Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective organocatalytic syntheses of α-selenated α- and β-amino acid derivatives†
Victoria
Haider‡
a,
Paul
Zebrowski‡
 a,
Jessica
Michalke
b,
Uwe
Monkowius
a,
Jessica
Michalke
b,
Uwe
Monkowius
 c and
Mario
Waser
c and
Mario
Waser
 *a
*a
aInstitute of Organic Chemistry, Johannes Kepler University Linz, Altenbergerstrasse 69, 4040 Linz, Austria. E-mail: mario.waser@jku.at
bInstitute of Catalysis, Johannes Kepler University Linz, Altenbergerstrasse 69, 4040 Linz, Austria
cSchool of Education, Chemistry, Johannes Kepler University Linz, Altenbergerstrasse 69, 4040 Linz, Austria
First published on 3rd January 2022
Abstract
Selenium-containing amino acids are valuable targets but methods for the stereoselective α-selenation of simple amino acid precursors are rare. We herein report the enantioselective electrophilic α-selenation of azlactones (masked α-amino acid derivatives) and isoxazolidin-5-ones (masked β-amino acids) using Cinchona alkaloids as easily accessible organocatalysts. A variety of differently substituted derivatives was accessed with reasonable levels of enantioselectivities and further studies concerning the stability and suitability of these compounds for further manipulations have been carried out as well.
Introduction
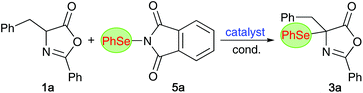
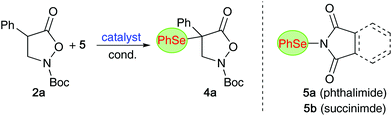
Non-natural α- and β-amino acids (AA) are compounds of high significance and value.1–3 Accordingly, the introduction of efficient and reliable strategies to access novel AA-derivatives in an enantioselective fashion has been a heavily investigated topic within the synthesis- and catalysis-oriented community.2–6 Hereby, the development of methods that allow for direct stereoselective functionalizations of simple (masked) α- or β-AA derivatives and precursors became an especially thoroughly explored field of research. More specifically, direct electrophilic α-functionalizations of suited (prochiral) AA-precursors emerged as versatile strategies to obtain valuable enantioenriched compounds straightforwardly. Besides asymmetric C–C bond formations, direct enantioselective Cα-heteroatom forming approaches to access diversely decorated chiral α-heterofunctionalized α- and β-AA-derivatives have been investigated in much detail.4 While (enantioselective) syntheses of α-halogenated, α-aminated, α-sulfanylated, and to some extent also α-oxygenated, α- and β-AA-derivatives have been regularly reported,4 conceptually similar asymmetric α-selenations of prochiral (masked) α- and β-AA precursors are, to the best of our knowledge, so far still missing.7 Considering the high value of (chiral) organoselenium compounds for medical applications,8–10 as well as their use in (or for) stereoselective syntheses and catalysis approaches,11 and the importance of Se-containing AA in particular,9 the lack in generally applicable methods to access novel α-selenated α-AA (α-Se-α-AA) or β-AA derivatives (α-Se-β-AA) comes as a surprise.Over the last years, our group has had a fundamental interest in the development of organocatalytic protocols to access enantioenriched α-(hetero)-functionalized α- and β-AA derivatives.12,13 Inspired by the value of organoselenium compounds and considering the availability of established electrophilic Se-transfer reagents,14 we now became interested in developing organocatalytic protocols to control the asymmetric α-selenation of easily accessible azlactones 1 (as α-AA precursors)6 and isoxazolidin-5-ones 2 (as β-AA building blocks)15,16 (Scheme 1).17
 | ||
| Scheme 1 Targeted organocatalytic electrophilic α-selenation of masked α- and β-AA derivatives 1 and 2. | ||
Results and discussion
Based on our own recent experience in the field of asymmetric organocatalysis, we opted for the use of chiral quaternary ammonium salts (compounds A–C)18 and chiral organobases (i.e. Cinchona alkaloids19 and Takemoto's bifunctional thiourea-containing amine D;20Fig. 1) as catalysts for the herein investigated electrophilic α-selenation reactions.α-Se-α-AA derivatives
