Controlling chirality in the synthesis of 4 + 4 diastereomeric amine macrocycles derived from trans-1,2-diaminocyclopentane and 2,6-diformylpyridine†
Abstract
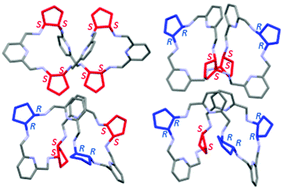
A few suitably long dialdehyde and primary diamine building blocks of a predetermined chirality have been designed and synthesized to enable controlled and efficient synthesis of all six possible diastereomers of 4 + 4 macrocyclic amine derived from trans-1,2-diaminocyclopentane (DACP) and 2,6-diformypyridine (DFP) units. Although two out of six diastereomers have been reported recently, their synthesis presented here is more direct and occurs with an improved yield. This family of 4 + 4 macrocycles contains one pair of homochiral enantiomers of identical RRRRRRRR and SSSSSSSS configurations of DACP units, two different meso forms (meso I of alternating RRSSRRSS and meso II of neighboring RRRRSSSS configuration of DACP moieties) as well as one pair of heterochiral enantiomers, where configuration of one diamine fragment is opposite to the other three diamine parts, RRRRRRSS and SSSSSSRR, respectively. The structures of each type of macrocycle in solid state have been confirmed by single crystal analyses of a macrocyclic amine in its suitable protonated form. The different symmetry of each type of macrocycle in solutions has been proved by 1H and 13C NMR spectra of their hydrochloride derivatives. The chiral nature of two different pairs of optically active enantiomers has been established by circular dichroism spectra. These chiral 4 + 4 diastereomeric macrocycles are receptors for chiral guests and recognize in solution 10-camphorsulfonic acid as well as chiral tartaric acid.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...