Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An ab initio investigation of the temperature-dependent energetic barriers towards CrAlB and (Mo,Cr)AlB formation in a metastable synthesis scenario
Dimitri
Bogdanovski
 *a,
Peter J.
Pöllmann
*a,
Peter J.
Pöllmann
 a and
Jochen M.
Schneider
a and
Jochen M.
Schneider
 *ab
*ab
aMaterials Chemistry, RWTH Aachen University, Kopernikusstraße 10, 52074 Aachen, Germany. E-mail: bogdanovski@mch.rwth-aachen.de; schneider@mch.rwth-aachen.de
bMax-Planck-Institut für Eisenforschung GmbH, Max-Planck-Str. 1, 40237 Düsseldorf, Germany
First published on 30th August 2022
Abstract
The orthorhombic CrAlB MAB phase has not been synthesized so far and was shown to be energetically unstable vs. the competing Cr2AlB2 phase in previous theoretical reports, which, however, did not explicitly investigate the magnitude of the energetic barrier towards CrAlB formation as a function of temperature. Temperature-dependent Gibbs energies of formation, obtained from density-functional-theory-based lattice dynamics simulations performed in this study, reveal that this barrier is very small (around 10 kJ mol−1 ≈ 0.008 eV per atom, on average) and may readily be overcome during high-energy synthesis scenarios, likely resulting in metastable phase formation. Furthermore, the electronic structures of MoAlB, a phase synthesized experimentally both in bulk and thin film form, and CrAlB are shown to be similar in direct comparison, with MoAlB exhibiting a higher electronic stability due to a local DOS minimum in proximity to the Fermi level, and quaternary compositions lying between the ternaries. Likewise, bonding characteristics are qualitatively very similar between both phases, with the transition metal–boron bonds being the dominant interaction in the entire unit cell, even though individual B–B bonds are stronger; quantitatively, all interactions are again stronger in MoAlB compared to CrAlB. It is reasonable to assume that, considering the successful synthesis of phase-pure MoAlB and known formation of metastable phases during physical vapor deposition, direct synthesis of metastable CrAlB thin films is possible due to the aforementioned small energy barrier. Furthermore, stability is enhanced upon alloying with Mo as this lowers the energy of formation, with a Mo/Cr ratio of approx. 0.33 sufficient to stabilize the Cr-rich (Mo,Cr)AlB solid solution vs. the primary competing phases, allowing for deposition of Mo-concentration-dependent stable and metastable (Mo,Cr)AlB solid solution phases.
Introduction
In recent years, layered ternary transition metal borides, commonly labelled MAB phases in analogy to the structurally similar MAX phases, have become a topic of intense interest, previously having been largely unexplored. In various investigations, properties such as low electrical resistivity (and corresponding good conductivity), especially for the best-studied MAB phase, MoAlB,1,2 acceptable electrocatalytic performance with simultaneous low toxicity for MoAlB and Cr2AlB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and high strength and fracture toughness values for several compositions1,2 have been reported. Another potentially highly application-relevant trait is the oxidation resistance of MoAlB in particular, which remains stable at temperatures up to 1600 °C due to formation of protective Al2O3 scales.2,4–6 However, only a few MAB phases are well-characterized, or even reported, so far, with data on other compositions frequently incomplete or entirely lacking.
3 and high strength and fracture toughness values for several compositions1,2 have been reported. Another potentially highly application-relevant trait is the oxidation resistance of MoAlB in particular, which remains stable at temperatures up to 1600 °C due to formation of protective Al2O3 scales.2,4–6 However, only a few MAB phases are well-characterized, or even reported, so far, with data on other compositions frequently incomplete or entirely lacking.
A convenient structural description of this class of materials is that of boron atoms coordinated by six transition metal (M) atoms, thus forming face-sharing M6B trigonal prisms extending along two crystallographic directions (typically a and c), and separated by dual layers of A atoms along the third direction (typically b).7 MAB phases are furthermore of high technological interest as precursors towards quasi-two-dimensional MBene materials in which the described A element layers are removed, obtained by chemical etching8,9 or directly as a thin film via physical vapor deposition.10
While MoAlB, as a prominent member of the MAB phases, has been synthesized and characterized already in the 1960s,11,12 with subsequent successful syntheses in both bulk2,4,5,13 and thin film6,14 form, the isostructural CrAlB phase has not been found so far. This is in stark contrast to other phases in the ternary Cr–Al–B system of non-equistoichiometric composition such as Cr2AlB2, Cr3AlB4 and Cr4AlB6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7,15 as well as Cr4AlB4,16 all of which have been obtained experimentally. This discrepancy has been partially explained by theoretical studies such as those by Bai et al.17 and Khazaei et al.,18 which have shown the hypothetical CrAlB phase to be unstable vs. the competing phase Cr2AlB2 and Al in the ground state at 0 K, while being stable upon formation from the elements, with the latter finding also reported by Rastogi et al.19 and Wei et al.20 It was also observed, via electronic structure analysis and force constant calculations, that the B–B bonds are strongest in CrAlB, followed by the Cr–B bonds, with Cr–Al and Al–B bonds being significantly weaker,17,18 allowing for extrapolations of the mechanical properties.17
7,15 as well as Cr4AlB4,16 all of which have been obtained experimentally. This discrepancy has been partially explained by theoretical studies such as those by Bai et al.17 and Khazaei et al.,18 which have shown the hypothetical CrAlB phase to be unstable vs. the competing phase Cr2AlB2 and Al in the ground state at 0 K, while being stable upon formation from the elements, with the latter finding also reported by Rastogi et al.19 and Wei et al.20 It was also observed, via electronic structure analysis and force constant calculations, that the B–B bonds are strongest in CrAlB, followed by the Cr–B bonds, with Cr–Al and Al–B bonds being significantly weaker,17,18 allowing for extrapolations of the mechanical properties.17
However, while reporting both enthalpies of formation (ΔH) and Gibbs reaction energies (ΔG) in the ground state, none of the hitherto published theoretical studies, to our knowledge, examined the temperature dependence ΔG(T) of the Gibbs energy, either vs. the elements or competing phases. ΔG(T) is crucial to estimate the magnitude of the energetic barrier towards CrAlB formation at different temperatures, as in certain synthesis scenarios, such as physical vapor deposition (PVD), comparatively small energetic barriers may be readily overcome, leading to the formation of off-equilibrium, metastable phases as thin films, as previously found for, e.g., fcc-TiAlN21 as well as for defects formed in MoAlB.10 Estimating the likelihood of metastable CrAlB phase formation by evaluation of the temperature-dependent energy barrier may be helpful for identifying possible synthesis pathways and is one of the rationales for the present study.
Looking beyond the ternary Cr–Al–B to the quaternary Mo–Cr–Al–B system, single crystals of various Mo-rich compositions, ranging from Mo0.93Cr0.07AlB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 to Mo0.61Cr0.39AlB
22 to Mo0.61Cr0.39AlB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 have been synthesized and characterized, establishing that Cr occupies the same lattice site as Mo22 and thus indicating that phase-pure CrAlB would indeed be isostructural to MoAlB. However, no Cr-rich quaternary phase has hitherto been found and, unlike for the ternary Cr–Al–B system, theoretical predictions are entirely absent here. Yet, given that phase-pure MoAlB has been shown to form upon PVD synthesis and its structural similarity to CrAlB, and stressing the aforementioned possibility of metastable phase formation in PVD, it should in principle be possible to obtain a Cr-rich (Mo,Cr)AlB phase (in other terms, Mo-stabilized CrAlB) upon addition of Mo.
23 have been synthesized and characterized, establishing that Cr occupies the same lattice site as Mo22 and thus indicating that phase-pure CrAlB would indeed be isostructural to MoAlB. However, no Cr-rich quaternary phase has hitherto been found and, unlike for the ternary Cr–Al–B system, theoretical predictions are entirely absent here. Yet, given that phase-pure MoAlB has been shown to form upon PVD synthesis and its structural similarity to CrAlB, and stressing the aforementioned possibility of metastable phase formation in PVD, it should in principle be possible to obtain a Cr-rich (Mo,Cr)AlB phase (in other terms, Mo-stabilized CrAlB) upon addition of Mo.
In this contribution, we have investigated both the energies of formation in the ground state of MoAlB, CrAlB and several quaternary Mo1−xCrxAlB phases of varying composition, and their temperature-dependent Gibbs energies, both vs. the elements and vs. stable competing phases (as detailed further below) via density-functional theory (DFT) approaches. We have identified a minimum Mo concentration to stabilize the Cr-rich (Mo,Cr)AlB phase, serving as a prediction for guiding PVD-based synthesis attempts. Furthermore, we performed electronic structure and bonding analysis for CrAlB and MoAlB, as well as for select (Mo,Cr)AlB compositions, in order to elucidate electronic arguments for improved (Mo,Cr)AlB stability compared to the ternary CrAlB phase.
Methodology/computational details
All ab initio calculations for this study were performed using density-functional theory24,25 as implemented in the Vienna ab initio Simulation Package (VASP, version 5.4.4., University of Vienna).26–28 Basis set generation was handled via the projector-augmented wave method (PAW),29,30 with a cut-off energy of 500 eV. Electronic exchange and correlation effects were accounted for using the well-established generalized gradient approximation functional as parametrized by Perdew, Burke and Ernzerhof (PBE).31 In addition to the valence electrons of each system, electrons in lower-lying levels were explicitly considered in the construction of the basis set (“semi-core state” approach) for the transition metals, resulting in electron configurations of 5s24d44s24p6 for Mo, 4s23d43p6 for Cr, 3s23p1 for Al and 2s22p1 for B according to the VASP potential library. Brillouin zone integration was handled with the method of Methfessel–Paxton32 for a k-mesh generated with the Monkhorst–Pack approach.33 The k-mesh sizes varied with the structures of the individual systems, which varied significantly depending on composition (as the use of supercells was necessitated in some cases), ranging from 17 × 5 × 17 for the formation energy calculations on the ternary systems to 1 × 1 × 1 Γ-point calculations for the lattice dynamics runs. A full list of k-mesh dimensions will not be given here; the k-mesh was in all cases set to be sufficiently dense to ensure energetic convergence, verified via trial runs. Unless otherwise specified, all systems were non-spin-polarized.The initial structural model for MoAlB was obtained from literature,7 and subsequently fully optimized with respect to lattice parameters and atomic positions. The thus far experimentally unknown CrAlB phase as well as the quaternary compositions were generated by successively replacing Mo with Cr, using 2 × 1 × 1 supercells (with Z = 8) to model the compositions where, in Mo1−xCrxAlB, x = 0.125, 0.375, 0.625, 0.875, and using the MoAlB unit cell (with Z = 4) if x = 0, 0.25, 0.5, 0.75, 1. This resulted in the existence of several distinct ordered configurations for a given composition, which were subsequently fully optimized and compared in terms of their total energy. While the energetic difference between the individual configurations proved to be negligible, typically below 10 meV per system, the configurations with the lowest energy for each composition were chosen for further investigations. Modelling of disordered structures using larger supercells was not performed due to the significantly higher computational cost. An exemplary structural model for the Mo0.5Cr0.5AlB quaternary system is shown in Fig. 1.
After optimization, the energies of formation at 0 K, ΔEf, were then calculated vs. the elements and the most stable competing phases according to:
| ΔEf (0 K) = EDFT (product) − ∑χiEDFT (educts) | (1) |
Analysis of the density of states and the crystal orbital Hamilton population (COHP)34 to estimate electronic stability and bonding characteristics were performed via the LOBSTER package (version 4.0.0, Institute of Inorganic Chemistry, RWTH Aachen University),35–38 post-processing wavefunctions obtained from VASP in single-point simulations of the optimized systems.
Lattice dynamics simulations based on finite displacement of atoms and phonon frequency sampling were performed to obtain ΔG(T) data using the phonopy package (version 2.11.0, University of Kyoto).39 This allows for automated generation of supercells of varying size (depending on stoichiometry), but always near-cubic structure with a, b, c >10 Å, in which symmetry-inequivalent atoms are displaced from their equilibrium positions along a given vector with a length of 0.01 Å. The exact number of displacements strongly depends on the space group of the system for which they are generated, with high-symmetry structures requiring lower amounts of displacements; as such, the quaternary compositions, being lower in symmetry, require more. For each such displacement, a single-point VASP calculation yielded interatomic force constants for the given configuration, which were then extracted and post-processed by phonopy across all displacements for a given system. From the changes in the force constant matrices resulting from the displacements of individual atoms, the dynamical matrix D was calculated via a Fourier transformation, and from this the phonon frequencies were obtained. These in turn yield the lattice vibration contribution to the Helmholtz energy, Aph, which is a dominant term, together with the electronic ground-state energy E0, in the calculation of A(T,V):
| A(T, V) = E0(V) + Aph(T, V) + ΔAel(T, V) + Aconf(T) + Avib, ah(T, V) |
The electronic excitation energy ΔAel can be summed with E0, while the configurational term Aconf can be omitted for strictly ordered systems and the anharmonic part of the vibrational energy Avib,ah is typically neglected due to computational cost. The volume dependence of the dominant terms E0 and Aph is accounted for by performing each set of simulations, containing all symmetry-inequivalent displacements for a given system, at several different volumes. Finally, fitting of the resulting data to the Birch–Murnaghan equation of state40,41 yields the temperature dependence, allowing the calculation of ΔG(T) functions for a given reaction, analogous to eqn (1), but for G (following from A) instead of EDFT. Further details for this approach are given elsewhere.42
Results and discussion
Formation energies of the Mo–Cr–Al–B system in the ground state
As a first step in this investigation, the formation energies ΔEf at T = 0 K were calculated for the ternary CrAlB and MoAlB phases as well as several quaternary compositions, corresponding to x = 0, 0.125, 0.25, 0.375, 0.5, 0.625, 0.75, 0.875 and 1 in Mo1−xCrxAlB. Formation from the constituting elements and from the most stable competing phases Mo2AlB2/MoB and Cr2AlB2 (as described in the methodology) were considered as scenarios, shown in Fig. 2a and b, respectively.Considering formation from the elements, it is evident that the ternary and quaternary (Mo,Cr)AlB product phases are stable across the entire probed compositional range, with the formation energy for MoAlB (known to exist both in bulk2,4,7 and as a phase-pure thin film6,14) being approx. −131 kJ mol−1 ≈ −0.113 eV per atom (as Z = 4). This is in excellent agreement with prior literature data by Khazaei et al. who reported a value of −0.114 eV per atom18 and Kota et al. with −132 kJ mol−1.43 While still negative, ΔEf increases with higher Cr content, indicating a less energetically favored reaction, in a quasi-linear manner up to x = 0.5, broadly following an energetics version of Vegard's law. At higher x, the slope changes and ΔEf reaches an energetic plateau at x ≈ 0.75, with further changes in the Mo/Cr ratio having no impact on the trend. This may serve as a first indicator that potential stabilization of the Cr-rich phase with Mo will likely begin to occur at Cr concentrations below that plateau, as the concentration of Mo at higher x is likely too dilute to significantly affect the potential energy surface of the system, with no discernible energetic gain. A linear decrease in equilibrium molar volume is also observed with increasing Cr content, which is to be expected due to the smaller radius -both atomic44 and ionic45- of Cr. The lattice parameters of MoAlB, CrAlB and the quaternary compositions (normalized to one constituting unit cell in the case of supercells to enable meaningful comparisons) are given in Table 1, likewise following Vegard's law.
| Composition | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) |
|---|---|---|---|---|---|---|
| MoAlB | 3.22 | 14.04 | 3.11 | 90 | 90 | 90 |
| Mo0.875Cr0.125AlB | 3.20 | 14.02 | 3.10 | 90 | 90 | 90 |
| Mo0.75Cr0.25AlB | 3.18 | 13.97 | 3.08 | 90 | 90 | 90 |
| Mo0.625Cr0.375AlB | 3.14 | 14.00 | 3.06 | 90 | 90 | 90 |
| Mo0.5Cr0.5AlB | 3.13 | 13.92 | 3.05 | 90 | 90 | 90 |
| Mo0.375Cr0.625AlB | 3.09 | 13.95 | 3.03 | 90 | 90 | 90 |
| Mo0.25Cr0.75AlB | 3.07 | 13.91 | 3.01 | 90 | 90 | 90 |
| Mo0.125Cr0.875AlB | 3.03 | 13.90 | 2.99 | 90 | 90 | 90 |
| CrAlB | 3.00 | 13.89 | 2.97 | 90 | 90 | 90 |
When, however, formation from the competing phases (Fig. 2b) is considered, it is immediately evident that several Cr-rich (Mo,Cr)AlB phases are unstable (ΔEf > 0), both for the ternary composition CrAlB (x = 1) and for quaternary compositions with low Mo content, such as Mo0.125Cr0.875AlB. This corresponds to prior findings for CrAlB17–20 and may partially explain the lack of successful experiments synthesizing that phase in equilibrium, as alluded to in the introduction. Conversely, it is also evident that addition of Mo does indeed stabilize the quaternary system, with x ≈ 0.8 (corresponding to a Mo/Cr ratio of 1/5) sufficient for phase formation if MoB and Cr2AlB2 are the competing phases. If Mo2AlB2 (along with Cr2AlB2) is considered as the competing phase on the Mo side instead, the Mo/Cr ratio required for stabilization shifts to ca. 1/3, at x ≈ 0.67. In this case, the energetic plateau observed for the Cr-rich phases in Fig. 2a is also present (blue curve), while disappearing for the former scenario (pink curve): the reason for this divergent behaviour is unclear at present. To our knowledge, this is the first study expressly identifying these concentration thresholds and yielding a stability window for the Cr-rich quaternary (Mo,Cr)AlB system. However, we reiterate that these findings pertain to the T = 0 K scenario only, and thus, dependence of the Gibbs formation energies on temperature must be considered to obtain the full thermodynamic picture, as seen in the next section.
Temperature-dependent Gibbs energies for CrAlB and MoAlB vs. competing phases
To extend the described observations made in the ground state towards application-relevant temperatures, ΔG(T) curves from lattice dynamics simulations, followed by fitting to the Birch–Murnaghan equation of state, were performed, as detailed in the methodological section. Both the formation of the ternary systems CrAlB and MoAlB from the elements and the most stable competing phases (from literature18) as well as the formation of different quaternary Mo1−xCrxAlB compositions from either Mo2AlB2 and Cr2AlB2 or MoB and Cr2AlB2 were considered. The results are depicted in Fig. 3.It is evident that the formation of CrAlB from Cr2AlB2 and Al is endergonic throughout the entire examined range (Fig. 3a), with ΔG ≈ 8 kJ mol−1 at 0 K, in very good agreement with the calculation of the energy of formation (roughly equivalent to the enthalpy) in the preceding section (see Fig. 2b), and ΔG slightly increasing with higher T. Formation from the next-stable competing phase, CrB, and Al is exergonic in the ground state and at temperatures up to approx. 725 K, after which CrB and Al are thermodynamically more stable. The steeper slope of the ΔG(T) curve for the latter reaction in comparison to formation from Cr2AlB2 also suggests that at higher T, the ternary diboride is less stable vs. CrB and Al; however, as these phases were not the focus of the present study, this was not investigated further. Formation from all other phases (as indicated in the figure) and the elements is exergonic throughout the temperature range. Thus, the observation that Cr2AlB2 has been experimentally synthesized,7 while CrAlB could not be obtained is reflected in the course of the Gibbs energies. However, the energetic barrier towards formation is very small, with the highest difference in the observed temperature range being approx. 12 kJ mol−1 ≈ 10 meV per atom at 1500 K. In a kinetically limited growth scenario such as during PVD synthesis, where the incident flux frequently exhibits high kinetic energies depending on the deposition parameters, this high-energy flux is rapidly quenched due to the difference in thermodynamic temperature between the substrate/growing film (in effect serving as a heat sink) and the incident particles. This may significantly kinetically limit atomic mobility and enable formation of CrAlB as a metastable phase (likely in coexistence with Cr2AlB2). Similar formation of metastable phases which were only slightly energetically disfavored has been previously observed for fcc-(Ti,Al)N with varying Ti/Al ratios, agreeing with theoretical predictions,21 as well as, recently, in orthorhombic MoAlB thin films, in which a two-dimensional MoB phase (MBene) and several metastable defects, which are energetically disfavored, coexist in the main phase.10,46
In the case of MoAlB (Fig. 3b), all examined competing phases are thermodynamically disfavored, explaining the stability of said compound and its experimentally observed phase-pure formation;6,7,14 however, the competing MoB phase is energetically very close at higher temperatures, and, if the curve is extrapolated, eventually likely forms instead of MoAlB, or as a main phase in a MoB/MoAlB/Al (or Al oxide) phase mixture. This agrees well with the prior finding that MoB formation in a two-dimensional MBene structure is possible not only through chemical etching and annealing,8,9 but, crucially, also in a direct PVD synthesis route.10 Nonetheless, for the examined temperature range, the stability of MoAlB is unequivocal and, coupled with the observations from the ground-state energies of formation detailed above, suggests that a temperature-dependent investigation of select quaternary systems is worthwhile to find a potential Mo-induced stabilization effect, as depicted in Fig. 3c and d.
Analogous to the ground-state formation energy calculations, two scenarios were considered: formation of the quaternary Mo1−xCrxAlB phases with varying compositions from Cr2AlB2, Mo2AlB2 and Al (Fig. 3c) or from Cr2AlB2, MoB and Al (Fig. 3d). The selection of Mo2AlB2 and MoB as competing phases on the Mo side is reinforced by their status of being the least energetically disfavored compared to MoAlB, as outlined in the preceding paragraph. As lattice dynamics calculations are computationally highly expensive, particularly for systems whose stoichiometric ratios necessitate low-symmetry supercells, only the Cr-rich side of the compositional space was sampled, i.e., the area around the cross-over point identified in Fig. 2b. Thus, x in Mo1−xCrxAlB was chosen as 0.5, 0.625, 0.75 and 0.875. The ΔG(T) curves for the formation of CrAlB and MoAlB (x = 1 and 0, respectively) are also shown again as boundaries.
Once again, the (Mo,Cr)AlB system exhibits a Vegard-like behavior in terms of its energetics, as the progressive addition of Mo continually lowers the Gibbs energy of formation: while the formation of the composition Mo0.125Cr0.875AlB is thermodynamically disfavored in both scenarios across the entire temperature range, Mo0.25Cr0.75AlB forms from MoB, Cr2AlB2 and Al at 0 K (Fig. 3d), agreeing well with the cross-over point identified for this scenario in Fig. 2b, while formation from Mo2AlB2, Cr2AlB2 and Al is still disfavored; however, the exergonic energy is very small for this composition. For Mo0.375Cr0.625AlB, formation occurs vs. both MoB and Mo2AlB2 (Fig. 3c), yet again in good agreement with the data in Fig. 2b, where the cross-over point, if formation from Mo2AlB2 is considered, lies around x ≈ 0.67. Furthermore, it is immediately evident that addition of Mo extends the stability of the system towards higher temperatures, regardless of choice of competing phases.
To summarize, our postulate is two-fold: first, due to the low energetic barrier for formation of ternary CrAlB, it is likely that this phase forms in high-energy synthesis routes, at least as a metastable side phase, and may be isolated there. Second, energetic stabilization occurs upon substitution of Cr with Mo, so that a quaternary (Mo,Cr)AlB phase ought to form spontaneously, allowing phase-pure isolation for a given composition if the metal–metal ratio is tightly controlled. However, we must stress that we are considering thermodynamic arguments only – potential kinetic barriers (or pathways) that may influence the actual stability of (Mo,Cr)AlB are not examined here, lying outside the scope of this study.
Electronic structure comparison and bonding analysis of MoAlB, CrAlB and (Mo,Cr)AlB
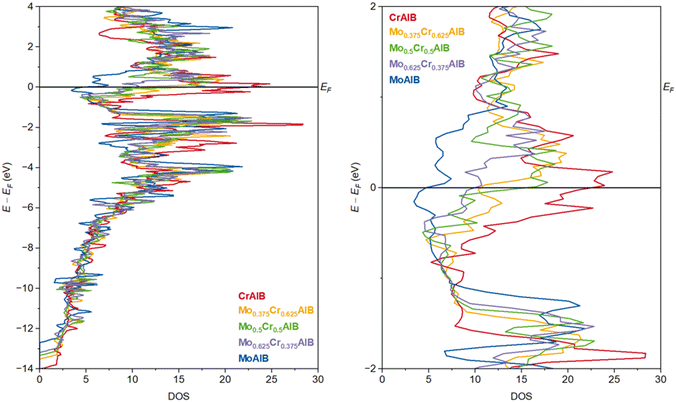
Having investigated the energetic trends in the ground state and at non-zero temperatures, we will briefly discuss the electronic structure of MoAlB, CrAlB and select quaternary systems as well as characteristic interatomic interactions to reinforce the outlined hypotheses from an electronic point of view. The total density of states (DOS) curves for MoAlB, CrAlB and the quaternary compositions Mo0.375Cr0.625AlB, Mo0.5Cr0.5AlB and Mo0.625Cr0.375AlB are depicted in Fig. 4, with the right-hand side figure magnifying the energetic area close to the Fermi level (EF). It can be clearly seen that the DOS for all systems qualitatively strongly resemble each other, as expected from the structural and electronic similarity: in particular, the DOS of the quaternary compositions lie roughly between those of CrAlB and MoAlB (red and blue curves, respectively), fulfilling yet again the expectation from Vegard's law, generalized to the electronic structure.However, particularly in proximity to EF (between −0.5 and 0.5 eV relative to it), the Mo0.5Cr0.5AlB DOS (green curve) is closer to that of CrAlB, with both exhibiting local near-maxima at EF, indicating localized states and, for metallic systems at EF, serving as a marker for electronic instability due to reduced orbital mixing between adjacent atoms.47 In contrast, the MoAlB DOS does not exhibit such a peak, with the electronic stability of the material in the ground state likely being higher. While this by no means precludes the formation of the other two phases per the arguments given above, it contributes to an explanation of why MoAlB is the more stable phase and the only one found experimentally so far despite the broad structural similarity of the phases. In the same vein, the Mo-rich quaternary phase Mo0.625Cr0.375AlB (lilac curve) lies closer to the MoAlB DOS at EF, in good agreement with the expectation of Mo stabilizing the quaternary system. However, the behaviour of the Cr-rich quaternary Mo0.375Cr0.625AlB composition is an outlier here, as its DOS value at EF, while lying above that of the Mo-rich phase, is markedly below that of Mo0.5Cr0.5AlB, and exhibits a local minimum. Nonetheless, generally speaking, the trend qualitatively follows the expectations.
While DOS plots are instructive to compare the electronic structures of different systems, they are less suited to a clear description of interatomic interactions, as antibonding and bonding character cannot readily be distinguished from the DOS alone. Thus, crystal orbital Hamilton population (COHP) analyses have been performed for MoAlB and CrAlB as the two ternary endpoints of the Mo–Cr–Al–B system, as well as for the equistoichiometric Mo0.5Cr0.5AlB, with the results shown in Fig. 5.
From the visualization of the interatomic distances in Fig. 5a, it is immediately evident that they are quite similar in all phases, with the highest deviation occurring for the homoatomic transition metal bonds (Mo–Mo/Cr–Cr, respectively) at approx. 10%. It should be noted here that, when spin polarization is taken into account for the CrAlB system, the interatomic distances increase and are nearly identical to those in the MoAlB system, yet the interaction strengths are broadly similar to those in the non-spin-polarized case depicted here. Thus, the difference in interaction (bond) strengths is, as a rule, strongly dependent on interatomic distance, and this case is observed here, yet it can be counteracted by magnetic effects. However, the CrAlB system is the only system exhibiting antiferromagnetic behaviour, with all other systems being nonmagnetic, and the difference in total energy is marginal (approx. 5 meV) to the non-spin-polarized case. Thus, for consistency of comparison, and due to the fact that magnetism was not considered for the G(T) calculations due to computational cost, we further discuss only the non-spin-polarized case for CrAlB here.
Visualization of the averaged integrated COHP (ICOHP) values of a single interaction of a given type in the 1st coordination sphere of an atom (Fig. 5b) and the summation of the ICOHP values per interaction type over the entire unit cell (Fig. 5c) reveals that all interactions are slightly stronger in MoAlB than in CrAlB, with the dominant interaction on a single, individual bond level (Fig. 5b) being the B–B bond, followed by the second-strongest individual interaction, Al–B. The other interactions, such as Al–Al, (Mo,Cr)–B etc., as given in the figure, are all significantly weaker, in good agreement with prior studies,18 with the homoatomic (Mo,Cr)–(Mo,Cr) interaction being weakest. This general trend is also well reflected in the case of the quaternary system Mo0.5Cr0.5AlB, in which the interatomic distances and averaged interactions typically lie between the values of the ternary endpoints, or are very close to the respective interaction in the ternary system with the corresponding metal. All interactions are of bonding character, as indicated by their negative ICOHP values. All interactions beyond the first coordination sphere are weaker by orders of magnitude and thus are not shown here. The individual ICOHP values for interactions in MoAlB and CrAlB, as well as the relative increase of the former vs. the latter, are shown in Table 2 in more detail, with the interactions in Mo0.5Cr0.5AlB not shown to improve readability.
| Interaction | Avg. ICOHP per bond (eV per bond) | Sum ICOHP per cell (eV) | ||||
|---|---|---|---|---|---|---|
| MoAlB | CrAlB | Δ rel (%) | MoAlB | CrAlB | Δ rel (%) | |
| B–B | −6.03 | −5.14 | 17.4 | −27.62 | −20.55 | 34.4 |
| Al–B | −3.29 | −2.73 | 20.3 | −15.48 | −13.29 | 16.5 |
| Al–Al | −2.12 | −1.87 | 13.2 | −29.20 | −27.43 | 6.5 |
| (Mo,Cr)–B | −1.88 | −1.37 | 37.1 | −46.04 | −33.24 | 38.5 |
| (Mo,Cr)–Al | −1.54 | −1.04 | 47.7 | −29.20 | −20.69 | 41.2 |
| (Mo,Cr)–(Mo,Cr) | −0.78 | −0.32 | 144 | −11.12 | −5.40 | 106 |
However, when the sum over all bonds of a given type is considered, as shown in Fig. 5c, the number of individual contacts plays a large role, which changes the picture dramatically. Besides the trivial observation that all interactions are bonding, as already shown, the bonding situation in the cell is dominated by the (Mo,Cr)–B interaction for both ternary systems, but dramatically stronger in MoAlB, as seen in Table 2. The (Mo,Cr)–Al interaction is second-strongest in MoAlB, tied with Al–Al, yet only in third place (clearly weaker than the Al–Al interaction) in CrAlB. The B–B interaction is much weaker in CrAlB compared to the MoAlB case, whereas the Al–Al interaction strength is largely unchanged between both systems. The dominant strength of the (Mo,Cr)–B interaction is in agreement with the findings of Bai et al.,17 who examined bond stiffness, typically well correlated with electronic bond strength; however, the Al–Al and (Mo,Cr)–Al interactions, when categorized by bond strength in this study, are stronger than suggested by the bond stiffness data. For the quaternary system, the ICOHP values split into contributions from Mo–X and Cr–X bonds, with X signifying all other species; when these values are summed, the resulting transition metal-heteroatom interactions again lie between the values in CrAlB and MoAlB.
Thus, summarizing, it can be said that the bonding contribution to MoAlB stability comes from the increased strength of all interatomic bond types, despite largely unchanged distances, with the main drivers being the interactions of Mo with Al and B. However, while these quantitative differences are significant, particularly in terms of increase when comparing CrAlB and MoAlB, the qualitative trends when identifying interactions from weakest to strongest are identical in both cases, yet again reflecting both systems’ similarity. Finally, we should add as a cautionary note that, strictly speaking, the COHP technique only yields the covalent portion of the interaction and is only an indirect (but strongly correlated) descriptor of the actual bond strength;47 nonetheless, the technique is regularly used to study metallic systems in literature and typically, trends are reproduced fairly well.48–50
Conclusions
We have carried out density functional theory-based theoretical studies of MoAlB, the hypothetical isostructural CrAlB and select quaternary Mo1−xCrxAlB compositions, with particular focus on their energies of formation and their temperature dependence. CrAlB is shown to be stable vs. the elements both in the ground-state at 0 K and across the investigated temperature range between 0 and 1500 K, but is unstable vs. the main competing phase Cr2AlB2. However, the energetic difference is very small, on the order of approx. 10 kJ mol−1 or 0.008 eV per atom, which can readily be overcome in a kinetically limited synthesis scenario, such as physical vapor deposition, where the presence of energetically disfavored defect phases has been experimentally observed for MoAlB before. Furthermore, already in the ground state at 0 K, alloying with small amounts of Mo lowers the formation energy of the quaternary phase vs. that of the most stable competing phases Cr2AlB2 and Mo2AlB2 or Cr2AlB2 and MoB, resulting in critical Mo/Cr ratios of 0.33 and 0.2, respectively, at which the quaternary phase is expected to form based on energetic arguments alone. This is also reflected in the temperature-dependent Gibbs energies of formation, with the composition Mo0.375Cr0.625AlB remaining stable at up to 600 K (vs. Mo2AlB2) or 1100 K (vs. MoB), and higher Mo concentrations enabling stability at yet higher temperatures. Thus, a predicted compositional threshold for stabilization via alloying is provided for future synthesis attempts. Finally, the experimentally known stability of MoAlB is rationalized via the electronic structure and bonding characteristics, which hints at stronger bonds of all types in MoAlB when compared to CrAlB and identifies the Mo–B interaction as the main stabilizing influence in that respect, even though the B–B interaction is stronger on an individual bond basis.Author contributions
D. B. and J. M. S.: Conceptualization, methodology. D. B: Investigation (lead), formal analysis, writing of original draft and editing. P. J. P: Visualization, investigation (supporting), writing (review/editing), data curation. J. M. S: Supervision, funding acquisition, resources, writing (review/editing).All authors have contributed and given approval to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. B. and J. M. S. thank the IT Center of RWTH Aachen University for the provision of computational resources and IT support via the Jülich-Aachen Research Alliance's (JARA) JARA0221 grant. J. M. S. acknowledges financial support from the MPG fellow program.Notes and references
- S. Kota, M. Sokol and M. W. Barsoum, Int. Mater. Rev., 2020, 65, 226–255 CrossRef CAS.
- L. Xu, O. Shi, C. Liu, D. Zhu, S. Grasso and C. Hu, Ceram. Int., 2018, 44, 13396–13401 CrossRef CAS.
- N. F. Rosli, M. Z. M. Nasir, N. Antonatos, Z. Sofer, A. Dash, J. Gonzalez-Julian, A. C. Fisher, R. D. Webster and M. Pumera, ACS Appl. Nano Mater., 2019, 2, 6010–6021 CrossRef CAS.
- S. Kota, E. Zapata-Solvas, A. Ly, J. Lu, O. Elkassabany, A. Huon, W. E. Lee, L. Hultman, S. J. May and M. W. Barsoum, Sci. Rep., 2016, 6, 26475 CrossRef CAS PubMed.
- O. Shi, L. Xu, A. Jiang, Q. Xu, Y. Xiao, D. Zhu, S. Grasso and C. Hu, Ceram. Int., 2019, 45, 2446–2450 CrossRef CAS.
- J.-O. Achenbach, R. Sahu, B. Völker, M. Hans, D. Primetzhofer, D. J. Miljanovic, C. Scheu and J. M. Schneider, Coatings, 2019, 9, 510 CrossRef CAS.
- M. Ade and H. Hillebrecht, Inorg. Chem., 2015, 54, 6122–6135 CrossRef CAS PubMed.
- L. T. Alameda, C. F. Holder, J. L. Fenton and R. E. Schaak, Chem. Mater., 2017, 29, 8953–8957 CrossRef CAS.
- L. T. Alameda, P. Moradifar, Z. P. Metzger, N. Alem and R. E. Schaak, J. Am. Chem. Soc., 2018, 140, 8833–8840 CrossRef CAS PubMed.
- R. Sahu, D. Bogdanovski, J. O. Achenbach, S. Y. Zhang, M. Hans, D. Primetzhofer, J. M. Schneider and C. Scheu, Nanoscale, 2021, 13, 18077–18083 RSC.
- W. Rieger, H. Nowotny and F. Benesovsky, Monatsh. Chem. Verw. Teile Anderer Wiss., 1965, 96, 844–851 CrossRef.
- W. Jeitschko, Monatsh. Chem. Verw. Teile Anderer Wiss., 1966, 97, 1472–1476 CrossRef CAS.
- S. Wang, Y. Xu, Z. Yu, H. Tan, S. Du, Y. Zhang, J. Yang and W. Liu, Ceram. Int., 2019, 45, 23515–23521 CrossRef CAS.
- S. Evertz, P. Pöllmann, D. M. Holzapfel, E. Mayer and J. M. Schneider, J. Eur. Ceram. Soc., 2021, 41, 6302–6308 CrossRef CAS.
- S. Kota, W. Wang, J. Lu, V. Natu, C. Opagiste, G. Ying, L. Hultman, S. J. May and M. W. Barsoum, J. Alloys Compd., 2018, 767, 474–482 CrossRef CAS.
- H. Zhang, F.-z. Dai, H. Xiang, Z. Zhang and Y. Zhou, J. Mater. Sci. Technol., 2019, 35, 530–534 CrossRef CAS.
- Y. Bai, X. Qi, X. He, D. Sun, F. Kong, Y. Zheng, R. Wang and A. I. Duff, J. Am. Ceram. Soc., 2019, 102, 3715–3727 CrossRef CAS.
- M. Khazaei, J. Wang, M. Estili, A. Ranjbar, S. Suehara, M. Arai, K. Esfarjani and S. Yunoki, Nanoscale, 2019, 11, 11305–11314 RSC.
- A. Rastogi, P. Rajpoot and U. P. Verma, Mater. Chem. Phys., 2018, 211, 242–248 CrossRef CAS.
- J. Wei, L. Zhang and Y. Liu, Solid State Commun., 2021, 326, 114182 CrossRef CAS.
- P. H. Mayrhofer, D. Music and J. M. Schneider, J. Appl. Phys., 2006, 100, 094906 CrossRef.
- S. Okada, K. Iizumi, K. Kudaka, K. Kudou, M. Miyamoto, Y. Yu and T. Lundström, J. Solid State Chem., 1997, 133, 36–43 CrossRef CAS.
- Y. Yu and T. Lundström, J. Alloys Compd., 1995, 226, 5–9 CrossRef CAS.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- M. Methfessel and A. T. Paxton, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 3616–3621 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- R. Dronskowski and P. E. Bloechl, J. Phys. Chem., 1993, 97, 8617–8624 CrossRef CAS.
- V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, J. Phys. Chem. A, 2011, 115, 5461–5466 CrossRef CAS PubMed.
- S. Maintz, V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, J. Comput. Chem., 2013, 34, 2557–2567 CrossRef CAS PubMed.
- S. Maintz, V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, J. Comput. Chem., 2016, 37, 1030–1035 CrossRef CAS PubMed.
- R. Nelson, C. Ertural, J. George, V. L. Deringer, G. Hautier and R. Dronskowski, J. Comput. Chem., 2020, 41, 1931–1940 CrossRef CAS PubMed.
- A. Togo and I. Tanaka, Scr. Mater., 2015, 108, 1–5 CrossRef CAS.
- F. D. Murnaghan, Proc. Natl. Acad. Sci. U. S. A., 1944, 30, 244–247 CrossRef CAS PubMed.
- F. Birch, Phys. Rev., 1947, 71, 809–824 CrossRef CAS.
- R. P. Stoffel, C. Wessel, M.-W. Lumey and R. Dronskowski, Angew. Chem., Int. Ed., 2010, 49, 5242–5266 CrossRef CAS PubMed.
- S. Kota, M. Agne, E. Zapata-Solvas, O. Dezellus, D. Lopez, B. Gardiola, M. Radovic and M. W. Barsoum, Phys. Rev. B, 2017, 95, 144108 CrossRef.
- J. C. Slater, J. Chem. Phys., 1964, 41, 3199–3204 CrossRef CAS.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- R. Sahu, D. Bogdanovski, J.-O. Achenbach, J. M. Schneider and C. Scheu, Nanoscale, 2022, 14, 2578–2585 RSC.
- R. Dronskowski, Computational chemistry of solid state materials a guide for materials scientists, chemists, physicists and others, Wiley-Blackwell, Weinheim, 2005 Search PubMed.
- G. A. Landrum and R. Dronskowski, Angew. Chem., Int. Ed., 2000, 39, 1560–1585 CrossRef CAS PubMed.
- A. Saksena, D. Bogdanovski, H. Sahasrabuddhe, D. Music and J. M. Schneider, Materials, 2020, 13, 2298 CrossRef CAS PubMed.
- S. Amano, D. Bogdanovski, H. Yamane, M. Terauchi and R. Dronskowski, Angew. Chem., Int. Ed., 2016, 55, 1652–1657 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |