Networks of as-dispersed, polymer-wrapped (6,5) single-walled carbon nanotubes for selective Cu2+ and glyphosate sensing†
Abstract
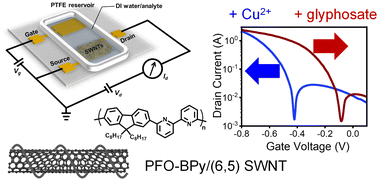
Networks of semiconducting single-walled carbon nanotubes (SWNTs) can be used as the transducing layer for sensors based on water-gated transistors. To add specific sensing capabilities, SWNTs are often functionalized with additional moieties or selective membranes are applied, thus increasing the complexity of the fabrication process. Here we demonstrate that drop-cast networks of monochiral (6,5) SWNTs, which are commonly dispersed in organic solvents with the polyfluorene–bipyridine copolymer PFO-BPy, can be employed directly and without additional functionalization or ion-selective membranes to detect Cu2+ ions over a wide range of concentrations in aqueous solutions. The observed voltage shifts of water-gated transistors with these (6,5) SWNT networks directly correlate with the cupric ion concentration. They result from induced n-doping due to the complexation of positive copper ions to the bipyridine units of the wrapping polymer. Furthermore, the competitive binding of Cu2+ to the herbicide glyphosate as well as to biologically relevant pyrophosphates can be used for the direct detection and quantification of these molecules at nano- to micromolar concentrations.
- This article is part of the themed collections: Nanomaterials for printed electronics and 2022 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...