Nanoarchitectonics horizons: materials for life sciences
V.
Karthick
 *ab,
Lok
Kumar Shrestha
*ab,
Lok
Kumar Shrestha
 bc,
V. Ganesh
Kumar
a,
Pranjali
Pranjali
de,
Dinesh
Kumar
bc,
V. Ganesh
Kumar
a,
Pranjali
Pranjali
de,
Dinesh
Kumar
 e,
Aniruddha
Pal
f and
Katsuhiko
Ariga
e,
Aniruddha
Pal
f and
Katsuhiko
Ariga
 *bg
*bg
aCentre for Ocean Research, Sathyabama Institute of Science and Technology, Jeppiaar Nagar, Rajiv Gandhi Salai, Chennai 600119, India. E-mail: vkarthick.cor@sathyabama.ac.in
bInternational Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: ARIGA.Katsuhiko@nims.go.jp
cDepartment of Materials Science, Faculty of Pure and Applied Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8573, Japan
dDepartment of Physics, Banaras Hindu University, Varanasi 221005, Uttar Pradesh, India
eCentre of Biomedical Research, SGPGIMS Campus, Lucknow 226014, Uttar Pradesh, India
fNanomaterials Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba Central 5, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan
gDepartment of Advanced Materials Science, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8561, Japan
First published on 6th July 2022
Abstract
Nanoarchitectonics relies on the fabrication of materials at the atomic/molecular level to achieve the desired shape and function. Significant advances have been made in understanding the characteristics and spatial assemblies that contribute to material performance. Biomaterials undergo several changes when presented with various environmental cues. The ability to overcome such challenges, maintaining the integrity and effective functioning of native properties, can be regarded as a characteristic of a successful biomaterial. Control over the shape and efficacy of target materials can be tailored via various processes, like self-assembly, supramolecular chemistry, atomic/molecular manipulation, etc. Interplay between the physicochemical properties of materials and biomolecule recognition sites defines the structural rigidity in hierarchical structures. Materials including polymers, metal nanoparticles, nucleic acid systems, metal–organic frameworks, and carbon-based nanostructures can be viewed as promising prospects for developing biocompatible systems. This review discusses recent advances relating to such biomaterials for life science applications, where nanoarchitectonics plays a decisive role either directly or indirectly.
1. Introduction
In nanotechnology, nanosized materials make the building blocks for nanoarchitectonics with respect to their physicochemical properties for a specific function. Nanomaterials allow exploitation of the fundamental peculiarity of quantum mechanics, which drives our interest in the fabrication of nanoscale units. The promising behavior of these nanoscale particles promotes applications in biomedicines with enhanced utilization as both therapeutic and diagnostic agents via nanoarchitectonics.1,2 Nanoscale structures are created to achieve hierarchical structures through biomolecular interactions such as hydrophobic/hydrophilic, π–π, and other interactions.3 A significant number of these nanoformulations comprise drug conjugates and complexes, dendrimers, vesicles, micelles, core–shell particles, and carbon nanotubes designed to carry off drugs or contrast agents.4,5 Developing a robust biocompatible system functionalized with biomolecules is essential for biomedical applications. The developed system will be required to sustain the foreign body reaction where macrophages attach, resulting in the formation of a dense collagen layer around it.6 However, owing to the multiple administrations of conventional therapies and/or diagnostic nanoparticles, increased concern over patient compliance and safety has obliged medical nanotechnology to set focal points in the theranostic paradigm.7 A tunable spatial architecture allows enhanced interaction between nanomaterials and a metal–organic framework where a surfactant plays a vital role in achieving tunability and precision.8 Nanostructures upon surface modifications are often used in diagnostic applications by incorporating diagnostic and therapeutic moieties via conjugation. Nanostructures, including nanoshells, nanoflowers, nanorods, nanocages, nanoplates, nanocubes, nanosheets, layered nanostructures, and quantum dots are some of the structural assemblies that can be created via nanoarchitectonics.9 This review highlights some of the latest research work involving nanoarchitectonics to develop nanostructured biomaterials with life science applications ranging from anticancer, to imaging, drug delivery, and other related aspects.2. Material nanoarchitectonics: a paradigm shift to create biomaterials
Nanoarchitectonics aims to construct complex functional biosystems made of nanostructures/materials using a combination of supramolecular chemistry, harmonization of molecules/atoms, chemical nanomanipulation, and self-assembly with nanotechnology.10,11 The term nanoarchitectonics was coined by Masakazu Aono in 2000 during the 1st International Symposium on Nanoarchitectonics Using Suprainteractions in Tsukuba, Japan.12 Functional architectonics in biosystems can be delicately modified by bottom-up construction from building blocks with controlled dimensions.13,14 Nanoscale structural assemblies can be manipulated via noncovalent interactions to make strong bio–nano interactions. Nanoassemblies are formed by conjugating them with functional groups such as di and tri-sulfide bonds to enhance drug loading capacity, stability, and sensitivity.15 Structural precision via self-regulation of molecules via non-covalent interactions is seen as the best example of a naturally occurring nanoarchitectonics process. A variety of intramolecular interactions, including electrostatic, van der Waals, biomolecular recognition, and complementary base pairing of nucleic acids, contribute to the making of assemblies of interest.16 The arrangement of nanostructures as monolayer and multilayer arrangements evolved significantly in drug delivery specific to the controlled release of drugs/molecules.17 A layer-by-layer (LbL) assembly approach uses the deposition of multivalent components with complementary parties having electrostatic interactions.18 An arrangement of atoms or molecules for the precise targeting of tumor cells/mass overcoming of immunological response is regarded as a better target system. A cancerous cell is targeted by using a nanosystem via two processes: active and passive targeting. Active targeting involves molecular recognition of a ligand with the receptor and antibody with the antigen. Tumor cells have defective physiology, and in passive targeting, nanostructures assist in enhanced permeability and a retention effect where the efficacy of nanosystems depends on their ability to linger in circulating body fluid, thereby targeting tumor cells.19,20 To target a tumor system, hierarchical structures are fabricated with the help of nanoarchitectonics, including harmonization of various assemblies to facilitate enhanced interaction with cells, particularly for imaging and therapies.21 Polymers self-assembled into supramolecular structures are effectively utilized for the modulation of cells that can adhere to hydrophobic and hydrophilic surfaces.22 Manipulating magnetic nanosystems is proven to offer some of the best available magnetic resonance imaging (MRI) contrast agents.23 Likewise, composite materials with higher mechanical strength via crosslinking and addition of soft/hard components will yield biocompatible scaffolds for the comfortable growth of desired cell types.24 Some of the possible nanostructures can be developed via nanoarchitectonics and have potential applications in life science (Fig. 1).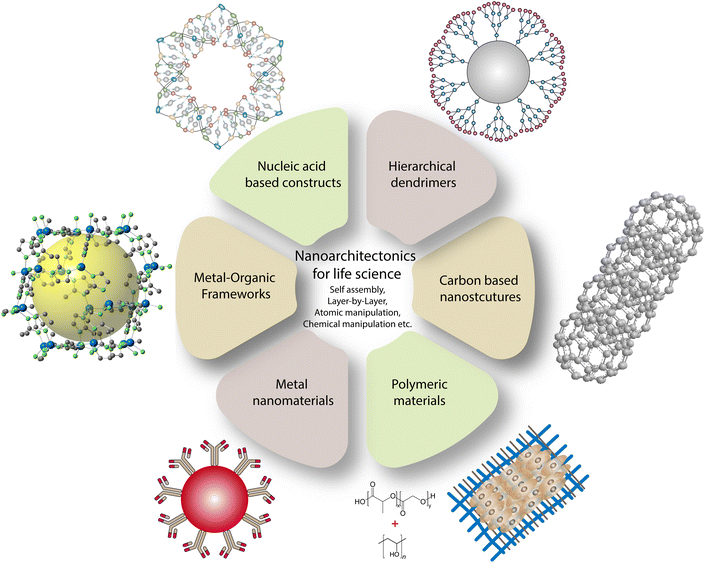 | ||
| Fig. 1 Prospects for developing biomaterials for life science applications via the nanoarchitectonics concept. | ||
2.1 Metal nanosystems
Fluorescent and plasmonic colloidal nanoparticles are widely used in imaging cells and sensing specific analytes. The multiplexed sensing concept involves functionalizing a nanomaterial with various types of element recognition (i.e., receptor identification on the cell surface).25 They are often designed with specific spatial and temporal changes due to cross-reactivity.26 Optical excitation of noble metal nanoparticles corresponding to their surface plasmon resonance results in the coherent oscillation of free electrons.27 The absorption of photons by metal nanoparticles results in the decay of a surface plasmon into hot electron/hole pairs. The pairs thermalize to a hot Fermi–Dirac-like distribution via electron–electron scattering, then equilibrate within the lattice through electron–phonon interactions.28 The size and shape of the gold nanoparticles play a crucial role in surface-enhanced Raman scattering effect. They possess an enhanced electric field at their edges where, various electric dipole and magnetic dipole are excited under irradiation.29 Capping of nanotriangles with biomolecules makes them much better for targeting applications as they easily penetrate cells by interacting with the ligands. Plasmonic nanoparticles can be organized into nanoparticle oligomers via self-organization by various interactions that are useful for the construction of 2D and 3D structures. Gold nanoparticles can be considered as a material-wise, biocompatible system, owing to lower toxicity among noble metal nanoparticles. Photothermal therapy for cancer treatment employing gold nanoparticles/clusters has been widely used in recent times by researchers worldwide. Recently, a graphene-oxide-based metal nanoparticle system has been employed to improve photothermal efficacy. In a recent study, decorated graphene oxide flower-shaped gold nanoparticles were conjugated to the nucleolin-specific aptamer and polyethylene glycol (PEG) for enhanced stability and specificity.30 The developed system is found to be effective in targeting tumors and requires the use of nanomaterials with extended π systems, which are active in the near-infrared-II (NIR-II) (1000–1700 nm) region to penetrate deep inside the tumor mass. A recent study combining boron difluoride formazanate dye with the surfactant Pluronic® F-127 yielded a stable photothermal agent with strong π–π stacking interaction.31 Qingqing Li and collaborators have developed a hydrogen-peroxide-driven Janus gold–platinum-based nanomotor for enhanced NIR-II-region photoacoustic imaging of tumor mass (deeper cells). The developed nanomotor was found to effectively penetrate deeper tumor cells via self-propulsion which then released platinum ions for nucleic acid and cell damage in tumor cells.32 Cancer immunotherapy is a promising area where scientists overcome the limitations of other cancer treatments, including chemotherapies and radiotherapies. To achieve immunotherapy, it is necessary to enhance the interactions between the effector and tumor cells via antibodies.33 A schematic illustration of how an immunomodulatory system can be developed out of a nanoparticle is shown in Fig. 2.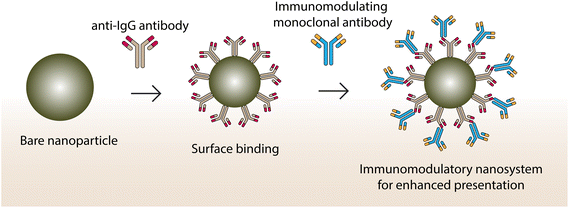 | ||
| Fig. 2 Immunomodulatory nanosystems conjugating two different monoclonal antibodies using Fc-specific recognition for targeting effector and tumor cells. | ||
Cheng-Tao Jiang and co-workers have developed a nanoparticle-based immunomodulating adaptor to act as a bridge between effector and tumor cells for enhanced interaction.34 Antibody functionalization on systems such as liposomes, polymeric nanoparticles, metal nanoparticles, dendrimer nanoparticles, carbon nanotubes, magnetic nanoparticles, and metal–organic frameworks are effective in pharmaceutical applications, including drug delivery and immunotherapy.35 Gold nanocubes have also been explored for their suitability in anticancer applications as they have high photoluminescence properties with good mechanical flexibility and stability. They can be tailored via nanoarchitectonics, such as the addition of surfactants, and can be utilized to encapsulate therapeutic molecules to serve as a diagnostic moiety.36 For conducting high-throughput translational proteomic analysis, superparamagnetic iron oxide nanoparticles were developed for rapid magnetic separation from plasma to study proteomic profiling in an effective manner. Decorated gold nanocubes have been used as a fluorescent probe for imaging human liver cancer cells and human embryo kidney cells using single-photon excitation.37 Li Zhang and collaborators used palladium nanotubes to induce autophagy and an autophagic flux blockade on HeLa cells. They used an autophagosome marker protein light chain 3 and measured its fluorescent intensity to denote the autophagosome level.38 To increase the selectivity and specificity, biomolecules of interest are attached to it for advanced imaging and targeting of cell types.39 Self-assembled peptide-based nanoparticles with genipin were used for enhanced dark-field hyperspectral imaging. These peptide-based supramolecular nanostructures show excellent light-scattering properties because of c-terminal amidation.40,41 Metal nanoparticles can penetrate a deeper tumor mass when tagged with specific peptides. In a recent study, biotinylated cell-penetrating peptides and PEG along with gold nanoparticles were used to form a complex. The developed complex was able to internalize into pancreatic and glioblastoma cancer cells by interacting with them.42 Tagging a specific moiety/peptide with nanoparticles can target cancer cells, thereby recruiting Treg cells for the specific expression of chemokine receptors for enhanced action (Fig. 3).
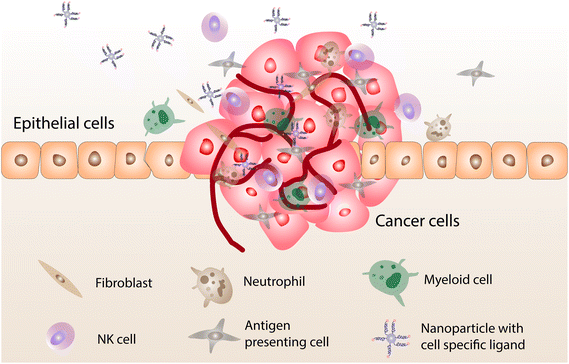 | ||
| Fig. 3 The recruitment of Treg cells at a tumor site and the expression of specific chemokine receptor cells which bind with ligands attached to the nanoparticle. | ||
Detecting cancer in early stages can help control the disease prognosis from a clinical point of view. Medical practitioners use microRNA (miRNA) as an effective way to detect a tumor in its early stages. Recently, Cairou Chen and collaborators developed target-triggered core–satellite nanocomposites made of gold nanorods and 4-sulfanylbenzonitrile for the surface-enhanced Raman spectroscopic detection of miRNA-21 (an oncogenic miRNA expressed in mammalian cells associated with various cancer types).43 Likewise, a gold-nanoparticle-oxidized multiwalled carbon nanotube composite is used as an electrochemiluminescence immunosensor to detect carcinoembryonic antigens.44 The authors suggested that although the detection sensitivity is lower, exploring this avenue can open up possibilities in developing highly sensitive immunosensors for the detection of carcinogenic antigens.
2.2 Nucleic acid-based nanostructures
The selectivity and programmability of Watson–Crick base pairs allow us to create a supramolecular DNA assembly with shape control, rigidity, and sensitivity. DNA is seen as an important building block for the construction of supramolecular assemblies due to its programmability and molecular recognition properties.45 Using DNA as a construction material for fabricating hierarchical structures was seen as a bright prospect when Seeman studied the possibility of creating nucleic acid junctions and lattices.46 This study paves the way to creating DNA nanostructures of various geometries and complex topologies that include truncated octahedral, icosahedral, tetrahedral, and dodecahedral.47–49 The upper hand for DNA nanostructures over other materials lies in their flexibility to remodel DNA strands dynamically using molecular simulations or reaction-diffusion modelling.50 Oligonucleotides, along with nucleotide linkers of various lengths and linker size, facilitate the development of complex DNA nanostructures.51 Integration of nanoarchitectonics to achieve DNA-based nanostructures has paved the way for the development of hierarchical structures by a bottom-up approach.52 Considering their advantages, many nucleic acid structures, and a plethora of nucleic acids, are available for possible applications of various sorts. Their stimuli-responsive behavior, drug loading capacity, and specificity have opened new biomedical research avenues.53 This section focusses on various studies that showcase their ability to be cited as effective biomaterials. Entry of drugs/carriers into cells is often challenging as the cell membrane is comprised of lipid bilayers with membrane proteins which obstruct the path. DNA, despite being polyanionic, can still enter the negatively charged cell membrane via a variety of interplays and interactions. Xueyu Peng and co-workers have developed DNA nanostructures with tetrahedron, triangular prism, and cube shapes for studying the cellular internalization mechanism. By performing both experiments and simulations, they found that the membrane and scavenger receptors were mediating the entry of DNA nanostructures via an endocytic pathway. Molecular simulation studies indicated that the presence of corner angles in the nanostructures helped to make a stronger interaction with scavenger receptors.54 Hanewich-Hollatz and co-workers developed a robust conditional regulator using RNA to direct the function of a clustered regularly interspaced short palindromic repeats (CRISPR) protein effector to the target gene. The authors also stressed the importance of understanding the structural and functional relationship between RNAs, protein effectors, switches, and triggers.55 Anchoring DNA to the cell membrane is generally achieved by tagging a hydrophobic agent such as cholesterol or tocopherol with a spacer arm and a sticky end (Fig. 4).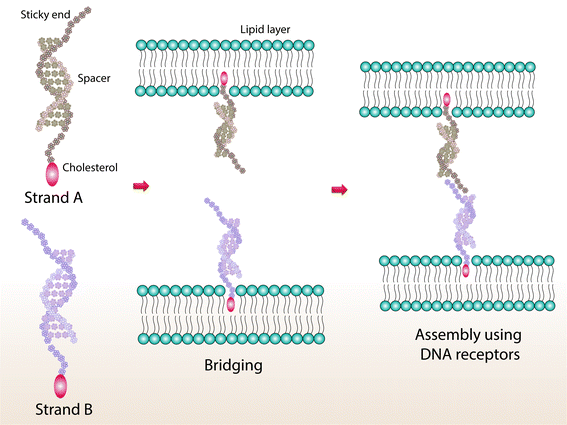 | ||
| Fig. 4 An illustration of a sticky region that allows DNA strands to bind with complementary base pairs via self-assembly to achieve a loop configuration. | ||
The sticky end facilitates its interaction with other biomolecules such as receptors through the complementarity of DNA strands.56 Imaging cellular components using fluorescent-based nucleic acids by covalent attachment of fluorophores with excellent fluorescence and stability has been extensively studied.57,58 Aggregation-induced emission, photoinduced electron transfer, and fluorescence-induced electron transfer are commonly used approaches to induce fluorescence in fluorophores.57 Immunotherapy holds great potential to combat cancer; however, the tumor microenvironment is immunosuppressive in nature. To overcome this, Kuan-Wei Huang and co-workers have developed a dual-targeted immunogene therapy using small interfering RNA against immunosuppressive factors and plasmid DNA encoding immunostimulating cytokines to modulate the immunosuppressive tumor microenvironment to activate the effector cells. This targeting was achieved using lipid-dendrimer-calcium phosphate nanoparticles with thymine-functionalized dendrimers for the enhanced delivery of genes and function.59 Loss of p53 function may lead to immunosuppression, and to restore its function, a C-X-C motif chemokine receptor type 4-targeted mRNA nanoplatform was designed by Yuling Xiao and collaborators to potentiate an immune checkpoint blockade response in hepatocellular carcinoma. For effective targeting, they conjugated a targeting peptide with the nanoplatform, which is specific to the receptor. The system is found to be effective for hepatocellular carcinoma targeting in a tumor microenvironment. Likewise, Kaixiang Zhang and collaborators have developed a pH-driven interlocked DNA nanospring to activate T-cell proliferation. They found that the developed system is more stable than that of double-stranded DNA, which regulates the nanoscale distribution of the cluster of differentiation (CD3) receptor on the cell surface at low pH (tumor microenvironment).60 In another study, pheophorbide A photosensitizers were grafted on a DNA backbone using phosphorothioate modification and small inhibitory RNAs (siRNA) along with a programmed death ligand were pooled together to form a supramolecular structure via self-assembly. The developed tetrahedron network was able to kill cells through photodynamic activity and simultaneously silence programmed death ligand expression for enhanced antitumor activity.61 Size-tunable ferrocene-based nucleic acid polymer structures along with glucose oxidase were developed to localize into tumor cells via an enhanced permeability and retention (EPR) effect. Ferrocene moieties in the polymer complex undergo a Fenton-like reaction to reduce its size, which helps the complex to enter tumor cells easily. Glucose oxidases present in the system react with glucose and form hydrogen peroxide to target cancer cells.62 The same group also developed an electron-transfer-triggered emission probe containing persistent luminescence nanoparticles along with ferrocene aptamers for imaging studies. The imaging is achieved when there is an electron transfer chain between the probe and the ligand, resulting in a fluorescent glow in the target organs.63
2.3 Dendrimer nanosystems
The ability to precisely manipulate the size, surface group, and molecular composition for targeted drug delivery and other applications makes dendrimers an excellent choice for biomedical scientists. Dendrimers are hyperbranched monodisperse complexed, which can be tailored to nanolevel for enhanced interaction with biological interfaces. In general, dendrimers comprise a central core, repeated binding units, and numerous terminals allowing them to carry molecules of interest. Tomalia and co-workers developed the first poly(amidoamine) (PAMAM) dendrimer family. After that, the PAMAM-based structures were widely used in biomedical applications.64 It has metal coordination sites that can be suited to accompany metal ions to increase the strength by the assembly of metal salts.65 To target activated glia in the central nervous system, Anjali Sharma and co-workers developed low-molecular-weight PEG-based building blocks using hypercore-hypermonomer and orthogonal approaches to present high surface hydroxyl density.66A dendrimer core is designed to accommodate the branching structures with the highest possible structural flexibility. Ling Peng and her research group have done pioneering work in developing amphiphilic dendrimers with hydrophobic alkyl chains and hydrophilic dendrons.67 Recently, the group used 1,4,7-triazacyclononane-1,4,7-triacetic acid-based dendrimer in advanced positron emission tomography imaging. They found that the conjugate offers enhanced specificity and superior image sensitivity to the reference 2-fluorodeoxyglucose. In addition, the developed dendrimer system can detect low-glucose-uptake tumors that are usually not detectable with the available systems.68 In a breakthrough study, an siRNA targeting system using an amphiphilic dendrimer conjugated to a dual targeting peptide bearing a tripeptide Arg–Gly–Asp head was developed for effective gene silencing. The stability of the conjugate was established by coating the positively charged siRNA/dendrimer conjugate with a negatively charged part of the tripeptide.69 In another study, an effective system to deliver siRNA for silencing genes in normal cells and hematopoietic CD34+ stem cells was explored.70 A schematic illustration of how a supramolecular dendrimer system can effectively deliver siRNA for a gene silencing application is shown in Fig. 5.
