Hydrogel-based printing strategy for high-performance flexible thermoelectric generators†
Abstract
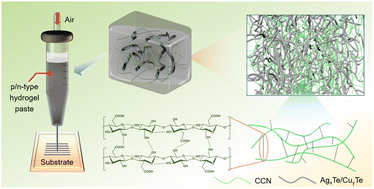
Flexible thermoelectric (TE) devices can utilize the slight temperature difference between curved surfaces and surroundings to generate TE potential, presenting great potential in microelectronic energy supply and wearable sensing. Printing method has been employed to fabricate high-performance flexible TE films by means of excellent capability of assembling nanomaterials, but the decrease in the electrical conductivity caused by organic matters in the thermoelectric pastes will significantly reduce the thermoelectric performance. Herein, we report a hydrogel-based printing strategy to deposit flexible TE generators on various flexible substrates. The hydrogel network formed by physical crosslinking and molecular chain entanglement at 0.498 wt% carboxylated cellulose nanofibers can effectively limit the fluidity of 1D nanorod dispersion, which produces only <5% decline in electrical conductivity and Seebeck coefficient compared to the pure inorganic nanorod films. The device with 72 couples constructed by printing presents a high power density of 1.278 W m−2 under a temperature difference of 50 K. The advantages of hydrogel-based printing can broaden application prospects in the field of wearable electronics.
- This article is part of the themed collections: Nanomaterials for printed electronics, Nanoscale Most Popular 2022 Articles and Nanoscale 2023 Lunar New Year Collection


 Please wait while we load your content...
Please wait while we load your content...