Ionic dielectrics for fully printed carbon nanotube transistors: impact of composition and induced stresses†
Abstract
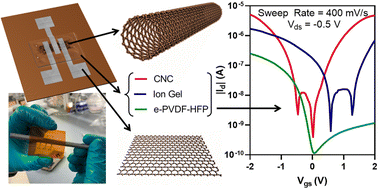
Printed carbon nanotube thin-film transistors (CNT-TFTs) are candidates for flexible electronics with printability on a wide range of substrates. Among the layers comprising a CNT-TFT, the gate dielectric has proven most difficult to additively print owing to challenges in film uniformity, thickness, and post-processing requirements. Printed ionic dielectrics show promise for addressing these issues and yielding devices that operate at low voltages thanks to their high-capacitance electric double layers. However, the printing of ionic dielectrics in their various compositions is not well understood, nor is the impact of certain stresses on these materials. In this work, we studied three compositionally distinct ionic dielectrics in fully printed CNT-TFTs: the polar-fluorinated polymer elastomer PVDF-HFP; an ion gel consisting of triblock polymer PS-PMMA-PS and ionic liquid EMIM-TFSI; and crystalline nanocellulose (CNC) with a salt concentration of 0.05%. Although ion gel has been thoroughly studied, e-PVDF-HFP and CNC printing are relatively new and this study provides insights into their ink formulation, print processing, and performance as gate dielectrics. Using a consistent aerosol jet printing approach, each ionic dielectric was printed into similar CNT-TFTs, allowing for direct comparison through extensive characterization, including mechanical and electrical stress tests. The ionic dielectrics were found to have distinct operational dependencies based on their compositional and ionic attributes. Overall, the results reveal a number of trade-offs that must be managed when selecting a printable ionic dielectric, with CNC showing the strongest performance for low-voltage operation but the ion gel and elastomer exhibiting better stability under bias and mechanical stresses.
- This article is part of the themed collection: Nanomaterials for printed electronics


 Please wait while we load your content...
Please wait while we load your content...
