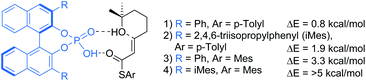Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective “clip-cycle” synthesis of di-, tri- and spiro-substituted tetrahydropyrans†
Khadra
Alomari
ab,
N.
Sai Pavan Chakravarthy
a,
Bastien
Duchadeau
a,
Kristaps
Ermanis‡
 *c and
Paul A.
Clarke
*c and
Paul A.
Clarke
 *a
*a
aDepartment of Chemistry, University of York, Heslington, York, North Yorks YO10 5DD, UK. E-mail: paul.clarke@york.ac.uk
bDepartment of Chemistry, Faculty of Science, Jazan University, Saudi Arabia
cCentre for Molecular Informatics, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: Kristaps.Ermanis1@nottingham.ac.uk
First published on 13th January 2022
Abstract
ω-Unsaturated alcohols were “clipped” via alkene metathesis to a thioester activating group, which was followed by a chiral phosphoric acid catalyzed intramolecular oxa-Michael cyclization to yield tetrahydropyrans and spiro-tetrahydropyrans with excellent enantioselectivity. The mechanism and origin of the enantioselectivity was probed by DFT calculations and kinetic isotope studies, where there was excellent correlation between the computational and synthetic investigations.
Substituted tetrahydropyrans appear in many biologically active natural products,1 and they are also the fifth most prevalent heterocycle in pharmaceutical molecules.2 As such the development of improved stereoselective methods for their synthesis has been a goal of many laboratories,3 including our own. Innovations in hetero-Diels–Alder,4 Prins5 and Maitland–Japp6 reactions have provided new methods for the enantioselective synthesis of tetrahydropyrans bearing a variety of substitution patterns. The intramolecular oxa-Michael reaction is also popular for the synthesis of substituted tetrahydropyrans,7,8 and recent studies have increased our understanding of the issues governing the diastereoselectivity of this cyclisation.9,10 However, the control of enantioselectivity in oxa-Michael reactions is less common.11,12
Our disclosure of a stereodivergent oxa-Michael reaction9 showed that when an α,β-unsaturated thioester was the Michael acceptor 2,6-cis-tetrahydropyrans were formed with high selectivity when a Brønsted acid was used to catalyse the oxa-Michael reaction. Alternatively, when buffered TBAF was used the 2,6-trans-diastereomer was predominantly formed. DFT studies indicated that the Brønsted acid acted as a proton shuttle simultaneously making the hydroxyl group more nucleophilic and the thioester more electrophilic (Scheme 1). As the DFT studies had indicated an intimate role for the Brønsted acid, we postulated that a suitable chiral Brønsted acid may be able to promote an enantioselective intramolecular oxa-Michael reaction leading to the formation of enantioenriched tetrahydropyrans (Scheme 1). We envisaged an olefin metathesis reaction immediately prior to the asymmetric oxa-Michael reaction to develop a “clip-cycle” process similar to the one we had successfully employed in the asymmetric synthesis of pyrrolidinines.13
In this paper we report the successful application of a two-step “clip-cycle” procedure for the enantioselective synthesis of substituted tetrahydropyrans and spirocyclic tetrahydropyrans, as well as to the kinetic resolution of racemic substrates leading to the enantioselective formation of 2,6-disubstituted tetrahydropyrans. The “clip-cycle” protocol offers the advantage that it is (i) modular, enabling a diverse range of tetrahydropyrans to be assembled from readily available hydroxy alkenes and acrylates, (ii) catalytic in both the “clip” and “cycle” steps, (iii) straightforward to perform, (iv) generates the tetrahydropyrans directly, and (v) provides a versatile handle in the form of the thioester for subsequent functionalization.
Chiral phosphoric acids (CPA) are a privileged chiral Brønsted acid scaffold.14 To validate the concept of a CPA catalysed oxa-Michael reaction, a simple molecular mechanics computational study with the MMFF15 force field was conducted. Conformational searches were run on the envisaged proton-shuttle TS-like structures (Fig. 1). Phosphoric acid catalysts with both phenyl and 2,4,6-triisopropylphenyl (iMes) 3,3′ substituents were tested in combination with substrates with Ph and Mes thioester substituents. In all cases TS-like structures leading to the S product enantiomer were found to be lower by up to more than 5 kcal mol−1. The substituent on the thioester was found to be a particularly powerful handle for boosting enantioselectivity. While this was at low level of theory, it indicated that achieving enantioselectivity should be possible.
