Multiplexed printed sensors for in situ monitoring in bivalve aquaculture†
Abstract
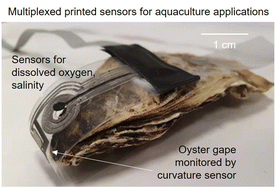
Non-intrusive sensors that can be attached to marine species offer opportunities to study the impacts of environmental changes on their behaviors and well-being. This work presents a thin, flexible sensor tag to monitor the effects of dissolved oxygen and salinity on bivalve gape movement. The measurement range studied was 0.5–6 ppm for the dissolved oxygen sensor and 4–40 g kg−1 for the salinity sensor. The curvature strain sensor based on electrodeposited semiconducting fibers enabled measurements of an oyster's gape down to sub-mm displacement. The multiplexed sensors were fabricated by low-cost techniques, offering an economical and convenient platform for aquaculture studies.
- This article is part of the themed collection: Nanomaterials for printed electronics


 Please wait while we load your content...
Please wait while we load your content...