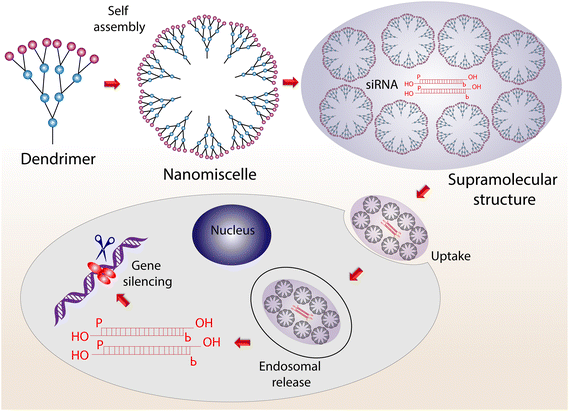 | ||
| Fig. 5 The process of moving from a dendrimer to a supramolecular structure via self-assembly for the cellular uptake of siRNA for gene silencing. | ||
Amphiphilic dendrimers have the advantage of multivalency using hydrophobic and hydrophilic heads through which strong interactions can be achieved between the host–guest system.71 Highly stable human membrane vesicles with dendrimersomes were fabricated using a co-assembly containing a human cell membrane and a Janus dendrimer for targeting bacterial adhesion proteins.72 Likewise, amine-terminated PAMAM dendrimers were conjugated with donepezil for effective delivery to the brain. The developed system was shown to possess a better half-life and uptake.73 Yongchao Wang and collaborators have developed a light-activable immunological adjuvant made of a hypoxia-responsive amphiphilic dendrimer conjugated with a photodynamic agent, chlorin e6, for accumulation in tumor cells. The developed conjugate was activated with the help of near-infrared light irradiation, resulting in the consumption of molecular oxygen to generate reactive oxygen species (ROS), which then destroy the tumor cells. Simultaneously, consumption of molecular oxygen by the dendrimer causes the transformation of 2-nitroimidazole to 2-aminoimidazole, which acts as an effective adjuvant.74
2.4 Biodegradable polymers
The utilization of polymers as biomaterials has attracted a lot of attention due to their flexibility and various mechanical properties. Materials in drug delivery applications often deliver drugs at the target site via stimuli-responsive functions, including pH, temperature, oxidation–reduction reactions, and hydrolysis. Various synthetic polymers like poly(lactic-co-glycolic acid) (PLGA), poly(glycolic acid), and poly(lactic acid) (PLA) were approved by the US Food and Drug Administration (FDA) for biomedical applications.76 Coordination polymers are naturally soft, so an external stimulus can deform their lattice structures. Temporally controlled supramolecular polymers, like functionalized naphthalene diimides, show great selectivity and can form helical supramolecular polymers upon binding adenosine phosphates (di and tri phosphates). Enzyme-like creatine phosphokinase and hexokinase can be used for switching the helicity of a supramolecular polymer.77 PLGA is an excellent stimuli-responsive biomaterial that is proven to have excellent biocompatibility. PLA, poly(propylene fumarate) (PPF), poly(glycerol sebacate) and poly(phosphane) can also be named as synthetic polymers which have been used for bone regeneration studies due to their excellent elasticity, ductility, and larger pore size.78 Polyesters, polyanhydrides, poly(amido amines), poly(β-amino esters), etc., are other biopolymers that have also been used as biomedicines. Mucoadhesive polymers such as poly(acrylic acid) are used for biomedical applications due to their large surface area and residence time.79 Polymers like poly(ethylene terephthalate) fibers and microsphere silicone rubbers are used for vascular prostheses and bypass surgery.75 Poly(N-isopropylacrylamide) (PNIPAm)-based thermoresponsive cell sheets control cell adhesion and detachment through temperature control. Fabrication of PNIPAm sheets were carried out using electron-beam-induced polymerization, atom transfer radical polymerization, reversible addition–fragmentation chain transfer polymerization, block copolymers, or ultraviolet-radiation-induced crosslinking.80 Erik C. Dreaden and co-workers have developed a dual-targeting hyaluronan-LbL assembly upon fluorescent polystyrene nanoparticles for pH-driven active targeting of hypoxic tumor pH and hyaluronan-directed targeting of stem cell receptor CD44.81 An accelerated degradation rate that causes the collapse of scaffolds and acid degradation products may lead to inflammation. Tuning the PLGA degradation rate allows us to achieve mechanically robust polymer structures suitable for various applications. Our group has successfully developed a system to release a drug in a sustained manner for an anticancer effect on breast cancer cells.82 Schematics on how PLGA delivers the drug to the target site and its aftermath are shown in Fig. 6.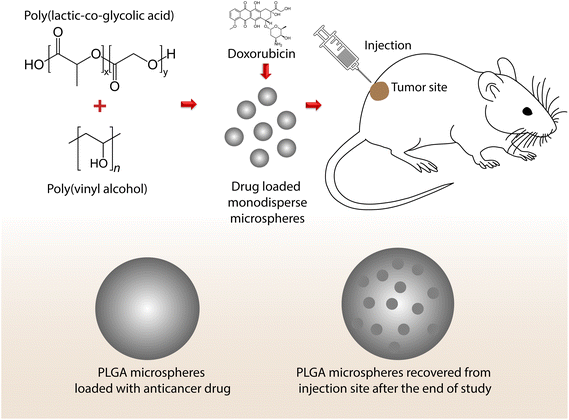 | ||
| Fig. 6 Drug-loaded PLGA microspheres showing changes in morphology after the delivery of a drug to a target site (i.e., from a smooth to a porous surface as proof of drug release). | ||
Biomolecules with a therapeutic nature are often entrapped within polymeric particles for sustained release. For example, intravenous administration of pitavastatin-loaded PLGA nanoparticles reduces the macrophage burden and matrix metallopeptidase 9 and chemokine ligand 2 levels in atherosclerotic plaques, decreasing plaque destabilization and increasing the fibrous cap area in the brachiocephalic artery in an apolipoprotein E-deficient mouse model.83 In addition, an extended-release profile for charged proteins without bursting were achieved using polymeric hydrogels through short-range electrostatic interactions.84 Many researchers affirmed that adding a minimal volume of nanoparticles to the polymer matrix could improve the physical and chemical properties of polymers. Hydroxyapatite (HAP), an inorganic component, is the principal constituent of human hard tissue and is used as a common biomaterial for bone tissue engineering and bone repair.85 HAP is osteoconductive in nature and can interact with tissue. The addition of tiny amounts of nano-HAP into the polymer matrix can drastically increase the elastic modulus of the polymer matrix. Porous HAP and collagen type I are found to be effective bone implant materials owing to their efficacy and safety.86 Porosity on the surface helps in targeted drug delivery and optimal biodistribution to facilitate particle permeation through epithelial tissues. Wei and collaborators demonstrated that poly(L-lactide) (PLLA), and HAP-based nanocomposite improved the protein adhesion property.87 Guoqiang Zhou and collaborators developed a one-step technique to achieve nanofibers made of HAP nanoparticles, PLLA and collagen. PLLA/collagen/HAP nanofiber scaffolds were found to enhance cell adhesion, proliferation, differentiation, and mineralization in mouse osteoblasts MC3T3-E1. They were also found to regulate the expression of various osteogenic markers.88 The incorporation of polymers and nanotube-based scaffolds has been studied for their combined benefits. Jell and co-workers stated that the thermally induced phase separation technique to prepare strong scaffolds from carbon nanotubes and polyurethane showed higher cell proliferation than pure polyurethane due to the modified surface structure. A PPF and single-wall carbon-nanotube-based nanocomposite showed that mesenchymal stem cells proliferated very nicely on the surface of scaffolds. The scaffolds reduced osteoblast cytotoxicity and increased the production of an angiogenic factor, the vascular endothelial growth factor.89 Sitharaman and collaborators have studied the biocompatibility of PPF-single-walled carbon-nanotube-based scaffolds in a rabbit model and showed significant bone growth. In addition, the surface chemistry of the developed scaffold provides excellent biocompatibility and bone cell growth.90 Control over channel, pore size, higher surface area and presence of active hydroxyl groups are some of the properties proving that mesoporous silica nanoparticles could be a promising drug delivery candidate. Owing to their very low toxicity, silica-based nanosystems have been preferred for human phase I clinical trials in recent times. To improve the burst release of drugs by mesoporous silica nanoparticles, several codelivery nanosystems have been studied. Recently, polyacrylic-acid-coated mesoporous silica nanoparticles along with a pH-sensitive lipid were used for the synergistic delivery and pH-responsive controlled release of arsenic trioxide and paclitaxel. Tumor-binding peptides were also tagged with the complex to achieve specific targeting as the complex showed both pH-responsive and sustained release of the drug.91 Minmin Chen and co-workers have developed an antibody-targeted and redox-responsive drug delivery system by conjugating anti-carbonic anhydrase iX antibody with mesoporous silica nanoparticles via disulfide bonding. The system effectively releases doxorubicin hydrochloric via redox-responsive reaction in the presence of glutathione by the cleavage of disulfide bonds.92 Likewise, a mesoporous silica-based biopolymer nanocomposite could be used as a drug delivery carrier for bone tissue engineering via interactions between silanol and functional groups of the drug.93 These studies indicate that a synergistic combination of chemical, physical, and biological properties of nanostructured biomaterials can be efficient for life science applications.