After these encouraging computational results, attention turned to an empirical investigation of the oxa-Michael cyclisation using the 2,2-gem-dimethyl substrate with three different thioesters (Table 1).
| Entry | Ar | Solvent | Temp. (°C) | Conv.b (%) | % eec |
|---|---|---|---|---|---|
| a CuI (10 mol%) was essential for good conversion (ref. 16). b Determined by 1H NMR for the cyclisation step. c Determined by chiral stationary phase HPLC. | |||||
| 1 | p-Tol | PhMe | rt | 6 | — |
| 2 | p-Tol | PhMe | 50 | 30 | 13 |
| 3 | p-Tol | Cyclohexane | 50 | 77 | 18 |
| 4 | Mes | PhMe | 50 | 69 | 60 |
| 5 | Mes | Cyclohexane | 50 | 96 | 69 |
| 6 | Mes | Cyclohexane | 75 | 100 | 66 |
| 7 | iMes | Cyclohexane | 50 | 85 | 98 |
The results in Table 1 show that cyclohexane is the solvent of choice (entries 2, 3, 4 and 5), while there is little reduction in enantioselectivity at elevated temperatures (entries 5 and 6). Increase in the steric bulk of the thioester group had a beneficial effect on the enantioselectivity of the reaction, with the highest enantioselectivity occurring with the use of the iMes thioester (compare entries 3, 5 and 7). A catalyst screen was also conducted, but no improvement was seen on either the conversion or the enantioselectivity of the reaction with chiral phosphoric acid catalysts 3b–d (Table 2).
With these results the optimal conditions were determined to be the use of the iMes thioester in cyclohexane at 50 °C with (R)-TRIP as the catalyst, and an investigation of substrate scope could begin. The first family of substrates to be investigated were 2,2-disubstituted systems (Fig. 2).
 | ||
| Fig. 2 Asymmetric ‘clip-cycle’ synthesis of 2,2,6-trisubstituted THPs. Yields are reported over two steps. Enantiomeric excesses were determined by chiral stationary phase HPLC. In the case of 5e the reaction was run for 48 hours. (a) One pot reaction in cyclohexane (see ESI†). | ||
All substrates underwent the clip-cycle reaction smoothly and generated THP products in good yields. The enantioselectivity of the reaction proved to be excellent with all substrates giving THP products in >90% ee. The exceptions to this was the spiro-cyclohexyl substrate which gave the product 5c in a moderate 60% ee and the unsubstituted substrate which gave 5g. We are unsure why the spiro-cyclohexyl substrate has a lower ee than the spiro-cyclopentyl or spiro-THP systems, and we suspect that the lack of substitution in the precursor to 5g is responsible for its reduced enantioselectivity.
Full oxa-Michael mechanistic pathway was then modelled, with the main goal of identifying the enantioselectivity determining step (Scheme 2). Thorough conformational searches were done for all starting materials, intermediates, and products. Carefully selected conformations for each were then optimized with B3LYP17/6-31G**18/SMD(cyclohexane),19 and single-point energies calculated with M06-2X20/def2-TZVP21/SMD(cyclohexane).
In the resulting pathway the lowest barriers for cyclization and tautomerization were very close, with activation free energies 16.7 and 17.1 kcal mol−1, respectively. The difference is less than the expected error of DFT methods, therefore either of the steps could in principle determine enantioselectivity. Cyclization could set the stereochemistry and be followed by a faster tautomerization. Alternatively, the reaction could exhibit Curtin–Hammett control, with a reversible cyclization followed by an enantioselective, rate-determining tautomerization. Therefore another way of determining the rate- and selectivity-determining step was necessary. To this end, H/D KIEs were calculated for both steps and found to be 1.8 and 6.4 for the cyclization and tautomerization, respectively. Experimentally, the KIE was determined to be 1.2 (see ESI†), clearly indicating that the cyclization is the RDS. Thus, further computational study was focused on this step to predict and understand the mechanism of enantioinduction (Fig. 3).
 | ||
| Fig. 3 Lowest energy cyclization TSs. Free energies shown in kcal mol−1 relative to the starting complex, calculated at M06-2X/def2-TZVP/SMD(cyclohexane)//B3LYP/6-31G**/SMD(cyclohexane). | ||
The alcohol can attack either Si or Re face of the conjugate double bond, and form either E or Z enol in the process. This gives 4 possible mechanistic pathways, and several cyclization TSs for each were identified. The S,E TS has the lowest activation free energy of 16.7 kcal mol−1. The lowest TSs leading to the R product are the R,Z and R,E, and have activation free energies of 17.9 kcal mol−1 and 18.6 kcal mol−1, which are 1.2 kcal and 1.9 kcal mol−1 higher, respectively. These higher energies are caused by unfavourable steric interactions with the 3,3′-substituents on the catalyst. For the R,Z TS another factor is the increased energetic cost to form a Z-enol, as the large thioester and alkyl substituents are on the same side in the forming enol and experience significant steric interaction. Based on these energy differences between the enantiomeric pathways, the major enantiomer is predicted to be S at expected 77% ee.