We started our investigations by optimizing the α-selenation of the parent azlactone 1a with the commercially available phthalimide-based PhSe-transfer agent 5a14,21 (Table 1 gives an overview of the most significant results obtained in an exhaustive screening of different organocatalysts and conditions). First attempts with the established chiral ammonium salt catalysts A–C18 were found to be discouraging, as illustrated by the non-selective examples summarized in entries 1–3. A variety of other ammonium salt derivatives and conditions/bases were tested as well, but racemic 3a was obtained in each case only. By switching to classical Cinchona alkaloids as chiral organobase catalysts next, results were more encouraging. First experiments utilizing the four naturally available alkaloids quinine (QN), quinidine (QD), cinchonine (CN), and cinchonidine (CD) gave promising results (entries 4–7), with QN and QD being more selective than their structural analogues CD and CN. Due to their pseudoenantiomeric relationship, the diastereomeric QN and QD gave access to both enantiomers of product 3a in an enantioenriched manner, albeit with a slightly higher enantioselectivity for the (−)-enantiomer (accessible by using QN; entry 4).| Entry | Cat. (mol%) | Base | Solvent | T [°C] | Yieldb [%] | erc |
|---|---|---|---|---|---|---|
| a Unless otherwise stated, reactions were run for 1 h using 0.05 mmol 1a and 0.055 mmol 5a in the presence of the given catalyst in the indicated solvent (c = 0.05 M with respect to 1a) under Ar and exclusion of light. b Isolated yields. c Determined by HPLC using a chiral stationary phase (given as (−)/(+)-enantiomeric ratio). d c = 0.0125 (based on 1a). e 1 mmol scale. | ||||||
| 1 | A (10%) | K3PO4 | Toluene | 25 | 85 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
| 2 | B (10%) | K3PO4 | Toluene | 25 | 34 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
| 3 | C (10%) | K3PO4 | Toluene | 25 | 78 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
| 4 | QN (10%) | — | Toluene | 25 | 82 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 5 | QD (10%) | — | Toluene | 25 | 96 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
| 6 | CN (10%) | — | Toluene | 25 | 84 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
| 7 | CD (10%) | — | Toluene | 25 | 95 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| 8 | QN (10%) | — | CH2Cl2 | 25 | 92 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 9 | QN (10%) | — | MTBE | 25 | 98 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 10 | Me-QN (10%) | — | Toluene | 25 | 90 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
| 11 | DHQN (10%) | — | Toluene | 25 | 90 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 12 | D (10%) | — | Toluene | 25 | 68 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 13 | DHQN (5%) | — | Toluene | 25 | 97 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 14 | DHQN (1%) | — | Toluene | 25 | 85 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 15 | DHQN (5%) | — | Toluened | 25 | 97 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 16 | DHQN (5%) | — | Toluened | 0 | 92(94)e | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (88 11 (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12)e 12)e |
| 17 | DHQN (5%) | — | Toluened | −20 | 95 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
Comparing different solvents next (entries 4, 8, and 9), toluene was found to be superior to halogenated or ether-based solvents (other aromatic solvents did not allow for any improvement anymore). Modified quinine-derivatives were screened as well but, as exemplified for Me-QN, O-alkylation had a detrimental effect on the enantioselectivity (entry 10). Encouragingly however, dihydroquinine (DHQN) allowed for a slightly higher selectivity than QN (compare entries 11 and 4). Considering the beneficial effect of free OH-groups within the tested Cinchona alkaloids, we also used Takemoto's bifunctional thiourea-containing catalyst D,20 which however did not allow for any enantioinduction (entry 12).
Having identified DHQN as the best-suited (easily accessible) chiral organobase catalyst for the asymmetric synthesis of the masked α-Se-α-AA 3a, we carried out a final optimization with this alkaloid (entries 13–17). Lowering of the catalyst loading to 5 mol% was possible without negatively effecting the outcome (see entries 13 and 14) and a slightly higher dilution had a beneficial effect on the enantioselectivity (entry 15). With respect to the reaction temperature, 0 °C was found to be the optimum (entry 16), while lower temperatures had a detrimental effect on the selectivity again (entry 17). Gratifyingly, the reaction could also successfully be carried out under the optimized conditions on 1 mmol 1a scale (entry 16), substantiating the robustness of the protocol.
One important observation that we made during this optimization process was that product 3a slowly decomposes when kept in solution, especially in the presence of light, by forming diphenyldiselenide. The stability is however significantly improved in the absence of light and the compound was found to be benchstable for several weeks when stored in substance under argon in the dark. With this obvious sensitivity noted, we next tested the suitability of compound 3a for further manipulations. Unfortunately however, we were not able to carry out the selective (nucleophilic) ring opening of this azlactone to analogous acyclic α-Se-α-AA derivatives, as compound 3a undergoes relatively rapid decomposition and deselenation reactions upon treatment with acid or base. For example, as outlined in the upper part of Scheme 2, the treatment of enantioenriched 3a with K2CO3 in MeOH lead to the formation of rac-6 in low yield only (accompanied with unidentified side-products). Opting for other bases and solvents turned out to be even worse and besides several not further specified decomposition products again the formation of diphenyldiselenide was observed.
As it was not possible to access more advanced α-Se-α-AA derivatives from 3a, we also explored the direct α-selenation of the dipeptide-based azlactone 7 (Scheme 2, lower reaction). Although it was possible to carry out this reaction with reasonable yields in the presence of either QN or QD (leading to opposite diastereoisomer preferences), product 8 was found to be very unstable and decomposed within a few hours when kept in solution.
Having identified suited conditions for the enantioselective α-selenation of 1a (entry 16, Table 1) and with a better understanding of the sensitivity of the hereby accessed product 3a at hand, we next investigated the application scope (Scheme 3) for a variety of differently substituted azlactones 1 (giving products 3a–u) as well as utilizing alternative phthalimide-based Se-transfer reagents 5 (products 3v–x). Unfortunately, the α-phenyl product 3b was found to be very sensitive and although its formation was indicated by crude product 1H NMR analysis, it was not possible to isolate this derivative at all because of its fast decomposition. In sharp contrast, the α-alkylated targets 3c–g were found to be more stable and could be obtained in satisfying isolated yields and with moderate to good enantioselectivities, depending on their substitution pattern (i.e. the presence of a bulky i-Pr group (3d) or a nitrobenzyl group (3i) had a negative effect on the enantioselectivity). Interesting effects were observed when varying the substituent in the 2-position of the azlactone skeleton (products 3j–p). While the presence of an electronrich methoxybenzene group (3l) had a positive effect on the enantioselectivity, electronpoor aryl substituents, i.e. –CF3 (3j) and –NO2 (3k), lead to significantly reduced selectivities. Moreover, the presence of alkyl substituents in this position resulted in more or less racemic product formation only (3o and 3p). Testing a few other 2-methoxybenzene containing substrates 1 with different residues in the α-position revealed a complex scenario of substituent effects. While the products 3q and 3r could be obtained with higher enantioselectivities than their 2-phenyl analogues 3h and 3i, the presence of an isobutyl group had a detrimental effect hereby (3tvs.3e).
A few alternative selenation reagents were prepared as well22 and tested herein (products 3v–x), showing a rather pronounced dependency of the overall reaction progress and the enantioselectivity based on the nature of these reagents.
α-Se-β-AA derivatives
Having investigated the α-selenation of the masked α-AA compounds 1, we next put our focus on the cyclic β-AA derivatives 2 (Table 2). We started again by using chiral ammonium salt catalysts for the α-selenation of the parent substrate 2a with the phthalimide-based reagent 5a (entries 1–3). Surprisingly, although Maruoka's catalyst A was found to be very well-suited for asymmetric α-(hetero)functionalizations of compounds 2 in the past,12,13,16 only low levels of enantioselectivities could be achieved for the α-selenation of 2a (entry 1 gives the best result obtained after screening a variety of different conditions). The alternative ammonium salts B and C as well as the bifunctional catalyst D were even less promising (entries 2–4) and we thus tested Cinchona alkaloids next. Interestingly, these chiral amines have so far only sparingly been used for asymmetric α-functionalizations of compounds 223 and we were glad to see that they allowed for promising initial levels of enantioselectivities accompanied with reasonable yields under conditions similar to those used for azlactones 1. Again, QN and DHQN performed slightly better than the other derivatives (entries 5–8) and the reaction was found to be carried out best in toluene in the presence of 10 mol% catalyst at temperatures between 0–25 °C (please see entries 9–14 for the optimization of conditions with QN as a catalyst). Interestingly, the enantioselectivity could be improved to some extent when using the succinimide-based reagent 5b instead of 5a (entry 15).24 Finally, DHQN was used with 5b under slightly diluted conditions, resulting in robust and scalable conditions allowing for moderate enantioselectivities (entry 17; in general, the reaction is complete within 2 h but can also be run overnight to ensure full conversion, i.e. for the larger scale experiment and for some other derivatives (vide infra)).| Entry | Cat. | 5 | Solvent | t [h] | T [°C] | Yieldb [%] | erc |
|---|---|---|---|---|---|---|---|
| a Unless otherwise stated, reactions were run using 0.05 mmol 2a and 0.055 mmol 5 in the presence of the given catalyst in the indicated solvent (c = 0.05 M with respect to 2a) under Ar and exclusion of light. b Isolated yields. c Determined by HPLC using a chiral stationary phase (given as (+)/(−)-enantiomeric ratio). d With 1.1 equiv. K2CO3. e c = 0.025 based on 2a. f 0.8 mmol scale. | |||||||
| 1 | A (5%)d | 5a | Toluene | 20 | 25 | 37 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 2 | B (5%)d | 5a | Toluene | 20 | 25 | 48 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
| 3 | C (5%)d | 5a | Toluene | 20 | 25 | 47 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
| 4 | D (5%) | 5a | Toluene | 2 | 25 | 81 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
| 5 | QN (10%) | 5a | Toluene | 2 | 25 | 68 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 6 | QD (10%) | 5a | Toluene | 2 | 25 | 65 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68 |
| 7 | CD (10%) | 5a | Toluene | 2 | 25 | 65 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 8 | DHQN (10%) | 5a | Toluene | 2 | 25 | 73 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 9 | QN (5%) | 5a | Toluene | 2 | 25 | 43 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 10 | QN (10%) | 5a | CH2Cl2 | 2 | 25 | 76 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 11 | QN (10%) | 5a | MTBE | 2 | 25 | 76 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
| 12 | QN (10%) | 5a | Toluene | 2 | 0 | 82 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 13 | QN (10%) | 5a | Toluene | 2 | −20 | 76 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 14 | QN (10%) | 5a | Toluenee | 2 | 25 | 78 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 15 | QN (10%) | 5b | Toluene | 2 | 25 | 74 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 16 | DHQN (10%) | 5b | Toluenee | 2 | 25 | 72 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 17 | DHQN (10%) | 5b | Toluenee | 14 | 0–25 | 69(72)f | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17(83 17(83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17)f 17)f |
Interestingly, it was also possible to increase the enantiopurity of product 4a up to er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by slowly crystallizing racemic 4a out of a solution of enantioenriched 4a (er = 83
2 by slowly crystallizing racemic 4a out of a solution of enantioenriched 4a (er = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17) in Et2O.25
17) in Et2O.25
We next tested the suitability of compound 4a for the transformations outlined in Scheme 4. Gratifyingly, the nucleophilic ring opening with benzylic amines (giving amides 9) as well as with MeOH in the presence of Amberlyst A21 (accessing ester 10) proceeded reasonably well without causing any erosion of er. The treatment of 4a with mCPBA on the other hand led to a direct elimination reaction, most presumably via syn-elimination of the in situ formed selenoxide-species, which allows for the straightforward synthesis of alkene 11 hereby.
Finally, we also investigated the application scope for the DHQN-catalysed α-selenation of a variety of differently substituted α-arylated derivatives 2 (Scheme 5). It should be noted that we also tested the analogous α-benzylated substrate (Bn instead of Ar). Unfortunately however, and in accordance with our previous α-halogenation results,13b this compound was found to be much less reactive than the α-aryl analogues, giving traces of targeted product only. Furthermore, we also observed a strong influence of the nature of the aryl substituent (Ar) on the outcome of the reaction in terms of yield, conversion, and enantioselectivity. The naphthalene-based 4b as well as the halogenated products 4d–4g could be obtained with complete conversion of the respective starting materials 2 and with moderate levels of enantioselectivities. In contrast, the thiophene-substituted 4c as well as the MeO- and the Me-containing 4h and 4i were formed much slower and in these cases the enantioselectivities were also lower (4h could not be isolated at all because of its low stability). Also, the use of alternative Se-transfer reagents (accessing products 4j–l) was found to be difficult herein, illustrating that our protocol for the α-selenation of compounds 2 is unfortunately rather sensitive to substituent effects.
Conclusions
Using simple Cinchona alkaloids as chiral organobase catalysts allowed for the, to the best of our knowledge so far unprecedented, enantioselective electrophilic α-selenation of azlactones 1 (which were used as masked α-amino acid derivatives) and isoxazolidin-5-ones 2 (representing masked β-amino acids). Utilizing the herein developed methodology gave access to a variety of differently substituted α-Se-amino acid derivatives 3 and 4 with reasonable levels of enantioselectivities and in good yields. Unfortunately, it was not possible to obtain suited crystals of enantioenriched 3 and 4 that allowed for single crystal X-ray analysis and therefore the absolute configuration of the products accessed herein remains yet unknown.Further studies concerning the stability and suitability of these compounds for further manipulations have been carried out as well, demonstrating a certain sensitivity, especially in the presence of light and/or in solution.
Experimental details
Full experimental procedures as well as analytical details can be found in the online ESI.†General procedure for the α-selenation of azlactones 1
To a solution of 1 (1 equiv.) and DHQN (5 mol%) in toluene (0.0125 M with respect to 1) under an atmosphere of argon was added the corresponding Se-transfer agent 5 (1.1 equiv.) at 0 °C and under exclusion of light. The reaction mixture was stirred for 1 h under these conditions. After filtration through a pad of Na2SO4 the crude product was subjected to silica gel column chromatography (eluent: heptanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc gradient) giving products 3 in the report yields and enantiopurities (compare with Scheme 3).
EtOAc gradient) giving products 3 in the report yields and enantiopurities (compare with Scheme 3).
Analytical details of product 3a
Obtained as a white amorphous solid in 92% yield with er = 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11. TLC (heptanes
11. TLC (heptanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3.5
EtOAc = 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): Rf = 0.49. [α]24D = −65.1 (c 1.00, CHCl3). 1H-NMR (300 MHz, CDCl3, 298.0 K): δ/ppm = 7.57 (d, J = 9.0 Hz, 2H), 7.50 (d, J = 6.0 Hz, 2H), 7.39 (t, J = 9.0 Hz, 1H), 7.26 (t, J = 9.0 Hz, 2H), 7.19–7.14 (m, 4H), 7.12–7.02 (m, 4H), 3.54 (d, J = 12.0 Hz, 1H), 3.42 (d, J = 15.0 Hz, 1H). 13C-NMR (75 MHz, CDCl3, 298.0 K): δ/ppm = 176.4, 160.7, 138.3, 134.6, 132.8, 130.2, 130.2, 129.2, 128.7, 128.5, 127.9, 127.6, 125.3, 73.9, 41.3. HRMS (ESI-QTOF, MeOH) m/z: [M + H]+ calculated for C23H17NO2Se, 408.0498; found, 408.0500. HPLC: Chiralpak AD-H (n-hexane
1): Rf = 0.49. [α]24D = −65.1 (c 1.00, CHCl3). 1H-NMR (300 MHz, CDCl3, 298.0 K): δ/ppm = 7.57 (d, J = 9.0 Hz, 2H), 7.50 (d, J = 6.0 Hz, 2H), 7.39 (t, J = 9.0 Hz, 1H), 7.26 (t, J = 9.0 Hz, 2H), 7.19–7.14 (m, 4H), 7.12–7.02 (m, 4H), 3.54 (d, J = 12.0 Hz, 1H), 3.42 (d, J = 15.0 Hz, 1H). 13C-NMR (75 MHz, CDCl3, 298.0 K): δ/ppm = 176.4, 160.7, 138.3, 134.6, 132.8, 130.2, 130.2, 129.2, 128.7, 128.5, 127.9, 127.6, 125.3, 73.9, 41.3. HRMS (ESI-QTOF, MeOH) m/z: [M + H]+ calculated for C23H17NO2Se, 408.0498; found, 408.0500. HPLC: Chiralpak AD-H (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-PrOH = 20
i-PrOH = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, flow rate 0.3 mL min−1, 10 °C, λ = 220 nm), retention times tR(minor) = 26.4 min, tR(major) = 27.8 min.
1, flow rate 0.3 mL min−1, 10 °C, λ = 220 nm), retention times tR(minor) = 26.4 min, tR(major) = 27.8 min.
General procedure for the α-selenation of isoxazolidinones 2
A mixture of 2 (1 equiv.), DHQN (10 mol%) and dry toluene (0.025 M with respect to 2) was stirred under Ar and cooled on an icebath. The selenation reagent 5 (1.1–1.2 equiv.) was added at once and the reaction flask was covered with aluminium foil. The reaction mixture was gradually warmed to room temperature and stirred for 14 h, whereupon it was concentrated under reduced pressure and directly subjected to column chromatography with gradient elution (silica gel, heptanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 1
EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 10
0 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain products 4 in the given yields and enantiopurities (compare with Scheme 5).
1) to obtain products 4 in the given yields and enantiopurities (compare with Scheme 5).
Analytical details of compound 4a
Obtained in 72% yield with er = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17. TLC (EtOAc
17. TLC (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) heptanes = 10
heptanes = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): Rf = 0.24 (UV). [α]23D = +90.2 (c 1.01, CHCl3). 1H-NMR (300 MHz, CDCl3, 298.0 K): δ/ppm = 7.41–7.34 (m, 3H), 7.30–7.18 (m, 7H), 4.75 (d, J = 12.7 Hz, 1H), 4.34 (d, J = 12.7 Hz, 1H), 1.56 (s, 9H). 13C-NMR (75 MHz, CDCl3, 298.0 K): δ/ppm = 172.1, 156.6, 138.3, 135.7, 130.6, 129.3, 128.8, 128.6, 127.5, 126.3, 84.7, 59.5, 49.3, 28.4. HRMS (ESI-QTOF, MeOH) m/z: [M + NH4]+ calculated for C20H25N2O4Se+, 437.0974; found, 437.0973. HPLC: Chiralpak AD-H (n-hexane
1): Rf = 0.24 (UV). [α]23D = +90.2 (c 1.01, CHCl3). 1H-NMR (300 MHz, CDCl3, 298.0 K): δ/ppm = 7.41–7.34 (m, 3H), 7.30–7.18 (m, 7H), 4.75 (d, J = 12.7 Hz, 1H), 4.34 (d, J = 12.7 Hz, 1H), 1.56 (s, 9H). 13C-NMR (75 MHz, CDCl3, 298.0 K): δ/ppm = 172.1, 156.6, 138.3, 135.7, 130.6, 129.3, 128.8, 128.6, 127.5, 126.3, 84.7, 59.5, 49.3, 28.4. HRMS (ESI-QTOF, MeOH) m/z: [M + NH4]+ calculated for C20H25N2O4Se+, 437.0974; found, 437.0973. HPLC: Chiralpak AD-H (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-PrOH = 20
i-PrOH = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, flow rate 1.0 mL min−1, 20 °C, λ = 254 nm), retention times tR(minor) = 9.7 min, tR(major) = 11.0 min.
1, flow rate 1.0 mL min−1, 20 °C, λ = 254 nm), retention times tR(minor) = 9.7 min, tR(major) = 11.0 min.
Author contributions
V. Haider and P. Zebrowski contributed equally to the experimental work as well as the preparation of the manuscript. J. Michalke carried out additional supporting experiments. U. Monkowius carried out single crystal X-ray diffraction analysis. M. Waser initiated and supervised the project and wrote the manuscript with input from all authors. All authors have given approval to the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was generously supported by the Austrian Science Funds (FWF): Projects P30237 and P31784. The used NMR spectrometers were acquired in collaboration with the University of South Bohemia (CZ) with financial support from the European Union through the EFRE INTERREG IV ETC-AT-CZ program (project M00146, “RERI-uasb”).Notes and references
- Amino Acids, Peptides and Proteins in Organic Chemistry, ed. A. B. Hughes, Wiley-VCH, 2009, Vol. 1–5 Search PubMed.
- Selected reviews for asymmetric α-AA syntheses: (a) C. Najera and J. M. Sansano, Chem. Rev., 2007, 107, 4584–4671 CrossRef CAS PubMed; (b) A. E. Metz and M. C. Kozlowski, J. Org. Chem., 2015, 80, 1–7 CrossRef CAS PubMed; (c) H. Vogt and S. Bräse, Org. Biomol. Chem., 2007, 5, 406–430 RSC; (d) C. Cativiela and M. Ordóñez, Tetrahedron: Asymmetry, 2009, 20, 1–63 CrossRef CAS PubMed; (e) Tetrahedron Organic Chemistry Vol. 7, ed. R. M. Williams, 2016 Search PubMed.
- Selected reviews for asymmetric β-AA syntheses: (a) S. Abele and D. Seebach, Eur. J. Org. Chem., 2000, 1–15 CrossRef CAS; (b) Enantioselective Synthesis of -Amino Acids, ed. E. Juaristi and V. A. Soloshonok, John Wiley & Sons, 2nd edn, 2005 Search PubMed; (c) B. Weiner, W. Szymanski, D. B. Janssen, A. J. Minnaard and B. L. Feringa, Chem. Soc. Rev., 2010, 39, 1656–1691 RSC; (d) M. Ashfaq, R. Tabassum, M. M. Ahmad, N. A. Hassan, H. Oku and G. Rivera, Med. Chem., 2015, 5, 295–309 Search PubMed; (e) H. Noda and M. Shibasaki, Eur. J. Org. Chem., 2020, 2350–2361 CrossRef CAS.
- I. Eder, V. Haider, P. Zebrowski and M. Waser, Eur. J. Org. Chem., 2021, 202–219 CrossRef CAS.
- M. J. O'Donnell, Tetrahedron, 2019, 75, 3667–3696 CrossRef.
- (a) A.-N. R. Alba and R. Rios, Chem. – Asian J., 2011, 6, 720–734 CrossRef CAS PubMed; (b) P. P. de Castro, A. G. Carpanez and G. W. Amarante, Chem. – Eur. J., 2016, 22, 10294–11031 CrossRef CAS PubMed; (c) I. F. S. Marra, P. P. de Castro and G. W. Amarante, Eur. J. Org. Chem., 2019, 5830–5855 CrossRef CAS.
- For single racemic examples: (a) A. Bhalla, P. Venugopalan, K. K. Bhasinand and S. S. Bari, Tetrahedron, 2007, 63, 3195–3204 CrossRef CAS; (b) J. Li, C. Ma, D. Xing and W. Hu, Org. Lett., 2019, 21, 2101–2105 CrossRef CAS PubMed; (c) S. Jana, P. Aseeva and R. M. Koenigs, Chem. Commun., 2019, 55, 12825–12828 RSC; (d) Z. Shang, Q. Chen, L. Xing, Y. Zhang, L. Wait and Y. Du, Adv. Synth. Catal., 2019, 361, 4926–4932 CrossRef CAS.
- (a) G. Mugesh, W.-W. Du Mont and H. Sies, Chem. Rev., 2001, 101, 2125–2179 CrossRef CAS PubMed; (b) M. P. Rayman, Proc. Nutr. Soc., 2005, 64, 527–542 CrossRef CAS PubMed; (c) C. Narajji, M. D. Karvekar and A. K. Das, Indian J. Pharm. Sci., 2007, 69, 344–351 CrossRef CAS; (d) Organoselenium Chemistry, ed. T. Wirth, Wiley-VCH, 2011 Search PubMed; (e) D. Bartolini, L. Sancineto, A. Fabro de Bem, K. D. Tew, C. Santi, R. Radi, P. Toquato and F. Galli, Selenocompounds in cancer therapy: an overview, in Advances in Cancer Research, ed. K. D. Tew and F. Galli, Academic Press, 2017, Vol. 136, pp. 259–302 Search PubMed; (f) Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments, ed. V. K. Jain and I. Priyadarsini, RSC, 2018 Search PubMed; (g) Organoselenium Chemistry, ed. B. C. Ranu and B. Banerjee, De Gruyter, 2020 Search PubMed.
- (a) A. Böck, K. Forchhammer, J. Heider and C. Baron, Trends Biochem. Sci., 1991, 16, 463–467 CrossRef; (b) D. L. Hatfield and V. N. Gladyshev, Mol. Cell Biol., 2002, 22, 3565–3576 CrossRef CAS PubMed; (c) G. V. Kryukov, S. Castellano, S. V. Novoselov, A. V. Lobanov, O. Zehtab, R. Guigo and V. N. Gladyshev, Science, 2003, 300, 1439–1443 CrossRef CAS PubMed; (d) V. M. Labunskyy, D. L. Hatfield and V. N. Gladyshev, Physiol. Rev., 2014, 94, 739–777 CrossRef CAS PubMed; (e) A. S. Rahmanto and M. J. Davies, IUBMB Life, 2012, 64, 863–871 CrossRef CAS PubMed.
- (a) B. Banerjee and M. Koketsu, Coord. Chem. Rev., 2017, 339, 104–127 CrossRef CAS; (b) P. N. Makhal, A. Nandi and V. R. Kaki, ChemistrySelect, 2021, 6, 663–679 CrossRef CAS.
- (a) T. Wirth, Angew. Chem., Int. Ed., 2000, 39, 3740–3749 CrossRef CAS; (b) F. V. Singh and T. Wirth, Catal. Sci. Technol., 2019, 9, 1073–1091 RSC; (c) L. Shao, Y. Li, J. Lu and X. Jiang, Org. Chem. Front., 2019, 6, 2999–3041 RSC.
- For selected C–C-bond forming examples: (a) M. Waser, K. Gratzer, R. Herchl and N. Müller, Org. Biomol. Chem., 2012, 10, 251–254 RSC; (b) V. Capaccio, K. Zielke, A. Eitzinger, A. Massa, L. Palombi, K. Faust and M. Waser, Org. Chem. Front., 2018, 5, 3336–3340 RSC; (c) A. Eitzinger, M. Winter, J. Schörgenhumer and M. Waser, Chem. Commun., 2020, 56, 579–582 RSC.
- For two recent α-heterofunctionalization examples: (a) A. Eitzinger, J. F. Brière, D. Cahard and M. Waser, Org. Biomol. Chem., 2020, 18, 405–408 RSC; (b) P. Zebrowski, I. Eder, A. Eitzinger, S. C. Mallojjala and M. Waser, ACS Org. Inorg. Au, 2021, 1 DOI:10.1021/acsorginorgau.1c00025.
- L. A. Wessjohann and U. Sinks, J. Prakt. Chem., 1998, 340, 189–203 CrossRef CAS.
- A. Macchia, A. Eitzinger, J.-F. Brière, M. Waser and A. Massa, Synthesis, 2021, 53, 107–122 CrossRef CAS.
- For pioneering examples: (a) T. Tite, M. Sabbah, V. Levacher and J.-F. Brière, Chem. Commun., 2013, 49, 11569–11571 RSC; (b) T. Cadart, C. Berthonneau, V. Levacher, S. Perrio and J.-F. Brière, Chem. – Eur. J., 2016, 22, 15261–15264 CrossRef CAS PubMed; (c) T. Cadart, V. Levacher, S. Perrio and J.-F. Brière, Adv. Synth. Catal., 2018, 360, 1499–1509 CrossRef CAS.
- For pioneering organocatalytic enantioselective α-selenation protocols: (a) M. Tiecco, A. Carlone, S. Sternativo, F. Marini, G. Bartoli and P. Melchiorre, Angew. Chem., Int. Ed., 2007, 46, 6882–6885 CrossRef CAS PubMed; (b) H. Sunden, R. Rios and A. Cordova, Tetrahedron Lett., 2007, 48, 7865–7869 CrossRef CAS.
- For selected reviews: (a) S. Shirakawa and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 4312–4348 CrossRef CAS PubMed; (b) S. Kaneko, Y. Kumatabara and S. Shirakawa, Org. Biomol. Chem., 2016, 14, 5367–5376 RSC; (c) D. Qia and J. Sun, Recent Progress in Asymmetric Ion–Pairing Catalysis with Ammonium Salts, Chem. – Eur. J., 2019, 25, 3740–3751 CrossRef PubMed.
- Cinchona Alkaloids in Synthesis & Catalysis, ed. C. E. Song, Wiley-VCH, 2009 Search PubMed.
- T. Okino, Y. Hoashi and Y. Takemoto, J. Am. Chem. Soc., 2003, 125, 12672–12673 CrossRef CAS PubMed.
- CAS: [71098-88-9].
- Further details can be found in the online ESI.†.
- J.-S. Yu, H. Noda and M. Shibasaki, Chem. – Eur. J., 2018, 24, 15796–15800 CrossRef CAS PubMed.
- Reagent 9 was also tested for the synthesis of azlactone 3a but found inferior compared to 5a (61% yield, er = 53
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47).
47). - Crystallographic details for rac-4a can be found in the online ESI. CCDC 2121570† (Cambridge Crystallographic Data Centre, http://www.ccdc.cam.ac.uk) contains the supplementary crystallographic data.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details and analytical data. CCDC 2121570. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ob02235k |
| ‡ These authors contributed equally (in alphabetical order). |
| This journal is © The Royal Society of Chemistry 2022 |