubMed; (b) A. D. Zdetsis, J. Phys. Chem. C, 2018, 122, 17526–17536 CrossRef CAS; (c) Y. Wang, J. Phys. Chem. A, 2022, 126, 164–176 CrossRef CAS PubMed.
- (a) E. Font-Sanchis, C. Aliaga, E. V. Bejan, R. Cornejo and J. C. Scaiano, J. Org. Chem., 2003, 68, 3199–3204 CrossRef CAS PubMed; (b) L. E. Harrington, J. F. Britten and M. J. McGlinchey, Tetrahedron Lett., 2003, 44, 8057–8060 CrossRef CAS.
- (a) R. C. Gostowski, T. Bailey, S. D. Bonner, E. E. Emrich and S. L. Steelman, J. Phys. Org. Chem., 2000, 13, 735–739 CrossRef CAS; (b) Y. Tian, K. Uchida, H. Kurata, Y. Hirao, T. Nishiuchi and T. Kubo, J. Am. Chem. Soc., 2014, 136, 12784–12793 CrossRef CAS PubMed; (c) D. T. Hogan and T. C. Sutherland, J. Phys. Chem. Lett., 2018, 9, 2825–2829 CrossRef CAS PubMed; (d) T. Nishiuchi, R. Ito, A. Takada, Y. Yasuda, T. Nagata, E. Stratmann and T. Kubo, Chem, – Asian J., 2019, 14, 1830–1836 CrossRef CAS PubMed.
- (a) C. Poriel, J.-J. Liang, J. Rault-Berthelot, F. Barrière, N. Cocherel, A. M. Z. Slawin, D. Horhant, M. Virboul, G. Alcaraz, N. Audebrand, L. Vignau, N. Huby, G. Wantz and L. Hirsch, Chem. – Eur. J., 2007, 13, 10055–10069 CrossRef CAS PubMed; (b) H. Usta, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2008, 130, 8580–8581 CrossRef CAS PubMed; (c) H. Usta, C. Risko, Z. Wang, H. Huang, M. K. Deliomeroglu, A. Zhukhovitskiy, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2009, 131, 5586–5608 CrossRef CAS PubMed; (d) D. T. Chase, A. G. Fix, S. J. Kang, B. D. Rose, C. D. Weber, Y. Zhong, L. N. Zakharov, M. C. Lonergan, C. Nuckolls and M. M. Haley, J. Am. Chem. Soc., 2012, 134, 10349–10352 CrossRef CAS PubMed; (e) X. Yang, D. Liu and Q. Miao, Angew. Chem., 2014, 126, 6904–6908 ( Angew. Chem., Int. Ed. , 2014 , 53 , 6786–6790 ) CrossRef.
- K. Fukuda, J.-y. Fujiyoshi, H. Matsui, T. Nagami, S. Takamuku, Y. Kitagawa, B. Champagne and M. Nakano, Org. Chem. Front., 2017, 4, 779–789 RSC.
- (a) K. Kamada, K. Ohta, A. Shimizu, T. Kubo, R. Kishi, H. Takahashi, E. Botek, B. Champagne and M. Nakano, J. Phys. Chem. Lett., 2010, 1, 937–940 CrossRef CAS; (b) S. Nobusue, H. Miyoshi, A. Shimizu, I. Hisaki, K. Fukuda, M. Nakano and Y. Tobe, Angew. Chem., 2015, 127, 2118–2122 ( Angew. Chem., Int. Ed. , 2015 , 54 , 2090–2094 ) CrossRef; (c) C. Liu, Y. Ni, X. Lu, G. Li and J. Wu, Acc. Chem. Res., 2019, 52, 2309–2321 CrossRef CAS PubMed; (d) Q. Jiang, Y. Han, Y. Zou, H. Phan, L. Yuan, T. S. Herng, J. Ding and C. Chi, Chem. – Eur. J., 2020, 26, 15613–15622 CrossRef CAS PubMed.
- X. Yang, D. Zhang, Y. Liao and D. Zhao, J. Org. Chem., 2020, 85, 5761–5770 CrossRef CAS PubMed.
- (a) T. Kubo, K. Yamamoto, K. Nakasuji, T. Takui and I. Murata, Angew. Chem., 1996, 108, 456–457 ( Angew. Chem., Int. Ed. Engl. , 1996 , 35 , 439–441 ) CrossRef; (b) T. Kubo, K. Yamamoto, K. Nakasuji, T. Takui and I. Murata, Bull. Chem. Soc. Jpn., 2001, 74, 1999–2009 CrossRef CAS; (c) R. G. Hicks, Org. Biomol. Chem., 2007, 5, 1321–1338 RSC; (d) Y. Morita and S. Nishida, in Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, ed. R. G. Hicks, John Wiley & Sons, Chichester, 2010, pp. 81–145 Search PubMed; (e) T. Kubo, Y. Katada, A. Shimizu, Y. Hirao, K. Sato, T. Takui, M. Uruichi, K. Yakushi and R. C. Haddon, J. Am. Chem. Soc., 2011, 133, 14240–14243 CrossRef CAS PubMed; (f) M. Abe, Chem. Rev., 2013, 113, 7011–7088 CrossRef CAS PubMed; (g) B. Prajapati, D.-K. Dang, P. J. Chmielewski, M. A. Majewski, T. Lis, C. J. Gómez-García, P. M. Zimmerman and M. Stępień, Angew. Chem., 2021, 133, 22670–22678 ( Angew. Chem., Int. Ed. , 2021 , 60 , 22496–22504 ) CrossRef.
- (a) H. Nishide and K. Oyaizu, Science, 2008, 319, 737–738 CrossRef CAS PubMed; (b) Y. Morita, S. Nishida, T. Murata, M. Moriguchi, A. Ueda, M. Satoh, K. Arifuku, K. Sato and T. Takui, Nat. Mater., 2011, 10, 947–951 CrossRef CAS PubMed.
- R. G. Hicks, in Stable radicals: fundamentals and applied aspects of odd-electron compounds, ed. R. G. Hicks, Wiley, Chichester, 2010, pp. 317–380 Search PubMed.
- G. P. Moss, P. A. S. Smith and D. Tavernier, Pure Appl. Chem., 1995, 67, 1307–1375 Search PubMed.
- H. A. Favre and W. H. Powell, Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013, The Royal Society of Chemistry, 2013 Search PubMed.
- T. Cadart, D. Nečas, R. P. Kaiser, L. Favereau, I. Císařová, R. Gyepes, J. Hodačová, K. Kalíková, L. Bednárová, J. Crassous and M. Kotora, Chem. – Eur. J., 2021, 27, 11279–11284 CrossRef CAS PubMed.
- B. M. Gross and M. Oestreich, Synthesis, 2021, 53, 2512–2516 CrossRef CAS.
- A. Weiß and J. Podlech, Eur. J. Org. Chem., 2019, 6697–6701 CrossRef.
- A. G. Fix, P. E. Deal, C. L. Vonnegut, B. D. Rose, L. N. Zakharov and M. M. Haley, Org. Lett., 2013, 15, 1362–1365 CrossRef CAS PubMed.
- D. C. Ebner, J. T. Bagdanoff, E. M. Ferreira, R. M. McFadden, D. D. Caspi, R. M. Trend and B. M. Stoltz, Chem. – Eur. J., 2009, 15, 12978–12992 CrossRef CAS PubMed.
- P. E. Eaton, G. R. Carlson and J. T. Lee, J. Org. Chem., 1973, 38, 4071–4073 CrossRef CAS.
- (a) Y. Miyake, M. Wu, M. J. Rahman, Y. Kuwatani and M. Iyoda, J. Org. Chem., 2006, 71, 6110–6117 CrossRef CAS PubMed; (b) Q. Perron, J. Praz and A. Alexakis, Tetrahedron: Asymmetry, 2009, 20, 1004–1007 CrossRef CAS; (c) Q. Perron and A. Alexakis, Adv. Synth. Catal., 2010, 352, 2611–2620 CrossRef CAS.
- Y.-Y. Jhang, T.-T. Fan-Chiang, J.-M. Huang and J.-C. Hsieh, Org. Lett., 2016, 18, 1154–1157 CrossRef CAS PubMed.
- S. Stoll and A. Schweiger, J. Magn. Reson., 2006, 178, 42–55 CrossRef CAS PubMed.
- (a) J. Kruszewski and T. M. Krygowski, Tetrahedron Lett., 1972, 13, 3839–3842 CrossRef; (b) T. M. Krygowski, J. Chem. Inf. Comput. Sci., 1993, 33, 70–78 CrossRef CAS; (c) T. M. Krygowski and M. K. Cyrański, Chem. Rev., 2001, 101, 1385–1420 CrossRef CAS PubMed.
- (a) J. A. N. F. Gomes and R. B. Mallion, Chem. Rev., 2001, 101, 1349–1383 CrossRef CAS PubMed; (b) Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef PubMed; (c) A. Stanger, J. Org. Chem., 2006, 71, 883–893 CrossRef CAS PubMed; (d) H. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Org. Lett., 2006, 8, 863–866 CrossRef CAS PubMed; (e) A. Stanger, Eur. J. Org. Chem., 2020, 3120–3127 CrossRef CAS.
- R. H. Mitchell, Chem. Rev., 2001, 101, 1301–1315 CrossRef CAS PubMed.
- K. H. Hausser, H. Brunner and J. C. Jochims, Mol. Phys., 1966, 10, 253–260 CrossRef CAS.
- (a) J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bässler, M. Porsch and J. Daub, Adv. Mater., 1995, 7, 551–554 CrossRef CAS; (b) C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367–2371 CrossRef CAS PubMed; (c) J. Sworakowski, Synth. Met., 2018, 235, 125–130 CrossRef CAS; (d) E. V. Tretyakov, P. V. Petunin, S. I. Zhivetyeva, D. E. Gorbunov, N. P. Gritsan, M. V. Fedin, D. V. Stass, R. I. Samoilova, I. Y. Bagryanskaya, I. K. Shundrina, A. S. Bogomyakov, M. S. Kazantsev, P. S. Postnikov, M. E. Trusova and V. I. Ovcharenko, J. Am. Chem. Soc., 2021, 143, 8164–8176 CrossRef CAS PubMed.
- D. Döhnert and J. Koutecký, J. Am. Chem. Soc., 1980, 102, 1789–1796 CrossRef.
- K. Yamaguchi, Chem. Phys. Lett., 1975, 33, 330–335 CrossRef CAS.
- (a) F. Cervantes-Navarro and D. Glossman-Mitnik, J. Mex. Chem. Soc., 2013, 57, 19–22 CAS; (b) J. Podlech, S. C. Fleck, M. Metzler, J. Bürck and A. S. Ulrich, Chem. – Eur. J., 2014, 20, 11463–11470 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of 1H and 13C NMR spectra for all new compounds, further characterisation data of 7, computational details. CCDC 2129930. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ob00172a |
| This journal is © The Royal Society of Chemistry 2022 |