The absolute stereochemistry of the cyclisation was determined experimentally as S by the conversion of THP 4b in 66% ee, to the methyl ester by the action of AgOTf and MeOH and compared to the known literature compound.22 This confirms the prediction of the DFT calculations and, remarkably, even the initial low-level MMFF calculations. Indirectly, it also adds support to cyclisation as the rate and stereochemical determining step, as the tautomerization was calculated to give the opposite enantioselectivity.
As can be seen from Fig. 4, the reaction tolerates 3,3-disubstitution well generating enantiomerically enriched products in good to excellent enantioselectivities. It also proved possible to run the “clip-cycle” process in a single pot without loss of yield or enantioselectivity. Alkenes 1b and 6c were “clipped” with 2c in cyclohexane at 50 °C, and when there was no alkene remaining by TLC, (R)-3a (20 mol%) was added and the reaction stirred at the same temperature for a further 24 h to give 5b in 71% yield and 95% ee, and 7c in 70% yield and 99% ee. With a reasonable substrate scope explored, attention turned to the kinetic resolution of racemic substrates23 with the aim of forming enantioenriched 2,6-disubstituted THPs 9 (Fig. 5).
 | ||
| Fig. 4 Asymmetric ‘clip-cycle’ synthesis of 3,3,6-trisubstituted THPs. Yields are reported over two steps. Enantiomeric excesses were determined by chiral stationary phase HPLC. (a) One pot reaction in cyclohexane (see ESI†). | ||
 | ||
| Fig. 5 ‘Clip-cycle’ kinetic resolution. Formation of 2,6-disubstituted tetrahydropyrans. Conversions and enantiomeric excesses were determined by chiral stationary phase HPLC. | ||
Cyclisation precursors 8a, b were subjected to the “cycle”-phase of the “clip-cycle” procedure and monitored by chiral stationary phase HPLC. The phenyl-containing substrate 8a underwent cyclisation to give THP 9a in 68% ee at 58% conversion after 8 h. Recovered metathesis product 8a was determined to have an ee of 97%. Iso-propyl-containing substrate 8b cyclized to give 9b in 92% ee after 6 h and 52% conversion. Recovered metathesis product 8b was left uncyclized in 85% ee.24 These initial studies in “clip-cycle” kinetic resolution are highly encouraging giving access to resolved precursors and THPs in good to excellent enantioselectivities at desirable levels of conversion.
In conclusion we have developed a highly enantioselective method for the synthesis of 2,2,6-, 3,3,6-trisubstituted and 2,2- and 3,3-spirocyclic THPs, as well as for the kinetic resolution of oxa-Michael precursors, which provides 2,6-disubstituted THPs with good to excellent enantioselectivities. Computational studies predicted that the reaction could be highly enantioselective and that either cyclisation or tautomerisation could be the enantio-determining step. DFT predicted that these different steps would lead to different enantiomers, the (S)-enantiomer for cyclisation and the (R)-enantiomer for tautomerisation. Synthetic and kinetic isotope studies showed that the (S)-enantiomer was the major product, and that cyclisation was most likely the enantio-determining step.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Jazan University (KA), ERASMUS+ (BD), the Leverhulme Trust and Isaac Newton Trust (KE) for financial support. The computational work has been performed using resources provided by the Cambridge Tier-2 system operated by the University of Cambridge Research Computing Service (http://www.hpc.cam.ac.uk) funded by EPSRC Tier-2 capital grant EP/P020259/1.Notes and references
- N. M. Nasir, E. Ermanis and P. A. Clarke, Org. Biomol. Chem., 2014, 12, 3323–3335 RSC.
- R. D. Taylor, M. MacCross and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed.
- M. A. Perry, S. D. Rychnovsky and N. Sizemore, Synthesis of saturated tetrahydropyrans. Synthesis of saturated oxygen heterocycles 1, Topics in Heterocyclic Chemistry 35, ed. J. Cossy, 2014, pp. 43–95 Search PubMed.
- (a) V. Laina-Martín, J. A. Fernández-Salas and J. Alemán, Chem. – Eur. J., 2021, 27, 12509–12520 CrossRef PubMed; (b) G. Blond, M. Gulea and V. Mamane, Curr. Org. Chem., 2016, 20, 2161–2210 CrossRef CAS.
- A. Budakoti, P. K. Mondaal, P. Verma and J. Khamrai, Beilstein J. Org. Chem., 2021, 17, 932–963 CrossRef CAS PubMed.
- (a) P. A. Clarke and K. Ermanis, Curr. Org. Chem., 2013, 17, 2025–2037 CrossRef CAS; (b) P. A. Clarke, S. Santos, N. Mistry, L. Burroughs and A. C. Humphries, Org. Lett., 2011, 13, 624–627 CrossRef CAS PubMed; (c) M. Iqbal, N. Mistry and P. A. Clarke, Tetrahedron, 2011, 67, 4960–4966 CrossRef CAS; (d) P. A. Clarke, N. M. Nasir, P. B. Sellars, A. M. Peter, C. A. Lawson and J. L. Burroughs, Org. Biomol. Chem., 2016, 14, 6840–6852 RSC.
- C. F. Nising and S. Bräse, Chem. Soc. Rev., 2012, 41, 988–999 RSC.
- (a) H. Fuwa, K. Noto and M. Sasaki, Org. Lett., 2010, 12, 1636–1639 CrossRef CAS PubMed; (b) H. Fuwa, K. Noto and M. Sasaki, Org. Lett., 2011, 13, 1820–1823 CrossRef CAS PubMed; (c) H. Fuwa, N. Ichinokawa, K. Noto and M. Sasaki, J. Org. Chem., 2012, 77, 2588–2607 CrossRef CAS PubMed; (d) R. W. Bates and T. G. Lek, Synthesis, 2014, 46, 1731–1738 CrossRef; (e) I. Paterson and G. W. Haslett, Org. Lett., 2013, 15, 1338–1341 CrossRef CAS PubMed.
- K. Ermanis, Y.-T. Hsiao, U. Kaya, A. Jeuken and P. A. Clarke, Chem. Sci., 2017, 8, 482–490 RSC.
- D. Csókás, A. X. Y. Ho, R. Ramabhadran and R. W. Bates, Org. Biomol. Chem., 2019, 17, 6293–6304 RSC.
- (a) W.-B. Xie and Z. Li, Synthesis, 2020, 52, 2127–2146 CrossRef CAS; (b) Y. Wang and D.-M. Du, Org. Chem. Front., 2020, 7, 3266–3283 RSC.
- (a) S. Lin, G.-L. Zhao, L. Deiana, J. Sun, Q. Zhang, H. Leijonmarck and A. Córdova, Chem. – Eur. J., 2010, 16, 13930–13934 CrossRef CAS; (b) R. Miyaji, K. Asano and S. Matsubara, Org. Biomol. Chem., 2014, 12, 119–122 RSC; (c) H. Eastman, J. Ryan, B. Maciá, V. Caprio and E. O'Reilly, ChemCatChem, 2019, 11, 3760–3762 CrossRef CAS; (d) X.-J. Lv, Y.-C. Ming, H.-C. Wu and Y.-K. Liu, Org. Chem. Front., 2021, 8, 6309–6316 RSC.
- C. J. Maddocks, K. Ermanis and P. A. Clarke, Org. Lett., 2020, 22, 8116–8121 CrossRef CAS PubMed.
- D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS.
- T. A. Halgren, Comput. Chem., 1996, 17, 490–519 CrossRef CAS.
- K. Voigtritter, S. Ghorai and B. H. Lipshultz, J. Org. Chem., 2011, 76, 4697–4702 CrossRef CAS PubMed.
- (a) A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS; (c) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- (a) W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS; (b) P. C. Hariharan and J. A. Pople, Theor. Chem. Acc., 1973, 28, 213–222 Search PubMed.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- Y. Zhao and D. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- (a) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC; (b) F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- E. Marotta, E. Foresti, T. Marcelli, F. Peri, P. Righi, N. Scardovi and G. Rosini, Org. Lett., 2002, 4, 4451–4453 CrossRef CAS PubMed.
- N. Yoneda, A. Matsumoto, K. Asano and S. Matsubara, Chem. Lett., 2016, 45, 1300–1303 CrossRef CAS.
- 2,6-cis-THP formation was determined by analysis of the 1H NMR (see ESI†). The absolute stereochemistry is assigned as the thioester substituent in the THP having the (S)-configuration assuming the same sense of enantioselectivity occurs in the KR as it does in the asymmetric reaction. This implies the absolute stereochemistry of the uncyclized alcohol is (S).
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental procedures and analysis data for all new compounds, overview of the computational methods and results (PDF). Full set of DFT output files with optimized structures, frequencies and high-level single-point energies can be found at http://doi.org/10.17639/nott.7156. Additional reference spectroscopic and reaction data can be found at DOI: 10.15124/8ff9123f-ee1a-4ae2-a60e-7626c989e5a0. See DOI: 10.1039/d2ob00023g |
| ‡ Current address: School of Chemistry, University of Nottingham, University Park, Nottingham, UK, NG7 2RD. |
| This journal is © The Royal Society of Chemistry 2022 |