The role of acetylated cyclooxygenase-2 in the biosynthesis of resolvin precursors derived from eicosapentaenoic acid†
Abstract
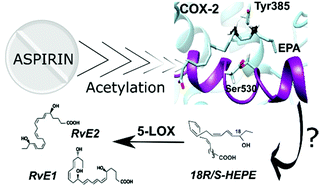
Specialized pro-resolving lipid mediators (SPMs) are natural bioactive agents actively involved in inflammation resolution. SPMs act when uncontrolled inflammatory processes are developed, for instance, in patients of COVID-19 or other diseases. The so-called resolution pharmacology aims at developing new treatments based on the use of SPMs as agonists, which promote inflammation resolution without unwanted side effects. It has been shown that the biosynthesis of SPMs called eicosapentaenoic acid (EPA)-derived E-series resolvins is initiated by aspirin-acetylated COX-2 from EPA, leading to 18-hydroperoxy-eicosapentaenoic acid (18-HpEPE). However, there are many open questions concerning the intriguing role of aspirin in the molecular mechanism of resolvin formation. Our MD simulations, combined with QM/MM calculations, show that the potential energy barriers for the H16-abstraction from EPA, required for forming 18-HpEPE, are higher than for the H13-abstraction, thus explaining why 18-HpEPE is a marginal product of COX-2 catalysis. By contrast, in the aspirin-acetylated COX-2/EPA complex, the H16proS-abstraction energy barriers are somewhat lower than the H13proS energy barriers and much smaller than the H16-transfer barriers in the wild type COX-2/EPA system. Those results agree with the experimental observation that aspirin favours the synthesis of several SPMs known as aspirin-triggered resolvins. In the following step of the catalytic mechanism, the calculated O2 addition to C18 is preferred versus the addition to C14 which also agrees with 18R-HEPE and 18S-HEPE being the main products from EPA in aspirin-acetylated COX-2.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...