2.5 Fullerene nanoarchitectonics
Ever since its discovery, the fullerene molecule, a member of the carbon family, has been extensively studied for excellent photochemical and electrochemical properties.94,95 It is an exceptional zero-dimensional molecule that can be assembled into 1D, 2D, and 3D supramolecular structures via nanoarchitectonics. Biomedical applications of fullerene have gained interest, as a morphological control over C60 fullerene via nanoarchitectonics to achieve supramolecular assembly is underway.96 Functionalization of C60 fullerene with the addition of specific groups opens new avenues in life science applications. Fullerenes can act as an effective scavenger for ROS and Yen Wei and collaborators developed a carnosine-conjugated fullerene complex via self-assembly. The developed complex showed great biocompatibility, and excellent ROS scavenging capability against hydrogen-peroxide-induced damage to human fetal hepatocytic cells.97 To create aligned C60 fullerene nanostructures, our group used the Langmuir–Blodgett technique, resulting in a monolayer film with aligned nanostructures.98 In 2015, aligned 1D fullerene whiskers were used to induce myogenic differentiation from myoblast to myotube on C2C12 cells. Markers denoting myogenic differentiation of MyoD and Myogenin were checked.99 Various techniques have been adapted to increase the differentiation efficiency of neural stem cells. Our group has successfully developed C60 fullerene nanowhiskers for aligning neural stem cells to differentiate them into neurons.100 Likewise, continuous tunable alignment of fullerene nanowhiskers was achieved using the Langmuir–Blodgett technique to enhance multipotency and self-renewal of human mesenchymal stem cells. It was found that the nanopattern could control the self-renewal process of human mesenchymal stem cells by mechanotransduction101 (Fig. 7).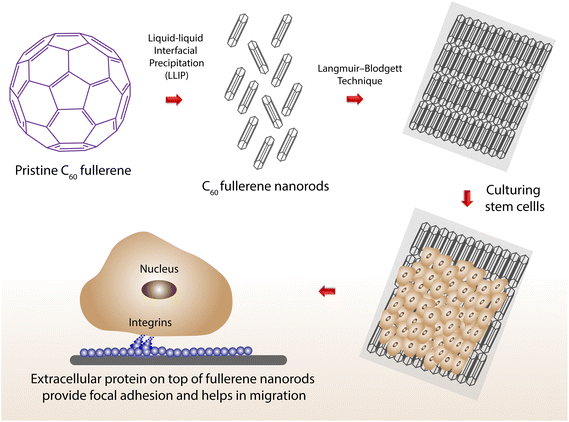 | ||
| Fig. 7 Stem cells grown on top of aligned C60 fullerene nanotubes (via the Langmuir–Blodgett technique) helps to achieve better migration of cells. | ||
The high aspect ratio of the fullerene nanowhiskers offered protein nanopatterns to allow the elongation of focal adhesion parallel to the long axes of these aligned structures.101 Maciej Serda and co-workers developed water-soluble glycofullerenes to inhibit non-receptor Src kinase inhibitors. They also showed that the decoration of protein corona on the surface of C60 fullerene was able to specifically target various kinase inhibitors.102 Water-soluble fullerene with an excellent fluorescent property in the near-infrared region has been synthesized for radiofrequency hyperthermia on breast adenocarcinoma cells, and non-tumor mammary fat pad cells.103 Matteo Di Giosia and collaborators have developed a C70-lysozyme conjugate to monitor its subcellular localization using two-photon excitation fluorescence microscopic and third-harmonic generation imaging techniques. They found that the conjugate induces singlet oxygen production upon irradiation with white light, thereby destroying the cells.104 Fullerenes are well known for their photodynamic and photoacoustic properties. Anna Grebinyk and co-workers have studied the efficacy of a doxorubicin-C60 fullerene conjugate on human leukemic lymphoblasts. The conjugate was delivered in ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for C60 fullerene as drug carrier and photosensitizer. Using fluorescence microscopy, doxorubicin and C60 fullerene localization were monitored where doxorubicin imparts its antiproliferative activity, and C60 fullerene imparts its pro-oxidant properties.105 Pei-Wen Luo and co-workers have studied the interaction of human fibroblasts transfected with highly expressible bacteriorhodopsin on the aligned C60 fullerenes. Upon expression, light helps in reprogramming them to neural cells without a neural induction medium.106 In another interesting study, a cationic aminated fullerene derivative was developed to specifically bind with the anionic myosin heavy chain 9 of cancer cells. After binding, the aminated fullerene complex initiates the transfer of cytoplasmic myosin heavy chain 9 to the cell edge, thereby inhibiting cancer cell migration and the epithelial-to-mesenchymal transition process.107 Kosuke Minami and co-workers have developed water-soluble cationic fullerenes, tetra-(piperazino)[60]fullerene epoxide-serum protein ternary complexes, for the effective delivery of highly unstable siRNA. The amphiphilic nature of the fullerene complex and its conjugation with protein forms a stable micelle that protects siRNA for safer delivery at the target site.108 Daisuke Iohara and collaborators have developed a highly stable hydrophilic C60 fullerene complex in combination with an anionic γ-cyclodextrin derivative, sugammadex. The complex was found to have excellent photodynamic activity, generating ROS in both in vitro and in vivo conditions.109 Likewise, Xiaochen Dong and co-workers have developed an NIR-light-harvesting fullerene-based nanosystem for photoacoustic imaging-guided photodynamic and photothermal therapy. To enhance the ROS production, fullerene molecules were combined with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine with conjugated methoxyl poly(ethylene glycol).110
1 for C60 fullerene as drug carrier and photosensitizer. Using fluorescence microscopy, doxorubicin and C60 fullerene localization were monitored where doxorubicin imparts its antiproliferative activity, and C60 fullerene imparts its pro-oxidant properties.105 Pei-Wen Luo and co-workers have studied the interaction of human fibroblasts transfected with highly expressible bacteriorhodopsin on the aligned C60 fullerenes. Upon expression, light helps in reprogramming them to neural cells without a neural induction medium.106 In another interesting study, a cationic aminated fullerene derivative was developed to specifically bind with the anionic myosin heavy chain 9 of cancer cells. After binding, the aminated fullerene complex initiates the transfer of cytoplasmic myosin heavy chain 9 to the cell edge, thereby inhibiting cancer cell migration and the epithelial-to-mesenchymal transition process.107 Kosuke Minami and co-workers have developed water-soluble cationic fullerenes, tetra-(piperazino)[60]fullerene epoxide-serum protein ternary complexes, for the effective delivery of highly unstable siRNA. The amphiphilic nature of the fullerene complex and its conjugation with protein forms a stable micelle that protects siRNA for safer delivery at the target site.108 Daisuke Iohara and collaborators have developed a highly stable hydrophilic C60 fullerene complex in combination with an anionic γ-cyclodextrin derivative, sugammadex. The complex was found to have excellent photodynamic activity, generating ROS in both in vitro and in vivo conditions.109 Likewise, Xiaochen Dong and co-workers have developed an NIR-light-harvesting fullerene-based nanosystem for photoacoustic imaging-guided photodynamic and photothermal therapy. To enhance the ROS production, fullerene molecules were combined with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine with conjugated methoxyl poly(ethylene glycol).110
2.6 Nanoarchitectonics of magnetic-based theranostic systems
Theranostics is a system that has developed at the juncture of therapeutics and diagnosis. In simple terms, theranostic nanoparticles facilitate simultaneous monitoring and treatment of diseases using a single platform.111 The performance of these nanoparticles stands on the joint ventures of passive and active targeting, controlled drug release, molecular imaging, and therapeutic functions. In addition, a classic theranostic nanoparticle must exhibit selective and rapid accumulation in the target of interest, exquisite delineation of biochemical and morphological characteristics of the disease, efficient drug delivery at the target site, rapid clearance, and excellent biocompatibility.112 Recently, magnetic-based theranostic nanoparticle (MBTN) systems containing magnetic nano-entities have gained much popularity in the diagnosis and treatment of various diseases, including cancers and cardiovascular diseases owing to their unique magnetic behavior. Moreover, due to their comparable sizes with various functional biomolecules, magnetic nanoparticles (MNPs) do not present any extravasations from normal vessels and accumulate at the pathological sites via the enhanced permeability and retention effect. The MNPs possess superparamagnetism, high field irreversibility, a high saturation field, and extra anisotropy contributions that can be further manipulated by an external magnetic field gradient and the ability to conjugate with many biological and drug entities, which lays a platform for multifunctional utility, such as contrast agents in magnetic resonance imaging,113 site-specific magnetic targeting,114 magnetic hyperthermia treatment,115 multimodal imaging,116 magnetic-field-dependent controlled drug delivery,117 magnetofection, and gene delivery.118 However, MNPs have hydrophobic surfaces, and due to hydrophobic interactions, these particles tend to agglomerate, resulting in poor performance due to the formation of larger clusters.119 Another common issue with such systems is the opsonization that renders the particles recognizable by the body's major defense system, the reticuloendothelial system.120 Therefore, the developed magnetic nanosystems need a surface coating to endow them with hydrophilic properties, ensure their stability in the reticuloendothelial system, non-toxicity, biocompatibility, and to prevent nanoparticle agglomeration. Several hydrophilic natural and synthetic polymers, such as dextran, chitosan, PEG, PLGA, poly(glyceryl monooleate), and PNIPAm have been successfully tried as surface-capping materials for MNPs.121–123,124 These coatings also facilitate the functionalization of MNPs with biomolecule ligands for active targeting of the diseased tissue and encapsulation of drugs for multifunctional performance. Dextran-coated MNPs have already been approved by FDA for the imaging of spleen, liver, and lymph nodes.125 A recent study from our lab investigated the effect of surface coatings on the proton relaxivity of MNPs and the results revealed that the MRI contrast enhancement abilities of MNPs strongly depend upon the type of surface-coating layers. In this study, a series of surface-capped gadolinium oxide nanoparticles were synthesized using polyols of different reductive abilities, such as diethylene, triethylene, tetraethylene, and PEG. The in vitro and ex vivo longitudinal (r1) and transverse (r2) proton relaxivities of synthesized nanoparticles showed a correlation with the chain length of polyols and increase with an increase in coating thickness i.e., with an increase in glycol chain length126 (Fig. 8).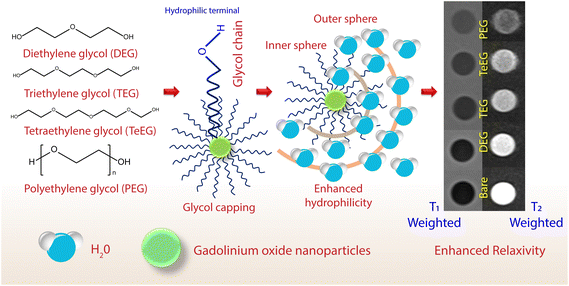 | ||
| Fig. 8 An illustration of how an enhancement in the surface hydrophilicity and an increase in the chain length of glycol on the gadolinium oxide nanoparticle surface can improve MR contrast. | ||
At present, Fe3O4-, Gd-, and Mn-based MNPs are the most commonly used imaging agents in MRI. Recently, superparamagnetic iron-oxide-based MBTNs, such as ferumoxides, and multifunctional manganese(II) oxide and PEG-functionalized gadolinium(III) oxide nanoparticles have been of keen interest for in vivo and in vitro imaging applications in MRI.127 Using mesoporous silica nanoparticles as templates, Wang and co-workers synthesized renal-clearable ultrasmall polyethylene-coated gadolinium nanoparticles to facilitate MR imaging in kidney disfunction. In addition, an in vivo MRI assisted by these nanoparticles was conducted on a mouse model with a damaged kidney to diagnose kidney dysfunction.123 Wei and collaborators constructed ultrasmall manganese oxide nanoparticles coated with zwitterionic dopamine sulfonate molecules for enhanced T1-weighted MRI contrast for the accurate diagnosis of brain diseases.128 Liang and co-workers developed a series of antiferromagnetic and paramagnetic nanoprobes to achieve ultra-high-field MRI performance abilities to detect small tumors and metastases.129 With advances in nanotechnology, MBTNs have now been tried as dual-mode imaging agents in clinical diagnosis. Smart nanotheranostics using iron oxide nanoparticles conjugated with a pH-low insertion peptide as a targeting unit to discern an acidic tumor microenvironment were used as a positron emission tomography PET/MRI dual-mode probe.73 In another study, a manganese(II) oxide/pyrargyrite based nanoplatform was designed to relieve tumor hypoxia and its diagnosis by MR and photoacoustic dual-mode imaging.130 Attempts have been made to incorporate fluorescent-active quantum dots and other fluorescents tags in MNPs to perform MRI-FI (fluorescence imaging) dual-mode imaging.131,132 Magneto-fluorescent nanoprobes produced by grafting magnetite nanorings with polyethyleneimine-capped quantum dots and gadolinium-based lipid-coated silica nanoparticles with a quantum dot core, respectively, serve as promising contrast agents for multimodal imaging.133,134 A current venture in this direction has also proposed the amalgamation of MNPs with rare earth metals, known for their optical properties, especially the heavier rare earth metals. Such MNP-based theranostic agents with high magnetization will demonstrate greater effectivity towards endowing highly accurate diagnostic information due to their increased relaxivity enabling local contrast enhancement, which will be many times higher than that provided by existing chelates.135 In addition, lead(II) selenide nanocrystals were anisotropically grown on gold–iron oxide hybrid nanoparticles by Shi and team for MRI and fluorescence-based labelling applications, along with magnetic-based targeting, delivery, and cell separation.133 Furthermore, to provide multimodal imaging capabilities with enhanced performance, contrast agents for PET have also been incorporated into MBTNs. Research has been carried out to integrate MNPs with radionuclide labels for single-photon emission computerized tomography to enhance the imaging sensitivity.136
MNPs also act as cargo carriers for chemotherapeutic drugs to enhance the drug specificity and reduce toxicity, owing to their multifunctional capabilities. Traditional chemotherapies are frequently reported to suffer various side effects due to the lack of specificity and systemic toxicities.137 The first clinical trial of magnetic drug targeting in humans was performed by Lübbe and co-workers using a ferrofluid with the drug epirubicin to treat solid tumors.138 Hence, MBTNs play a vital role in cancer management by delivering the drugs only to the target cells via magnetic targeting.137 Here, the MNPs are either attached with related receptors via antibodies to inhibit tumor growth or are loaded with the drugs for targeted therapy or through targeted hyperthermia.139 For example, Qian and co-workers conjugated near-infrared radiation-830 dye labelled insulin-like growth factor 1 with iron oxide NPs to carry doxorubicin into insulin-like growth factor 1 receptor-positive pancreatic cancer cells for targeted delivery.140 Sun and collaborators used methotrexate- and chlorotoxin-conjugated MNPs for targeted drug delivery to tumor cells. They observed extended particle retention and decreased survival rate of tumor cells by MR monitoring of nanoparticle accumulation at the target site.141 Manganese(II) oxide-disguised and tumor-microenvironment-triggered nanomedicine was constructed to achieve activable MI-guided diagnosis and a synergistic cascade chemo/chemo-dynamic cancer effect.142 In another study, temozolomide and lactoferrin-conjugated and dihydroxyhydrocinnamic-acid-coated cubic MNPs were prepared and utilized as an excellent actuator for drug delivery at the tumor site with enhanced penetration.142
Lately, magnetic-nanoparticle-induced hyperthermia treatment has become a topic of interest for many researchers due to their interesting behavior under external magnetic fields. Hyperthermia is a therapeutic modality to treat malignant tumors by the selective killing of tumor cells via heating.143 MNP-based hyperthermia works on the principles of heat generation through magnetic hysteresis loss, Néel relaxation, and Brown relaxation developed in magnetic nanoparticles exposed to an alternating current magnetic field.144 In addition, their nanoscale size aided by the guided magnetic field, gives them easy access to tumors and facilitates targeting of the specific tumor tissue when tailored with cancer-specific binding ligands. In hyperthermia treatment, MNPs are generally coated with specific stabilizers or encapsulated into delivery nanocarriers like liposomes for localized minimally invasive effects.145 There are many other examples where superparamagnetic iron oxide nanoparticles were conjugated with specific tumor cell targeting ligands to boost cellular internalization and safely increase the intercellular concentration of MNPs.146 Likewise, dextran-coated MNPs were conjugated with chimeric L6 monoclonal antibodies to target breast cancer cells.147 To date, various clinical reports are now available on MNP-mediated thermotherapies utilized to treat prostate carcinoma and other tumor entities. The studies have also revealed that at the high temperatures developed in hyperthermia, cancer cells become vulnerable, and hence an accelerated response is seen for chemotherapeutic drugs or radiation. Therefore, synergistic treatment using hyperthermia with other therapies has suggested better treatment efficacies. For example, a significant inhibitory effect was observed on tumors in mice bearing a xenograft of human hepatocarcinoma when treated with MNP-encapsulated As2O3 nanoparticles under simultaneous thermal and chemotherapy.148 Sun and co-workers developed surface-modified iron oxide nanoparticles loaded with Indocyanine green as a novel nanoprobe for targeted imaging and photodynamic/photothermal therapy for cancer treatment.149 Since MNPs are less toxic at the recommended doses, MNP-assisted transfection systems have been researched extensively in recent decades to combat internalization and intracellular trafficking problems in gene therapy to combat genetic disorders.150 It has outdone other nonviral transfection methods on the grounds of both transfection efficacy and cell viability, and the oscillating magnetic field promotes the MNP-mediated transfection. This method can also aid in delivering a therapeutic gene to malfunctioning human cells through magnetoreception.151 Preclinical experiences in MNP-mediated gene therapy have shown promising results. However, various technical, logistic, and conceptual hurdles still need to be overcome in further research.152
Therefore, the genesis of MBTNs can be traced to create an advanced medical tool to facilitate solo platforms for the precise and quick detection of diseases and treatments. The primary focus of developing MBTNs is to combine existing therapeutic and imaging agents to achieve multifunctionality. Although much work has been done on biomedical and biological applications of MBTNs, the clinical setting of these multifunctional MBTNs faces some real challenges concerning the design, safety, and biocompatibility of nanoparticles.153 The morphology, surface charge, and surface modifications of nanoparticles significantly affect cellular internalization, toxicity and in vivo clearance and hence require attention when designing MBTNs.154 For example, the effects of particle size and chemical composition are strongly reflected in the magnetic behavior of the nanoparticles.155 Therefore, MBTNs can be decorated with various suitable functionalities for active targeting and multi-action mechanisms for disease management. Furthermore, the surface modification of the nanoparticles plays a crucial role in the stability, cellular uptake, and clearance of MBTNs along with their magnetic response, owing to the altered surface charge and hence magnetic relaxivities.155 Therefore, advances in nanotechnology and the biomaterials field can provide techniques and tools to improve the performance of MBTNs for clinical use.
2.7 Metal–organic framework-based structures
Metal–organic frameworks (MOFs) are recently explored novel materials which contain metal ions and organic ligands with control over the function, porosity, and size of the assembly.156 Their programmability and hybrid organic–inorganic nature make them suitable candidate for biomedical applications. Delivering drugs to target site without affecting their function is difficult as they tend to lose their structure and function on their way due to several factors. MOFs can be used as an excellent cargo to deliver bioactive molecules and for the development of biodegradable polymers.157 Kang Liang and co-workers have developed a zeolitic imidazolate framework-8 for the encapsulation of nucleic acids, enzymes, and proteins.158 The developed system was exceptionally stable under harsh conditions and facilitated the release of biomolecules by a change in pH under physiological conditions. pH-Responsive chitosan immobilized MOFs were developed for the targeted delivery of doxorubicin. The MOF system was able to deliver doxorubicin at a slow and continuous pace in a tumor microenvironment (pH 6.8) compared to normal physiological conditions.159 MOF encapsulated with DNAzymes and metal ions plays a dual role in the in situ synthesis of cancer drugs and to perform gene therapy. In a tumor microenvironment, MOFs were expected to simultaneously release both ions and DNAzymes. Metal ions like copper catalyze the synthesis of chemotherapeutic drug through a copper-catalyzed azide–alkyne cycloaddition reaction. Likewise, zinc ions help in the activation of the cleavage activity of the DNAzyme, resulting in gene therapy160 (Fig. 9).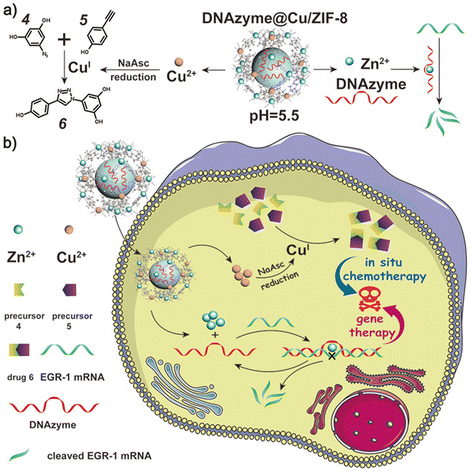 | ||
| Fig. 9 (a) The pH-responsive degradation behavior of DNAzyme@Cu/ZIF-8, the corresponding CuAAC reaction for in situ chemotherapy, and the mRNA cleavage reaction for gene therapy. (b) A smart DNAzyme@Cu/ZIF-8 nanoplatform for synergistic chemo-gene therapy. Reproduced from ref. 160 with permission from John Wiley and Sons, Copyright 2022. | ||
MOFs are highly pH sensitive with a high loading capacity and have an ability to escape lysosomes. Chunbai and collaborators developed a MOF-based platform for the co-delivery of cisplatin and siRNAs to attain both chemo and gene therapy. The developed system was effectively internalized into cancer cells and performed gene silencing on multiple drug-resistant genes.161
Combining MOFs with biomolecules ultimately reduces system toxicity and makes them more biocompatible. Polymers can be inserted into MOFs when they are heated to their melting temperature via nanochannels present on the surface of MOFs. Alternatively, nucleic acids, proteins and other biomolecules can be loaded and delivered for targeted drug delivery applications.156 To improve the mechanical strength and elasticity of hydrogels, crosslinking with inorganic structures, such as metal nanoparticles, MOFs and carbon nanostructures, is performed. Our group recently explored the possibility of incorporating zeolitic imidazolate framework-8 with a biodegradable polyurethane hydrogel to improve the printability of a bioink. The composites were treated with calcium chloride for reinforcement and the developed bioink showed great biocompatibility when cultured with human mesenchymal stem cells and helped in the proliferation of cells162 (Fig. 10).
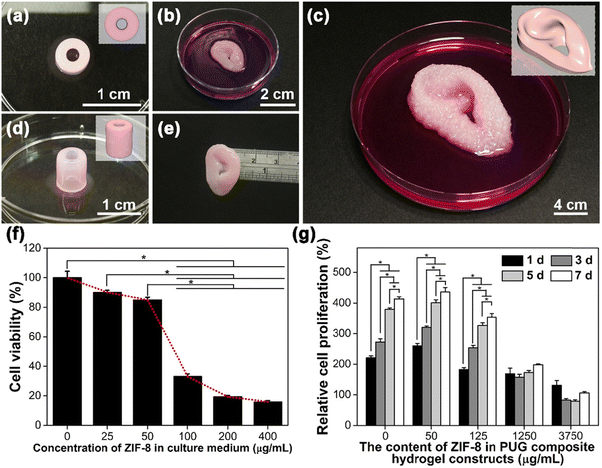 | ||
| Fig. 10 Printability, cell viability, and cell proliferation of MSC-laden PUG-ZIF-8 composite bioinks. (a and d) A blood-vessel-like construct printed with a PUG composite bioink containing 1250 μg mL−1 ZIF-8 crystals through a needle-like nozzle (inner diameter = 210 μm). (b, c and e) Small- and large-sized ear-shaped PUG composite hydrogel constructs containing 125 μg mL−1 ZIF-8 crystals printed through a 210 μm cone-like nozzle. (f) Viability of MSCs with a culture medium containing ZIF-8 at different concentrations after 24 h of direct incubation. The values are expressed as percentages relative to the blank control (cell culture medium) after 24 h. (g) The long-term proliferation of MSCs embedded in the composite hydrogel constructs for a period of 7 days. The values are expressed as percentages relative to the initial cell number (at day 0). Cell viability was evaluated via WST-8 analysis in all experiments. “*” represents p-values <0.05. Reproduced from ref. 162 with permission from American Chemical Society, Copyright 2022. | ||
Multifunctional amino-functionalized Materials of Institut Lavoisier-101 (Fe) were conjugated with camptothecine via noncovalent interaction. The assembly was further tagged with folic acid as the target and chlorine e6 labelled lysosomal cysteine endopeptidase substrate peptide as the signal and recognition moiety and photosensitizer. The developed MOF system could effectively perform cathepsin B-activated cancer cell imaging as well as chemo-photodynamic dual-therapy.163 Kyriakos C. Stylianou and co-workers developed a MOF containing unobstructed Watson–Crick faces of adenine in its cavity. The prepared MOFs enable the diffusion of thymine molecules which then pair with the adenine in the cavity, which acts as a nanoreactor for the dimerization of thymine via site-specific binding.164 These examples suggest that a MOF with its dynamic properties and enhanced entrapment efficiency may be suitable for strengthening biodegradable polymers, drug carriers and so on.
3. Conclusions and outlook
Based on prevailing sentiment, it is concluded that nanoarchitectonics can be of great use when creating nanoscale structures of particular interest from atoms or molecules via supramolecular chemistry, self-assembly, the chemical harmonization of molecules/atoms, nanomanipulation, etc. Some of the studies highlighted in this review showcase the role of nanoarchitectonics in developing a versatile tool to achieve various nanostructured biomaterials. The regulation of living cells via self-assembly on material platforms, such as fullerene, for control over biological processes shows excellent potential. Nucleic-acid-based nanostructures do not limit their role to drug-carrying but they also show promise in delivering advanced tools such as gene silencing via CRISPR technology. The bioimaging of cells and the self-assembly of complementary base pairs via lipid molecules can be performed via nanoarchitectonics involving DNA-based constructs. Likewise, the near-infrared radiation imaging of metal nanoparticles and ligand binding capacity help in attaching therapeutics/biomolecules to target tumor systems. Magnetic nanosystems enable the precise imaging of deeper tissues and serve as excellent MRI contrast and theranostic agents. Decorating primary and secondary antibodies onto nanoparticles result in immunomodulatory nanosystems for enhanced presentation. For drug-targeting applications, MOFs can hold a cargo under normal physiological conditions and release it in the tumor microenvironment. Thus, the creation of hierarchical structures, their use for biomedical applications, such as imaging, cancer studies, and drug delivery, and continuous effort will enhance the sophistication of life science studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
V. K. would like to thank the Takeda Science Foundation, Osaka, Japan for his research fellowship to work with the Supermolecules Group at National Institute for Materials Science (NIMS), Tsukuba, Ibaraki, Japan. V. K. also thanks the management of Sathyabama Institute of Science and Technology, Chennai for its staunch support in research activities. This study was partially supported by JSPS KAKENHI Grant Number JP20H00392, JP20H00316, JP21H04685, JP21F50068, and JP20K05590.References
- K. Ariga and R. Fakhrullin, RSC Adv., 2021, 11, 18898–18914 RSC.
- S. C. Baetke, T. Lammers and F. Kiessling, Br. J. Radiol., 2015, 88, 20150207 CrossRef CAS PubMed.
- K. Ariga, S. Watanabe, T. Mori and J. Takeya, NPG Asia Mater., 2018, 10, 90–106 CrossRef.
- K. Ariga, K. Kawakami, M. Ebara, Y. Kotsuchibashi, Q. Ji and J. P. Hill, New J. Chem., 2014, 38, 5149–5163 RSC.
- C. Zavaleta, D. Ho and E. J. Chung, SLAS Technol., 2018, 23, 281–293 CrossRef CAS PubMed.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- G. Bao, S. Mitragotri and S. Tong, Annu. Rev. Biomed. Eng., 2013, 15, 253–282 CrossRef CAS PubMed.
- Z. Wang, H. Zhao, Y. Zhang, A. Natalia, C. A. J. Ong, M. C. C. Teo, J. B. Y. So and H. Shao, Nat. Commun., 2021, 12, 1–12 CrossRef PubMed.
- K. Ariga, M. Nishikawa, T. Mori, J. Takeya, L. K. Shrestha and J. P. Hill, Sci. Technol. Adv. Mater., 2019, 20, 51–95 CrossRef CAS PubMed.
- K. Ariga, T. Mori and J. Li, Langmuir, 2019, 35, 3585–3599 CrossRef CAS PubMed.
- K. Ariga, ChemNanoMat, 2016, 2, 333–343 CrossRef CAS.
- M. Aono and K. Ariga, Adv. Mater., 2016, 28, 989–992 CrossRef CAS PubMed.
- M. Komiyama, K. Yoshimoto, M. Sisido and K. Ariga, Bull. Chem. Soc. Jpn., 2017, 90, 967–1004 CrossRef.
- K. Ariga, K.-C. Tsai, L. K. Shrestha and S. Hsu, Mater. Chem. Front., 2021, 5, 1018–1032 RSC.
- T. Muraoka, Bull. Chem. Soc. Jpn., 2020, 93, 138–153 CrossRef CAS.
- E. Piccinini, D. Pallarola, F. Battaglini and O. Azzaroni, Mol. Syst. Des. Eng., 2016, 1, 155–162 RSC.
- O. N. Oliveira, L. Caseli and K. Ariga, Chem. Rev., 2022, 122, 6459–6513 CrossRef PubMed.
- E. Piccinini, J. S. Tuninetti, J. I. Otamendi, S. E. Moya, M. Ceolín, F. Battaglini and O. Azzaroni, Phys. Chem. Chem. Phys., 2018, 20, 9298–9308 RSC.
- Y. Nakamura, A. Mochida, P. L. Choyke and H. Kobayashi, Bioconjugate Chem., 2016, 27, 2225–2238 CrossRef CAS PubMed.
- R. Bazak, M. Houri, S. El Achy, S. Kamel and T. Refaat, J. Cancer Res. Clin. Oncol., 2015, 141, 769–784 CrossRef CAS PubMed.
- K. Ariga, Nanoscale Horiz., 2021, 6, 364–378 RSC.
- N. E. Muzzio, M. A. Pasquale, W. A. Marmisollé, C. Von Bilderling, M. L. Cortez, L. I. Pietrasanta and O. Azzaroni, Biomater. Sci., 2018, 6, 2230–2247 RSC.
- K. Kikuchi, Bull. Chem. Soc. Jpn., 2015, 88, 518–521 CrossRef CAS.
- K. Ariga, X. Jia, J. Song, J. P. Hill, D. T. Leong, Y. Jia and J. Li, Angew. Chem., Int. Ed., 2020, 59, 15424–15446 CrossRef CAS PubMed.
- S. Carregal-Romero, E. Caballero-Díaz, L. Beqa, A. M. Abdelmonem, M. Ochs, D. Hühn, B. S. Suau, M. Valcarcel and W. J. Parak, Annu. Rev. Anal. Chem., 2013, 6, 53–81 CrossRef CAS PubMed.
- H. Mitomo and K. Ijiro, Bull. Chem. Soc. Jpn., 2021, 94, 1300–1310 CrossRef CAS.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- M. L. Brongersma, N. J. Halas and P. Nordlander, Nat. Nanotechnol., 2015, 10, 25–34 CrossRef CAS PubMed.
- M. T. Yaraki and Y. N. Tan, Chem. – Asian J., 2020, 15, 3180–3208 CrossRef CAS PubMed.
- J. Liu, F. Zhai, H. Zhou, W. Yang and S. Zhang, Adv. Healthcare Mater., 2019, 8, 1–11 Search PubMed.
- H. Xiang, L. Zhao, L. Yu, H. Chen, C. Wei, Y. Chen and Y. Zhao, Nat. Commun., 2021, 12, 218 CrossRef CAS PubMed.
- Q. Li, L. Liu, H. Huo, L. Su, Y. Wu, H. Lin, X. Ge, J. Mu, X. Zhang, L. Zheng and J. Song, ACS Nano, 2022, 16, 7947–7960 CrossRef CAS PubMed.
- A. D. Waldman, J. M. Fritz and M. J. Lenardo, Nat. Rev. Immunol., 2020, 20, 651–668 CrossRef CAS PubMed.
- C.-T. Jiang, K.-G. Chen, A. Liu, H. Huang, Y.-N. Fan, D.-K. Zhao, Q.-N. Ye, H.-B. Zhang, C.-F. Xu, S. Shen, M.-H. Xiong, J.-Z. Du, X.-Z. Yang and J. Wang, Nat. Commun., 2021, 12, 1359 CrossRef CAS PubMed.
- Z. Edis, J. Wang, M. K. Waqas, M. Ijaz and M. Ijaz, Int. J. Nanomed., 2021, 16, 1313–1330 CrossRef PubMed.
- K. Minami, J. Song, L. K. Shrestha and K. Ariga, Appl. Mater. Today, 2021, 23, 100989 CrossRef.
- X. Wu, T. Ming, X. Wang, P. Wang, J. Wang and J. Chen, ACS Nano, 2010, 4, 113–120 CrossRef CAS PubMed.
- L. Zhang, X. Chen, J. Wu, S. Ding, X. Wang, Q. Lei and W. Fang, RSC Adv., 2018, 8, 4130–4141 RSC.
- S. Eom, G. Choi, H. Nakamura and J.-H. Choy, Bull. Chem. Soc. Jpn., 2020, 93, 1–12 CrossRef CAS.
- Y. Liu, E. Naumenko, F. Akhatova, Q. Zou, R. Fakhrullin and X. Yan, Chem. Eng. J., 2021, 424, 130348 CrossRef CAS.
- S. Li, Q. Zou, R. Xing, T. Govindaraju, R. Fakhrullin and X. Yan, Theranostics, 2019, 9, 3249–3261 CrossRef CAS PubMed.
- C. Arib, A. Griveau, J. Eyer and J. Spadavecchia, Nanoscale Adv., 2022, 20–24 Search PubMed.
- C. Chen, J. Wang, D. Lu, R. You, Q. She, J. Chen, S. Feng and Y. Lu, Nanoscale, 2022, 14, 8103–8111 RSC.
- R. Wang, Y. Huang and Y. Chi, Analyst, 2022, 147, 3096–3100 RSC.
- X. Liang, L. Li, J. Tang, M. Komiyama and K. Ariga, Bull. Chem. Soc. Jpn., 2020, 93, 581–603 CrossRef CAS.
- N. C. Seeman, J. Theor. Biol., 1982, 99, 237–247 CrossRef CAS PubMed.
- R. P. Goodman, I. A. T. Schaap, C. F. Tardin, C. M. Erben, R. M. Berry, C. F. Schmidt and A. J. Turberfield, Science, 2005, 310, 1661–1665 CrossRef CAS PubMed.
- J. Chen and N. C. Seeman, Nature, 1991, 350, 631–633 CrossRef CAS PubMed.
- Y. He, T. Ye, M. Su, C. Zhang, A. E. Ribbe, W. Jiang and C. Mao, Nature, 2008, 452, 198–201 CrossRef CAS PubMed.
- K. Jahnke, H. Grubmüller, M. Igaev and K. Göpfrich, Nucleic Acids Res., 2021, 49, 4186–4195 CrossRef CAS PubMed.
- X. Liang, H. Chen, L. Li, R. An and M. Komiyama, Bull. Chem. Soc. Jpn., 2021, 94, 141–157 CrossRef CAS.
- X. Liang, M. Liu and M. Komiyama, Bull. Chem. Soc. Jpn., 2021, 94, 1737–1756 CrossRef CAS.
- B. L. Li, R. Li, H. L. Zou, K. Ariga, N. B. Li and D. T. Leong, Mater. Horiz., 2020, 7, 455–469 RSC.
- X. Peng, S. Fang, B. Ji, M. Li, J. Song, L. Qiu and W. Tan, Nano Lett., 2021, 21, 6946–6951 CrossRef CAS PubMed.
- M. H. Hanewich-Hollatz, Z. Chen, L. M. Hochrein, J. Huang and N. A. Pierce, ACS Cent. Sci., 2019, 5, 1241–1249 CrossRef CAS PubMed.
- B. M. Mognetti, P. Cicuta and L. Di Michele, Rep. Prog. Phys., 2019, 82, 116601 CrossRef CAS PubMed.
- A. Podder, H. J. Lee and B. H. Kim, Bull. Chem. Soc. Jpn., 2021, 94, 1010–1035 CrossRef CAS.
- Y. Sato, Bull. Chem. Soc. Jpn., 2020, 93, 406–413 CrossRef CAS.
- K.-W. Huang, F.-F. Hsu, J. T. Qiu, G.-J. Chern, Y.-A. Lee, C.-C. Chang, Y.-T. Huang, Y.-C. Sung, C.-C. Chiang, R.-L. Huang, C.-C. Lin, T. K. Dinh, H.-C. Huang, Y.-C. Shih, D. Alson, C.-Y. Lin, Y.-C. Lin, P.-C. Chang, S.-Y. Lin and Y. Chen, Sci. Adv., 2020, 6, eaax5032 CrossRef CAS PubMed.
- K. Zhang, Y. Ma, D. Wang, J. Liu, J. An, Y. Li, C. Ma, Y. Pei, Z. Zhang, J. Liu and J. Shi, Nano Lett., 2022, 22, 1937–1945 CrossRef PubMed.
- E. H. Kim, S. Park, Y. K. Kim, M. Moon, J. Park, K. J. Lee, S. Lee and Y.-P. Kim, Sci. Adv., 2020, 6, 1–14 Search PubMed.
- J. Tan, H. Li, X. Hu, R. Abdullah, S. Xie, L. Zhang, M. Zhao, Q. Luo, Y. Li, Z. Sun, Q. Yuan and W. Tan, Chem, 2019, 5, 1775–1792 CAS.
- J. Tan, H. Li, C. Ji, L. Zhang, C. Zhao, L. Tang, C. Zhang, Z. Sun, W. Tan and Q. Yuan, Nat. Commun., 2022, 13, 594 CrossRef CAS PubMed.
- D. A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J., 1985, 17, 117–132 CrossRef CAS.
- T. Kambe and K. Yamamoto, Polym. J., 2022, 54, 97–105 CrossRef CAS.
- A. Sharma, R. Sharma, Z. Zhang, K. Liaw, S. P. Kambhampati, J. E. Porterfield, K. C. Lin, L. B. DeRidder, S. Kannan and R. M. Kannan, Sci. Adv., 2020, 6, 1–15 Search PubMed.
- Z. Lyu, L. Ding, A. Tintaru and L. Peng, Acc. Chem. Res., 2020, 53, 2936–2949 CrossRef CAS PubMed.
- P. Garrigue, J. Tang, L. Ding, A. Bouhlel, A. Tintaru, E. Laurini, Y. Huang, Z. Lyu, M. Zhang, S. Fernandez, L. Balasse, W. Lan, E. Mas, D. Marson, Y. Weng, X. Liu, S. Giorgio, J. Iovanna, S. Pricl, B. Guillet and L. Peng, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11454–11459 CrossRef CAS PubMed.
- Y. Dong, T. Yu, L. Ding, E. Laurini, Y. Huang, M. Zhang, Y. Weng, S. Lin, P. Chen, D. Marson, Y. Jiang, S. Giorgio, S. Pricl, X. Liu, P. Rocchi and L. Peng, J. Am. Chem. Soc., 2018, 140, 16264–16274 CrossRef CAS PubMed.
- C. Chen, P. Posocco, X. Liu, Q. Cheng, E. Laurini, J. Zhou, C. Liu, Y. Wang, J. Tang, V. D. Col, T. Yu, S. Giorgio, M. Fermeglia, F. Qu, Z. Liang, J. J. Rossi, M. Liu, P. Rocchi, S. Pricl and L. Peng, Small, 2016, 12, 3667–3676 CrossRef CAS PubMed.
- A. F. Smeijers, K. Pieterse, P. A. J. Hilbers and A. J. Markvoort, Macromolecules, 2019, 52, 2778–2788 CrossRef CAS PubMed.
- S. S. Yadavalli, Q. Xiao, S. E. Sherman, W. D. Hasley, M. L. Klein, M. Goulian and V. Percec, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 744–752 CrossRef CAS PubMed.
- A. K. Singh, A. Gothwal, S. Rani, M. Rana, A. K. Sharma, A. K. Yadav and U. Gupta, ACS Omega, 2019, 4, 4519–4529 CrossRef CAS.
- Y. Wang, N. Gong, C. Ma, Y. Zhang, H. Tan, G. Qing, J. Zhang, Y. Wang, J. Wang, S. Chen, X. Li, Q. Ni, Y. Yuan, Y. Gan, J. Chen, F. Li, J. Zhang, C. Ou, Y. Zhao, X. Liu and X.-J. Liang, Nat. Commun., 2021, 12, 4964 CrossRef CAS PubMed.
- J. Premkumar, K. SonicaSree and T. Sudhakar, Handbook of Polymer and Ceramic Nanotechnology, Springer International Publishing, Cham, 2021, pp. 1–28 Search PubMed.
- E. M. Elmowafy, M. Tiboni and M. E. Soliman, J. Pharm. Invest., 2019, 49, 347–380 CrossRef CAS.
- S. Dhiman and S. J. George, Bull. Chem. Soc. Jpn., 2018, 91, 687–699 CrossRef CAS.
- B. Liang, Q. Shi, J.-G. Xu, Y.-M. Chai and J.-G. Xu, Front. Chem., 2020, 8, 603577 CrossRef CAS PubMed.
- S. Bashir, M. Hina, J. Iqbal, A. H. Rajpar, M. A. Mujtaba, N. A. Alghamdi, S. Wageh, K. Ramesh and S. Ramesh, Polymers, 2020, 12, 2702 CrossRef CAS PubMed.
- K. Nagase, M. Yamato, H. Kanazawa and T. Okano, Biomaterials, 2018, 153, 27–48 CrossRef CAS PubMed.
- E. C. Dreaden, S. W. Morton, K. E. Shopsowitz, J.-H. Choi, Z. J. Deng, N.-J. Cho and P. T. Hammond, ACS Nano, 2014, 8, 8374–8382 CrossRef CAS PubMed.
- V. Karthick, S. Panda, V. G. Kumar, D. Kumar, L. K. Shrestha, K. Ariga, K. Vasanth, S. Chinnathambi, T. S. Dhas and K. S. U. Suganya, Appl. Surf. Sci., 2019, 487, 211–217 CrossRef CAS.
- S. Katsuki, T. Matoba, S. Nakashiro, K. Sato, J. I. Koga, K. Nakano, Y. Nakano, S. Egusa, K. Sunagawa and K. Egashira, Circulation, 2014, 129, 896–906 CrossRef CAS PubMed.
- M. M. Pakulska, I. E. Donaghue, J. M. Obermeyer, A. Tuladhar, C. K. McLaughlin, T. N. Shendruk and M. S. Shoichet, Sci. Adv., 2016, 2, e1600519 CrossRef PubMed.
- J. G. Lyons, M. A. Plantz, W. K. Hsu, E. L. Hsu and S. Minardi, Front. Bioeng. Biotechnol., 2020, 8, 922 CrossRef PubMed.
- S. Sotome, K. Ae, A. Okawa, M. Ishizuki, H. Morioka, S. Matsumoto, T. Nakamura, S. Abe, Y. Beppu and K. Shinomiya, J. Orthop. Sci., 2016, 21, 373–380 CrossRef PubMed.
- S. Wei, J.-X. Ma, L. Xu, X.-S. Gu and X.-L. Ma, Mil. Med. Res., 2020, 7, 54 CAS.
- G. Zhou, S. Liu, Y. Ma, W. Xu, W. Meng, X. Lin, W. Wang, S. Wang and J. Zhang, Int. J. Nanomed., 2017, 12, 7577–7588 CrossRef CAS PubMed.
- G. Jell, R. Verdejo, L. Safinia, M. S. P. Shaffer, M. M. Stevens and A. Bismarck, J. Mater. Chem., 2008, 18, 1865–1872 RSC.
- B. Sitharaman, X. Shi, X. F. Walboomers, H. Liao, V. Cuijpers, L. J. Wilson, A. G. Mikos and J. A. Jansen, Bone, 2008, 43, 362–370 CrossRef CAS PubMed.
- B.-B. Zhang, X.-J. Chen, X.-D. Fan, J.-J. Zhu, Y.-H. Wei, H.-S. Zheng, H.-Y. Zheng, B.-H. Wang, J.-G. Piao and F.-Z. Li, Acta Pharmacol. Sin., 2021, 42, 832–842 CrossRef CAS PubMed.
- M. Chen, J. Hu, L. Wang, Y. Li, C. Zhu, C. Chen, M. Shi, Z. Ju, X. Cao and Z. Zhang, Sci. Rep., 2020, 10, 1–12 CrossRef PubMed.
- M. Vallet-Regí, Chem. – Eur. J., 2006, 12, 5934–5943 CrossRef PubMed.
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- J. Song, T. Murata, K. Tsai, X. Jia, F. Sciortino, R. Ma, Y. Yamauchi, J. P. Hill, L. K. Shrestha and K. Ariga, Adv. Mater. Interfaces, 2022, 9, 2102241 CrossRef CAS.
- K. Ariga and L. K. Shrestha, Materials, 2020, 13, 2280 CrossRef CAS PubMed.
- H. Ma, J. Zhao, H. Meng, D. Hu, Y. Zhou, X. Zhang, C. Wang, J. Li, J. Yuan and Y. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 16104–16113 CrossRef CAS PubMed.
- K. Ariga, Acc. Mater. Res., 2022, 3, 404–410 CrossRef CAS.
- K. Minami, Y. Kasuya, T. Yamazaki, Q. Ji, W. Nakanishi, J. P. Hill, H. Sakai and K. Ariga, Adv. Mater., 2015, 27, 4020–4026 CrossRef CAS PubMed.
- F.-Y. Hsieh, L. K. Shrestha, K. Ariga and S. Hsu, Chem. Commun., 2017, 53, 11024–11027 RSC.
- J. Song, X. Jia, K. Minami, J. P. Hill, J. Nakanishi, L. K. Shrestha and K. Ariga, ACS Appl. Nano Mater., 2020, 3, 6497–6506 CrossRef CAS.
- M. Serda, K. Malarz, A. Mrozek-Wilczkiewicz, M. Wojtyniak, R. Musioł and S. A. Curley, Sci. Rep., 2020, 10, 260 CrossRef CAS PubMed.
- N. A. Lapin, M. Krzykawska-Serda, S. Dilliard, Y. Mackeyev, M. Serda, L. J. Wilson, S. A. Curley and S. J. Corr, J. Controlled Release, 2017, 260, 92–99 CrossRef CAS PubMed.
- M. Di Giosia, A. Soldà, M. Seeger, A. Cantelli, F. Arnesano, M. I. Nardella, V. Mangini, F. Valle, M. Montalti, F. Zerbetto, S. Rapino, M. Calvaresi and V. Ntziachristos, Adv. Funct. Mater., 2021, 31, 2101527 CrossRef CAS.
- A. Grebinyk, S. Prylutska, O. Chepurna, S. Grebinyk, Y. Prylutskyy, U. Ritter, T. Y. Ohulchanskyy, O. Matyshevska, T. Dandekar and M. Frohme, Nanomaterials, 2019, 9, 1540 CrossRef CAS PubMed.
- P.-W. Luo, H.-W. Han, C.-S. Yang, L. K. Shrestha, K. Ariga and S. Hsu, Adv. Biosyst., 2019, 3, 1800254 CrossRef PubMed.
- W. Zhou, J. Huo, Y. Yang, X. Zhang, S. Li, C. Zhao, H. Ma, Y. Liu, J. Liu, J. Li, M. Zhen, J. Li, X. Fang and C. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 56862–56873 CrossRef CAS PubMed.
- K. Minami, K. Okamoto, K. Harano, E. Noiri and E. Nakamura, ACS Appl. Mater. Interfaces, 2018, 10, 19347–19354 CrossRef CAS PubMed.
- D. Iohara, F. Hirayama, M. Anraku and K. Uekama, ACS Appl. Nano Mater., 2019, 2, 716–725 CrossRef CAS.
- H. Shi, R. Gu, W. Xu, H. Huang, L. Xue, W. Wang, Y. Zhang, W. Si and X. Dong, ACS Appl. Mater. Interfaces, 2019, 11, 44970–44977 CrossRef CAS PubMed.
- B. Sumer and J. Gao, Nanomedicine, 2008, 3, 137–140 CrossRef PubMed.
- V. Karthick and K. Ariga, Concepts and Design of Materials Nanoarchitectonics, 2022, pp. 135–151 Search PubMed.
- M. A. Busquets, J. Estelrich and M. J. Sánchez-Martín, Int. J. Nanomed., 2015, 10, 1727 CrossRef PubMed.
- M. Mohseni, J. J. Connell, C. Payne, P. S. Patrick, R. Baker, Y. Yu, B. Siow, M. Zaw-Thin, T. L. Kalber, Q. A. Pankhurst and M. F. Lythgoe, Mater. Des., 2020, 191, 108610 CrossRef CAS.
- D. Chang, M. Lim, J. A. C. M. Goos, R. Qiao, Y. Y. Ng, F. M. Mansfeld, M. Jackson, T. P. Davis and M. Kavallaris, Front. Pharmacol., 2018, 9, 831 CrossRef PubMed.
- X. Wang, H. Hu, H. Zhang, C. Li, B. An and J. Dai, Nano Res., 2018, 11, 1069–1081 CrossRef CAS.
- J. F. Liu, B. Jang, D. Issadore and A. Tsourkas, WIREs Nanomed. Nanobiotechnol., 2019, 11, e1571 Search PubMed.
- G. N. Pandian and H. Sugiyama, Bull. Chem. Soc. Jpn., 2016, 89, 843–868 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- D. Chenthamara, S. Subramaniam, S. G. Ramakrishnan, S. Krishnaswamy, M. M. Essa, F.-H. Lin and M. W. Qoronfleh, Biomater. Res., 2019, 23, 20 CrossRef CAS PubMed.
- A. Guleria, P. Pranjali, M. K. Meher, A. Chaturvedi, S. Chakraborti, R. Raj, K. M. Poluri and D. Kumar, J. Phys. Chem. C, 2019, 123, 18061–18070 CrossRef CAS.
- Y. Li, L. Zhang, Y. Shi, J. Huang, Y. Yang and D. Ming, Polymers, 2020, 12, 2565 CrossRef CAS PubMed.
- A. Gagliardi, D. Cosco, B. P. Udongo, L. Dini, G. Viglietto and D. Paolino, Pharmaceutics, 2020, 12, 1017 CrossRef CAS PubMed.
- E. Swider, O. Koshkina, J. Tel, L. J. Cruz, I. J. M. de Vries and M. Srinivas, Acta Biomater., 2018, 73, 38–51 CrossRef CAS PubMed.
- P. C. Naha, Y. Liu, G. Hwang, Y. Huang, S. Gubara, V. Jonnakuti, A. Simon-Soro, D. Kim, L. Gao, H. Koo and D. P. Cormode, ACS Nano, 2019, 13, 4960–4971 CrossRef CAS PubMed.
- A. Chaturvedi, P. Pranjali, M. K. Meher, R. Raj, M. Basak, R. K. Singh, K. M. Poluri, D. Kumar and A. Guleria, J. Appl. Phys., 2020, 128, 034903 CrossRef CAS.
- A. Dougherty, C. Harper, F. Iskandar, I. Arif and G. Dougherty, J. Sci.: Adv. Mater. Devices, 2018, 3, 419–427 Search PubMed.
- R. Wei, K. Liu, K. Zhang, Y. Fan, H. Lin and J. Gao, ACS Appl. Mater. Interfaces, 2022, 14, 3784–3791 CrossRef CAS PubMed.
- Z. Liang, Q. Wang, H. Liao, M. Zhao, J. Lee, C. Yang, F. Li and D. Ling, Nat. Commun., 2021, 12, 1–11 CrossRef PubMed.
- Q. Wang, B. Qu, J. Li, Y. Liu, J. Dong, X. Peng and R. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 4980–4994 CrossRef CAS PubMed.
- H. Arora, M. Ramesh, K. Rajasekhar and T. Govindaraju, Bull. Chem. Soc. Jpn., 2020, 93, 507–546 CrossRef CAS.
- H.-V. Tran, N. M. Ngo, R. Medhi, P. Srinoi, T. Liu, S. Rittikulsittichai and T. R. Lee, Materials, 2022, 15, 503 CrossRef CAS PubMed.
- H.-M. Fan, M. Olivo, B. Shuter, J.-B. Yi, R. Bhuvaneswari, H.-R. Tan, G.-C. Xing, C.-T. Ng, L. Liu, S. S. Lucky, B.-H. Bay and J. Ding, J. Am. Chem. Soc., 2010, 132, 14803–14811 CrossRef CAS PubMed.
- R. Koole, M. M. van Schooneveld, J. Hilhorst, K. Castermans, D. P. Cormode, G. J. Strijkers, C. de Mello Donegá, D. Vanmaekelbergh, A. W. Griffioen, K. Nicolay, Z. A. Fayad, A. Meijerink and W. J. M. Mulder, Bioconjugate Chem., 2008, 19, 2471–2479 CrossRef CAS PubMed.
- P. Farinha, J. M. P. Coelho, C. P. Reis and M. M. Gaspar, Nanomaterials, 2021, 11, 3432 CrossRef CAS PubMed.
- J. Cheon and J. H. Lee, Acc. Chem. Res., 2008, 41, 1630–1640 CrossRef CAS PubMed.
- S. Eom, G. Choi, H. Nakamura and J.-H. Choy, Bull. Chem. Soc. Jpn., 2020, 93, 1–12 CrossRef CAS.
- A. S. Lübbe, C. Bergemann, W. Huhnt, T. Fricke, H. Riess, J. W. Brock and D. Huhn, Cancer Res., 1996, 56, 4694–4701 Search PubMed.
- Y. Yao, Y. Zhou, L. Liu, Y. Xu, Q. Chen, Y. Wang, S. Wu, Y. Deng, J. Zhang and A. Shao, Front. Mol. Biosci., 2020, 7, 193 CrossRef CAS PubMed.
- H. Zhou, W. Qian, F. M. Uckun, L. Wang, Y. A. Wang, H. Chen, D. Kooby, Q. Yu, M. Lipowska, C. A. Staley, H. Mao and L. Yang, ACS Nano, 2015, 9, 7976–7991 CrossRef CAS PubMed.
- N. Kohler, C. Sun, A. Fichtenholtz, J. Gunn, C. Fang and M. Zhang, Small, 2006, 2, 785–792 CrossRef CAS PubMed.
- B. Chen, S. Hatamie, H. Chiu, Z. Wei, S. Hu and D. Yao, Adv. Mater. Interfaces, 2022, 10, 2200022 CrossRef.
- L. Beola, V. Grazú, Y. Fernández-Afonso, R. M. Fratila, M. de las Heras, J. M. de la Fuente, L. Gutiérrez and L. Asín, ACS Appl. Mater. Interfaces, 2021, 13, 12982–12996 CrossRef CAS PubMed.
- I. M. Obaidat, V. Narayanaswamy, S. Alaabed, S. Sambasivam and C. V. V. Muralee Gopi, Magnetochemistry, 2019, 5, 67 CrossRef.
- K. M. Aguilar-Pérez, J. I. Avilés-Castrillo, D. I. Medina, R. Parra-Saldivar and H. M. N. Iqbal, Front. Bioeng. Biotechnol., 2020, 8, 1441 Search PubMed.
- P.-E. Le Renard, O. Jordan, A. Faes, A. Petri-Fink, H. Hofmann, D. Rüfenacht, F. Bosman, F. Buchegger and E. Doelker, Biomaterials, 2010, 31, 691–705 CrossRef CAS PubMed.
- S. J. DeNardo, G. L. DeNardo, L. A. Miers, A. Natarajan, A. R. Foreman, C. Gruettner, G. N. Adamson and R. Ivkov, Clin. Cancer Res., 2005, 11, 7087s–7092s CrossRef CAS PubMed.
- Z. Y. Wang, J. Song and D. S. Zhang, World J. Gastroenterol., 2009, 15, 2995–3002 CrossRef CAS PubMed.
- X. Sun, Y. Xu, Q. Guo, N. Wang, B. Wu, C. Zhu, W. Zhao, W. Qiang and M. Zheng, Langmuir, 2022, 38, 1360–1367 CrossRef CAS PubMed.
- M. Gogoi, Curr. Pathobiol. Rep., 2021, 9, 71–78 CrossRef.
- J. Dobson, Gene Ther., 2006, 13, 283–287 CrossRef CAS PubMed.
- S.-D. Li and L. Huang, Gene Ther., 2006, 13, 1313–1319 CrossRef CAS PubMed.
- P. M. Price, W. E. Mahmoud, A. A. Al-Ghamdi and L. M. Bronstein, Front. Chem., 2018, 6, 619 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 1565–1573 CrossRef CAS PubMed.
- S. Natarajan, K. Harini, G. P. Gajula, B. Sarmento, M. T. Neves-Petersen and V. Thiagarajan, BMC Mater., 2019, 1, 2 CrossRef.
- N. Hosono and T. Uemura, Bull. Chem. Soc. Jpn., 2021, 94, 2139–2148 CrossRef CAS.
- K. Ariga and R. Fakhrullin, Bull. Chem. Soc. Jpn., 2022, 95, 774–795 CrossRef CAS.
- K. Liang, R. Ricco, C. M. Doherty, M. J. Styles, S. Bell, N. Kirby, S. Mudie, D. Haylock, A. J. Hill, C. J. Doonan and P. Falcaro, Nat. Commun., 2015, 6, 4–11 Search PubMed.
- R. Abazari, A. R. Mahjoub, F. Ataei, A. Morsali, C. L. Carpenter-Warren, K. Mehdizadeh and A. M. Z. Slawin, Inorg. Chem., 2018, 57, 13364–13379 CrossRef CAS PubMed.
- Z. Wang, J. Niu, C. Zhao, X. Wang, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2021, 60, 12431–12437 CrossRef CAS PubMed.
- C. He, K. Lu, D. Liu and W. Lin, J. Am. Chem. Soc., 2014, 136, 5181–5184 CrossRef CAS PubMed.
- C.-T. Hsieh, K. Ariga, L. K. Shrestha and S. Hsu, Biomacromolecules, 2021, 22, 1053–1064 CrossRef CAS PubMed.
- J. Liu, L. Zhang, J. Lei, H. Shen and H. Ju, ACS Appl. Mater. Interfaces, 2017, 9, 2150–2158 CrossRef CAS PubMed.
- S. L. Anderson, P. G. Boyd, A. Gładysiak, T. N. Nguyen, R. G. Palgrave, D. Kubicki, L. Emsley, D. Bradshaw, M. J. Rosseinsky, B. Smit and K. C. Stylianou, Nat. Commun., 2019, 10, 1612 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |