Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solution and interfacial self-assembly of Bacillus subtilis bacterial lipoteichoic acid (LTA): nanoclustering, and effects of Ca2+ and temperature†
Bhavesh
Bharatiya
 a,
Magdalena
Wlodek
b,
Robert
Harniman
a,
Ralf
Schweins
c,
Judith
Mantell
d,
Gang
Wang
a,
Magdalena
Wlodek
b,
Robert
Harniman
a,
Ralf
Schweins
c,
Judith
Mantell
d,
Gang
Wang
 a,
Piotr
Warszynski
a,
Piotr
Warszynski
 b and
Wuge H.
Briscoe
b and
Wuge H.
Briscoe
 *a
*a
aSchool of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, UK. E-mail: wuge.briscoe@bristol.ac.uk; Tel: +44 (0)117 3318256
bJerzy Haber Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences, Niezapominajek 8, PL-30239 Krakow, Poland
cInstitut Laue-Langevin, DS/LSS, 71 Avenue des Martyrs, Grenoble 38000, France
dWolfson Bioimaging Facility, University of Bristol, Medical Sciences Building, University Walk, Bristol BS8 1TD, UK
First published on 18th July 2022
Abstract
Lipoteichoic acid (LTA) is a major structural and functional molecule in the Gram-positive bacteria membrane. Knowledge of LTA adsorption at interfaces and its solution self-assembly is crucial to understanding its role in bacterial adhesion and colonisation, infections and inflammations. Here, we report the self-assembly behaviour of LTA extracted from Bacillus subtilis, a Gram-positive bacterium, in an aqueous solution using cryogenic transmission electron microscopy (Cryo-TEM) and small-angle neutron scattering (SANS) and its adsorption behaviour at the solid–liquid interface using atomic force microscopy (AFM) imaging and quartz crystal microbalance with dissipation monitoring (QCM-D). The Cryo-TEM results indicated the formation of spherical LTA micelles that decreased in size on addition of calcium chloride (CaCl2), attributed to charge neutralisation and possible formation of stable Ca2+-bridges between the phosphate groups on neighbouring LTA chains. Analysis of the SANS data from the polydisperse LTA aggregates in solution using the two Lorentzian model revealed the existence of two correlation lengths, which could respectively account for the presence of LTA micelle clusters and the local structure arising from LTA intra-molecular interactions. In the presence of CaCl2, the decrease in the correlation lengths of the clusters indicated possible disruption of H-bonding by Ca2+, leading to poorer water-LTA interactions. At higher temperatures, the correlation length corresponding to the clusters increased, indicating a temperature assisted growth caused by the fluidization of micellar core and dehydration of the polar LTA chains. AFM imaging showed that adsorption of LTA aggregates at the SiO2–water interface was significantly prompted by the addition of CaCl2, also confirmed by QCM-D measurements. These unprecedented nanoscopic structural details on the morphology of LTA aggregates in solution and at the solid–liquid interface add to our fundamental understanding of its self-assembly behaviour hitherto underexplored.
Introduction
Lipoteichoic acid (LTA) is an important biopolymer present in the Gram-positive bacteria membrane, playing both structural and functional roles. LTA is anchored to the phospholipid membrane through its diacylglycerol and its glycerophosphate chain expands over the extracellular peptidoglycan layer (Fig. 1A). The glycerophosphate chain comprises ∼25–30 phosphodiester-linked repeating units (Fig. 1B), which may also contain ∼5–10 mol% glycosyl or D-alanyl ester units depending on bacterial growth conditions.1 The interfacial interactions leading to bacterial adhesion and colonisation are reportedly influenced by bacterial surface proteins and LTAs that expand towards the cell exterior.2–4 LTA is considered as a major virulence factor for various infections and inflammations caused by Gram positive bacteria. It is released from bacterial cells after the bacteriolysis and upon interactions with cationic peptides or β-lactam antibiotics. LTA can attach to target cells though CD14 and toll-like receptors, whilst non-specific binding with the membrane phospholipids also occurs. The fundamental understanding of self-assembled LTA structures is essential, considering its amphiphilic character, which may influence several biological functions.1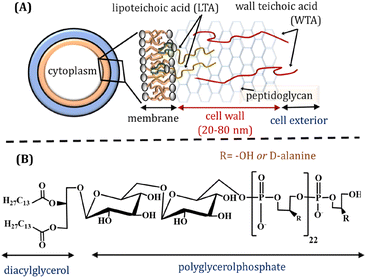 | ||
| Fig. 1 (A) Schematic representation of Gram-positive bacteria cell wall. (B) Structure of Bacillus subtilis lipoteichoic acid (LTA). | ||
The molecular self-assembly behaviour of LTA both in the solution and at the interfaces, such as the aggregate morphology and its response to solution conditions, is not well understood, despite the importance of such fundamental knowledge to the LTA role in infectivity, inflammation, and septic shock. It has been previously reported that surface active LTA molecules self-assemble to form aggregates in aqueous solutions driven by their amphiphilic properties. Wicken et al.5 measured the critical micelle concentration (CMC) of LTA extracted from Lactobacillus fermentum by fluorescence spectroscopy using merocyanine dye as a probe, obtaining CMC values of 1–10 μg mL−1 (∼0.2 × 10−6 M to 2 × 10−6 M). Courtney et al.6 calculated the CMC of LTA extracted from different cocci in phosphate buffered saline (PBS) solutions as 28–60 μg mL−1 (∼5.6 × 10−6 M to 12.1 × 10−6 M). These values are considerably higher than the CMC of ∼10−10 M observed for membrane phospholipids, which is due to the presence of the bulky hydrophilic glycerophosphate chain in the LTA structure, increasing its partitioning in aqueous bulk solutions.7,8
Several findings have implicated the function of monomeric LTA at low concentrations of ∼0.3 to 2 μg mL−1 – below its reported CMC – in various biological processes, e.g. maximum sensitisation of erythrocytes9 and stimulation of lysosomal enzymes from the macrophages.10 On the other hand, Streptococcus pyogenes LTA at concentrations higher than its CMC has been reported to induce disruption of erythrocytes in sheep and cause cytotoxicity to Girardi heart cells.6 These reports suggest significantly different responses to various biological processes by LTA molecules in both monomeric and aggregated states.
Labischinski et al.11 investigated micellar structures formed by LTA extracted from Bifidobacterium bifidum DSM 20239, Enterococcus hirae (Streptococcus faecium) ATCC 9790, Lactococcus garviue NCDO 2155, Listrria welshimeri SLCC 5354, and Staphylococcus aureus DSM 20233. Their small angle X-ray scattering (SAXS) analysis revealed spherical micelles with the core formed by the lipophilic chains in an α-type conformation and the shell formed by the hydrophilic glycerophosphate chains. Approximately 150 LTA molecules aggregated to form a micelle with an average diameter of ∼23 nm and a hydrophilic shell ∼8.5 nm in thickness. The chains were assumed to aggregate in a coiled conformation to satisfy steric requirements and rationalise the obtained micelle size. Fischer et al.12 determined the aggregation parameters for LTA extracted from Streptococcus pneumoniae using SAXS, reporting the formation of micelles with identical dimensions as observed by Labischinski et al.11 The hydrophilic chains were assumed to adopt a fully extended conformation based on the calculations from the structural dimension of the micelle and the LTA molecule. These findings indicate that the supramolecular structure of LTA is controlled mainly by the cross-sectional area of lipophilic diacylglycerol moieties and presumably less influenced by the hydrophilic chain structure. On the other hand, our recent report13 showed that the large molecular footprint and electrostatic characteristics of LTA also influenced the stability and integrity of the model phospholipid membrane. We found that mixed vesicles comprising Bacillus subtilis LTA and phospholipids showed an increase in the size as more LTA was added up to 6.5 mol%, while further LTA addition resulted in the partition of LTA from the mixed liposomes, leading to the coexistence of nascent LTA micelles and mixed LTA-lipid vesicles.
Understanding intermolecular interactions and self-assembled structures of bacterial lipids at various interfaces as well as in solution is of fundamental relevance to advancing our knowledge of bacterial functions.1,14,15 To the best of our knowledge, the self-assembly behaviour of LTA extracted form Bacillus subtilis has not been reported previously. In this study, we have investigated the aggregation of LTA in aqueous solution and their adsorption at the solid–liquid interface using cryogenic transmission electron microscopy (Cryo-TEM), small angle neutron scattering (SANS), atomic force microscopy (AFM) imaging, and quartz crystal microbalance with dissipation monitoring (QCM-D). The effects of the addition of Ca2+ at different concentrations and the solution temperature have also been investigated. We note that these observations were made in a model system, whereas the real biological system is much more complex. The nanoscopic morphological and structural information on LTA aggregates obtained using quantitative physical methods in the model system, however, is valuable to understanding the functional role of LTA in complex biological processes.
Materials and methods
The lipoteichoic acid (LTA) extracted from Bacillus subtilis and CaCl2 were purchased from Sigma-Aldrich, UK. Ultrapure water (resistivity 18.2 MΩ cm and total organic content (ToC) < 4 ppb) used for sample preparation was purified using Millipore® water purification system. Samples for SANS have been prepared in D2O instead of H2O.Cryogenic-transmission electron microscopy (Cryo-TEM)
Cryo-TEM samples were prepared by plunge freezing performed using a LEICA GP, into liquid N2-cooled liquid ethane. Droplets (∼4–8 μL) of LTA in pure and mixed solutions with CaCl2 were placed on glow discharged lacey carbon grids and left for 2 s before blotting (2 s) and plunging. This yielded samples with LTA aggregates embedded in the vitreous ice suspended inside the holes of the carbon grid. The sample grid was then transferred into a Gatan 626 cryo-holder and visualized at 200 kV in a Tecnai T20, FEI transmission electron microscope fitted with an FEI CETA camera. The images were analyzed using FIJI ImageJ® programme to generate the size histogram. All the images were taken using the same operational parameters to allow direct comparison of the structural changes.Quartz crystal microbalance with dissipation monitoring (QCM-D)
The deposition of 0.2 mg mL−1 LTA in ultrapure Milli-Q water and in 10 mM aqueous CaCl2 solution was studied in situ using the QCM-D (Q-Sense AB Gothenburg, Sweden; present name: Biolin Scientific). The QCM-D technique provides real-time information about the mass and structural properties of adsorbed species. It is based on measuring the resonant frequency of a disk-shaped piezoelectric quartz crystal with metal electrodes deposited on both sides. For a rigid film on the crystal, a small mass of adsorbate added to the electrodes induces a decrease in the resonant frequency (Δf), which is proportional to the mass deposited (Δm), according to Sauerbrey's relationship:16–18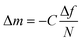 | (1) |
The SiO2-coated 5 MHz QCM-D crystals were purchased from Q-Sense AB (Sweden) and cleaned with piranha solution (a mixture of equivalent volumes of concentrated sulfuric acid and hydrogen peroxide) before the first use. After being dipped into the piranha solution for 2 min, the crystal substrates were then rinsed thoroughly with Milli-Q water, followed by 30 min immersion in hot (ca. 70 °C) Milli-Q water before dried in a stream of pure nitrogen prior to measurements. The crystals were mounted in the flow chamber of the instrument. After resonance frequency calibration, the chamber was filled with Milli-Q water to obtain a baseline. Then, the LTA dispersion was introduced, and the adsorption kinetics was monitored in situ. After obtaining a constant value of the resonance frequency, the chamber was rinsed. The Sauerbrey and Voigt viscoelastic model, implemented in QTools 3 software (Q-Sense AB)16,18 was used to analyse the experimental data of LTA in ultrapure Milli-Q water and in 10 mM aqueous CaCl2 solution, respectively. All the measurements were performed at 25 °C.
Atomic force microscopy (AFM)
AFM (Bruker Multimode 8, Peakforce feedback control) was used to image the morphology of LTA aggregates absorbed on SiO2 wafers. The ∼1 cm × 1 cm SiO2 substrate pieces were cleaned by rinsing with ethanol, dried with a nitrogen flow and UV-ozoned (UVO CLEANER®, Jelight Company Inc., US) for 20 min. It was then glued onto an AFM magnetic stub using Epon 1004 (Shell), and the substrate was incubated overnight in ∼30 mL of sonicated 2 mg mL−1 LTA aqueous dispersion (either in Milli-Q water or 10 mM CaCl2 solution) at 25 or 40 °C. The stub was then transferred to the AFM with a drop of the solution deposited atop to ensure the surface was not dried and exposed to air. A liquid cell containing water or CaCl2 solution was then mounted above the surface to keep it submerged throughout the measurement. PeakForce atomic force microscopy was conducted utilising a Multi-mode VIII microscope with Nanoscope V controller. SCANASYST-FLUID+ cantilevers (Bruker, CA, US) were used with a nominal tip radius and spring constant of 2 nm and 0.7 N m−1, respectively. The Peakforce amplitude and setpoint were 50 nm and 0.8–1 nN, respectively. Images were obtained in tapping mode, at 512 × 512 pixels at a scan rate of 0.5 Hz except for 250 nm × 250 nm scans which were collected at 256 × 256 pixels at a scan rate of 1 Hz. Images were recorded and processed, including flattening to account for sample tilting and low pass filters to smoothen the image, using the Bruker NanoScope Analysis 1.80 software package.Small angle neutron scattering (SANS) measurements
SANS data was obtained from 2 mg mL−1 LTA samples contained in quartz cells with a 2 mm path length on the D1120–22 small-angle diffractometer at the Institut Laue-Langevin, ILL, (Grenoble, France). A neutron beam with wavelength (λ = 5.5 Å with a FWHM of 9%) and sample-to-detector distance distances of 39 m, 8 m and 1.4 m were used to obtain a q-range of ∼0.0016–0.5 Å−1, where q = 4π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(2θ/2)/λ is the momentum transfer with 2θ the scattering angle. The neutron beam used had a size of 7 × 10 mm2. A 3He MWPC detector with 256 × 256 pixels of 3.75 × 3.75 mm2 each was used to record the scattered intensities. The raw scattering data were corrected for the detector efficiency, sample transmission, and background scattering and converted to scattering cross-section data (∂Σ/∂Ω vs. q) using LAMP software. The data was converted to an absolute scale (cm−1) using the secondary calibration standard H2O of 1 mm path length (cross-calibrated against an h/d polymer blends sample) with a differential scattering cross-section of 0.956 cm−1.
sin(2θ/2)/λ is the momentum transfer with 2θ the scattering angle. The neutron beam used had a size of 7 × 10 mm2. A 3He MWPC detector with 256 × 256 pixels of 3.75 × 3.75 mm2 each was used to record the scattered intensities. The raw scattering data were corrected for the detector efficiency, sample transmission, and background scattering and converted to scattering cross-section data (∂Σ/∂Ω vs. q) using LAMP software. The data was converted to an absolute scale (cm−1) using the secondary calibration standard H2O of 1 mm path length (cross-calibrated against an h/d polymer blends sample) with a differential scattering cross-section of 0.956 cm−1.
Analysis of SANS data
The SANS data were analysed using the two-Lorentzian model23 with the following equation, | (2) |
Results and discussion
First, we would like to comment on the complementarity between the different techniques and their limitations. Whilst AFM imaging provide high resolution (1–5 nm) nanomorphology, the sampling area (∼0.5–50 μm2) is relatively small and localised. SANS probes a relatively much larger volume of the sample with a beam size of 7 × 10 mm and a sample thickness of 2 mm. The recorded scattering intensity is thus an average of that from a large number of aggregates of different sizes and geometries and the SANS analyses can yield structural information on the length scale of 5–100 nm (cf.Table 1). Cryo-TEM in this case provides an indication of the morphology with a limited resolution due to modest contrast and a very small sample size.| [CaCl2] (mM) | ξ 1 (Å) | ξ 2 (Å) | n | m | χ 2 |
|---|---|---|---|---|---|
| 25 °C | |||||
| 0 | 517 ± 14 | 16.8 ± 0.6 | 3.4 ± 0.02 | 4.2 ± 0.04 | 1.9 |
| 2 | 388 ± 12 | 34.7 ± 0.9 | 4.0 ± 0.07 | 2.6 ± 0.05 | 1.5 |
| 5 | 381 ± 14 | 33.9 ± 0.9 | 3.9 ± 0.08 | 2.7 ± 0.05 | 1.2 |
| 10 | 377 ± 13 | 33.1 ± 0.8 | 3.9 ± 0.07 | 2.8 ± 0.05 | 1.3 |
| 40 °C | |||||
| 0 | 773 ± 35 | 17.9 ± 1.2 | 3.30 ± 0.02 | 3.1 ± 0.04 | 1.4 |
| 2 | 506 ± 27 | 32.0 ± 0.9 | 3.30 ± 0.07 | 2.7 ± 0.06 | 1.4 |
| 5 | 471 ± 28 | 32.4 ± 0.9 | 3.34 ± 0.08 | 2.8 ± 0.06 | 1.3 |
| 10 | 453 ± 22 | 31.5 ± 0.8 | 3.35 ± 0.07 | 3.0 ± 0.06 | 1.3 |
| 60 °C | |||||
| 0 | 764 ± 25 | 19.7 ± 1.6 | 3.5 ± 0.03 | 2.7 ± 0.03 | 1.2 |
| 2 | 457 ± 19 | 32.8 ± 0.9 | 3.5 ± 0.07 | 2.7 ± 0.07 | 1.5 |
| 5 | 453 ± 21 | 30.3 ± 0.7 | 3.5 ± 0.07 | 3.1 ± 0.06 | 1.3 |
| 10 | 453 ± 21 | 30.0 ± 0.7 | 3.4 ± 0.06 | 3.2 ± 0.07 | 1.3 |
LTA micellization in solution and the effect of Ca2+ ions
The Cryo-TEM measurements provided information on the size and morphology of the formed aggregates at LTA concentration of 2 mg mL−1 as shown in Fig. 2. The image suggests the formation of spherical structures with an average diameter of D ∼ 12 ± 4.1 nm in water (cf.Fig. 2A). The variations in the size distribution of LTA aggregates are shown in Fig. 2D. Due to its amphiphilicity, it is not surprising that LTA self-assembled into micelles at a concentration well about its reported CMC in aqueous solutions,5,6 although the LTA micelle morphology has not been visualised previously.With addition of CaCl2 (cf.Fig. 2B and C), a decrease in the size of aggregates to ∼10 nm is evident presumably due to the electrostatic interactions between the negative charges of phosphate groups and Ca2+ ions, leading to tighter packing of the chains in the shell region and the consequent shrinkage in the micelle size. The Ca2+ ions can induce charge neutralisation by bridging the phosphate groups of neighbouring LTA chains.24 The schematic in Fig. 2E illustrates the formation of LTA aggregates driven by the hydrophobic interactions among the hydrocarbon chains that formed the micelle core, while negatively charged hydrated polyglycerophosphate chains formed the shell. Earlier SAXS studies by Labischinski et al.11 and Fischer et al.12 also showed the formation of spherical micelles by LTA extracted from Staphylococcus aureus and Streptococcus pneumoniae, both with an average diameter of ∼23 nm but with the polymer chains adopting a highly coiled and a helical conformation, respectively, in the shell. Such structural assumptions about the coiled conformation of the polymer chain could be relevant to our study, considering the structural similarities in the LTA extracted from Bacillus subtilis and Staphylococcus aureus.
The microscopic evidence from the Cryo-TEM images in Fig. 2 on the formation of LTA micelles have not been reported previously. Pollack et al.25 investigated the Benzylpenicillin assisted secretion of vesicles from the peripheral cell exterior of Lactobacillus casei ATCC 7469 using Cryo-TEM. The results revealed vesicles formed by lipids and LTA mimicking the actual bacterial membrane compositions, although they formed distinct morphologies in the presence of different types of proteins. The balance between the electrostatic and steric forces between the LTA molecules and other membrane constituents, e.g. phospholipids and surface proteins, facilitates the formation of micelles or mixed vesicles. Our recent study13 reported LTA induced structural changes in the mixed vesicles formed by LTA and phospholipids in a mixture representing their molecular composition in the Bacillus subtilis bacteria membrane. The LTA incorporation in the mixed vesicles up to 6.5 mol% concentration increased their size. As more LTA was added to the mixture, steric repulsion between LTA molecules favoured their partial partition from the mixed liposomes, forming LTA micelles in coexistence with the mixed liposomes.
Nano-structural insights from small-angle neutron scattering (SANS) measurements
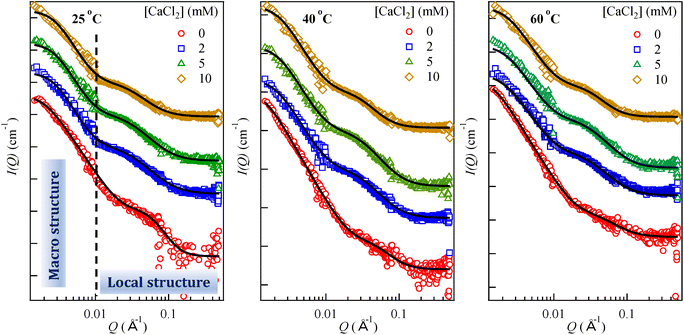
LTA aggregation behaviour in the presence of CaCl2 was further investigated by SANS. Fig. 3 shows the variations in scattering intensities for 2 mg mL−1 aqueous LTA solution in the presence of CaCl2 at three different temperatures (25, 40, and 60 °C). The scattering intensity decreased with the addition of CaCl2 up to 5 mM concentration and remains constant upon further increase to 10 mM (cf. Fig. S1(a) in ESI†). We attempted to fit this data using several models, e.g. the gel fit, the Guinier Porod approximation, the correlation length model etc. (cf. S1 in ESI†), but no physically relevant parameters could be achieved. Table 1 demonstrates fitted aggregation parameters using the two-Lorentzian model, which fits an empirical functional form to the SANS data characterised by two Lorentzian type functions. This model calculates two correlation lengths corresponding to the macro structure or clusters (low-q) and the local structure (high-q) as discussed below. With the presence of two main scattering regimes, it is conceivable that the high-q regime corresponds to the LTA molecules and the LTA aggregates account for the scattering in the low-q regime.The Lorentzian exponents n and m (eqn (2)) are related to the extent of LTA–water interactions and an increase in these values account for unfavourable solvent–biopolymer interactions.26 The calculated correlations lengths in the low-q scattering data are much higher than the reported sizes of nascent LTA micelles,11,12 which confirms the presence of clusters described by the correlation length (ξ1). The gradual down-turn (cf. Fig. S1(a) in ESI†) in the scattering intensity curve on increasing the CaCl2 concentration (0–10 mM) indicates the decrease in the size of the formed clusters.
Fig. 4 plots the fitted correlation lengths (ξ1 and ξ2) as a function of CaCl2 concentration at different temperatures. In the presence of 2 mM CaCl2, the ξ1 value decreased to 388 ± 12 Å from 517 ± 14 Å for LTA in water at 25 °C. Lorentzian exponent n (low-q) increased to 4.0 ± 0.07 from 3.4 ± 0.02, indicating poorer LTA–water interaction on CaCl2 addition, consistent with the formation of more compact and rigid structures due to the relatively long-lived salt-bridges between negatively charged phosphate centres of neighbouring LTA chains facilitated by Ca2+ ions.24 This high n value thus suggests stronger inter-LTA interactions compared to those between water and LTA. These results support the Cryo-TEM data for pure LTA (cf.Fig. 2) depicting polydisperse large aggregates that decreased in the number density and size upon addition of CaCl2. The ξ1 and n values are not sensitive to further increase in the CaCl2 concentration to 5 mM and 10 mM.
A previous SANS analysis27 on the aggregation of polyhedral oligomeric silsesquioxane dendrimers led to the interpretation of the presence of the structures with two length scales, attributed to global dendrimer aggregates and the intra-dendrimer local structure. In another report,28 SANS characterization of globular polyprotein hydrogels revealed the presence of two length-scales with the scattering centres attributed to the regions of clustered polyproteins. The low-q Lorentzian exponent values suggested surface fractals with a dimension in the range of 3 and 4, indicating rough surfaces of the scattering centres. Hammouda et al. also applied the correlation model29 and the two-Lorentzian model30 to their neutron scattering data from aqueous polyethyleneoxide (PEO) solutions to explain the clustering effect. Their results indicated an increase in the correlation length in the low-q region with increasing PEO concentration due to PEO cluster growth, while the value in the high-q region decreased, indicating shorter-range interactions. In another investigation, the microphase separation in the N,N-diethylacrylamide polymer hydrogels31 after temperature-jump was analysed using the two-Lorentzian model that alluded to the presence of two phases: a dense phase corresponding to the equilibrium structure at the final temperature and a dilute nonequilibrium phase showing swollen a network after the temperature jump from 25 to 30 °C.
The average aggregate size obtained by Cryo-TEM (cf.Fig. 2D) may be compared with ξ1. The correlation lengths (ξ1 and ξ2) are not the real size of aggregates but are indicative of the length scale of the spatial density/concentration fluctuations. For instance, for the semi-dilute polymer regime in hydrogels, the correlation length refers to the size of a “blob” or “mesh” of the polymer network. It may characterize the spatial correlation of concentration fluctuation in the system, i.e. the distance between entanglement points in the three-dimensional aggregate assemblies.26 The calculated correlation length in the high-q region (ξ2) for pure LTA aggregates is 16.8 ± 0.6 Å, while the Lorentzian exponent m is 4.2 ± 0.04. The former represents the dimension of a thermal blob originating from the fluid nature of the local concentration fluctuation in a semi-dilute polymer solution.32 The fitted value for ξ2 is much smaller than the molecular length of fully hydrated LTA molecule (∼11 nm). Earlier SAXS investigations on aqueous aggregates of LTAs from S aureus11 and S. Pneumoniae12 calculated the radius of the spherical micelles ∼11 nm (comparable to the size of fully extended LTA molecule), which indicates that the observed ξ2 values are not correlated with the length of single LTA molecules. The structure of B. subtilis LTA studied here is similar to S. aureus LTA.33 The small ξ2 value arises from the intra-LTA local structure, instead of the free LTA molecules. With addition of 2 mM CaCl2, the m values decrease (4.2 ± 0.04 for 0 mM CaCl2 to 2.6 ± 0.05). On the other hand, ξ2 increased to 34.7 ± 0.9 Å from an initial value of 16.8 ± 0.6 Å in water, which indicates an expansion of the polymer. Thus, ξ2 represents the short-range interactions involving LTA. Further increase of the concentration to 10 mM CaCl2 led to only minor changes in the fitted parameters, suggesting relative insensitivity of the LTA local structure above 2 mM CaCl2.
Effect of temperature on LTA self-assembly
The SANS experiments were also performed at 40 and 60 °C. The higher temperature would promote the thermal motion and affect the solubility of the lipophilic chains in the LTA molecule and the mean molecular area.13 With an increase in temperature, the scattering intensity in the low-q region increased considerably, which indicates formation of large aggregates. In pure water, the fitted ξ1 increased to 773 ± 34 Å at 40 °C from 517 ± 14 Å at 25 °C, and then decreased marginally at 60 °C. The corresponding n value remained almost constant at ∼3.4, indicating no further change in the LTA–water interactions up to 60 °C. The H-bonding formation could be disrupted at the higher temperature, which should result in reduced interactions between the polyglycerophopshate chains and water molecules. This should promote the hydrophobic interactions and favour the clustering of micelles. To counterbalance this, the increase in the temperature would also promote the fluidity of the LTA lipophilic chains, leading to a more open local structure of the polar chains. This is borne out in the trend of the increase in ξ1 and decrease in m with temperature in water (i.e. ξ1 = 16.8 ± 0.6 Å, 17.9 ± 1.2 Å, and 19.7 ± 1.6 Å and m = 4.2 ± 0.04, 3.1 ± 0.04, and 2.7 ± 0.03 at 25 °C, 40 °C, and 60 °C, respectively). The marginal decrease in the ξ1 value at 60 °C could also be attributed to the delicate balance between dehydration and chain fluidization, which led to more compact packing of the micelles due to dehydration of polar chains and the overall slightly smaller cluster size compared to 40 °C.However, the trends of the ξ2, n and m values with the temperature are not clear-cut in the presence of different CaCl2 concentrations of 2, 5 and 10 mM (cf.Table 1). Nonetheless, the decrease in ξ1 with temperature under all the CaCl2 concentrations indicates more compact cluster size, attributed to neutralisation and bridging of LTA phosphates by Ca2+. The ξ2 values at 40 and 60 °C were also consistently larger compared to those at 25 °C under all the CaCl2 concentrations, indicative of a more open network structure due to enhanced chain fluidity, whilst the increasing Ca2+ concentration again caused ξ2 to decrease slightly at all the temperatures. Similarly, the low-q Lorentzian exponent n values were consistently smaller at 40 °C and 60 °C as compared to 25 °C, which is particularly pronounced in the presence of Ca2+; whilst the high-q Lorentzian exponent m value remained slightly changed (2.6–3.2) at all temperatures in the presence of Ca2+, with the exception of m ∼ 4.2 in pure water at 25 °C indicating poorer water–LTA interactions. These structural changes in response to temperature and Ca2+, as revealed by SANS, point to the intricate interplays between hydration and chain flexibility which are made more complex by temperature and divalent cations.
Interfacial adsorption behaviour of LTA aggregates
Control AFM measurement using 2 mg mL−1 LTA aggregates indicated little adsorption at the mica–water interface (featureless images not shown), which may be attributed to the highly negatively charged mica surface repelling similar charged LTA,34,35 pointing to the importance of the electric double layer interactions in LTA surface adsorption. With the addition of 10 mM CaCl2, the adsorption of the LTA aggregates on the mica surface increased due to neutralisation and likely overcompensation of the negative LTA charges by Ca2+ (cf. Fig. S2(a) and S2(b) in ESI†). Redeker and Briscoe15 similarly reported enhanced adsorption of negatively charged lipopolysaccharides (LPS) on mica in the presence of Ca2+, which was attributed to the bridging effect between LPS molecules and between LPS and mica.Further AFM imaging was attempted on the SiO2 substrate in the presence of CaCl2 to correlate with the QCM-D results. To compare the effect of lower LTA concentration and establish a correlation with the QCM-D experiments (discussed later, cf. Fig. 7), AFM images of 0.2 mg mL−1 LTA aggregates adsorbed on SiO2 substrates are shown in Fig. 5. In pure water, limited adsorption of LTA aggregates was observed (Fig. 5A and B) at 25 and 40 °C, and the LTA aggregates also appeared to be easily removed by the scanning AFM tip during the measurements, indicating weak adsorption at the interface due to strong repulsion minimising the contacts between the negatively charged LTA glycerophosphate chains and the SiO2 surface. At 40 °C, the presence of a few large aggregates is evident, confirming the temperature assisted clustering as observed at higher LTA concentrations.
 | ||
| Fig. 5 AFM images showing the topography of 0.2 mg mL−1 LTA aggregates adsorbed at the SiO2–water interface in pure water at (A) 25 °C; (B) 40 °C, and 10 mM CaCl2 at (C) 25 °C; (D) 40 °C. | ||
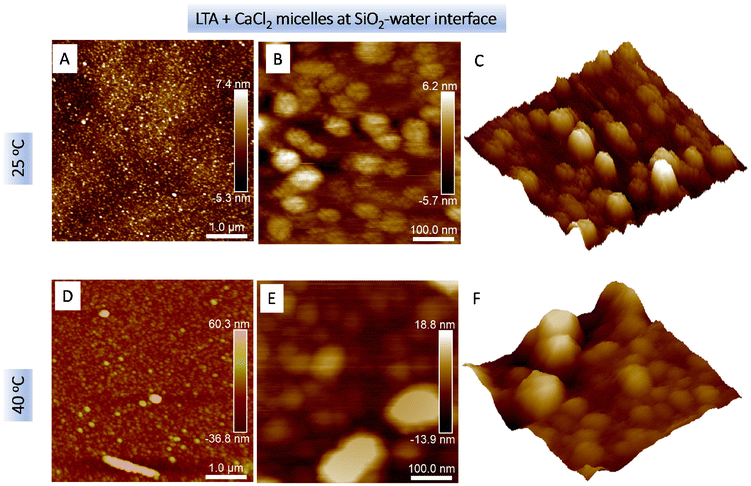
Due to the limited resolution of these images, the lateral sizes could not be estimated precisely. The LTA aggregates shown in Fig. 5(B) indicated the presence of two size distributions around 8–10 nm and ∼30 nm. As shown in Fig. 5C and D, the addition of 10 mM CaCl2 greatly promoted LTA aggregate adsorption, with spherical structures evident on the surface. At 25 °C, the calculated average size of these structures was 29.6 ± 5.2 nm. Interestingly, the average size decreased to 16.0 ± 2.1 nm at 40 °C indicating shrinking of the aggregates.
Fig. 6 shows the topography of 2 mg mL−1 LTA aqueous aggregates in the presence of 10 mM CaCl2 at the SiO2–water interface at 25 °C (Fig. 6A–C) and 40 °C (Fig. 6D–F). The calculated Z-axis height h of these adsorbed structures at the SiO2–water interface was 6.5 nm at 25 °C, which increased to 9.2 nm at 40 °C. Fig. 6A reveals a dense coverage by spherical LTA aggregates within the scan area of 5 μm × 5 μm at 25 °C. On increasing the incubation temperature to 40 °C, the surface coverage by the LTA aggregates increased and further adsorbed layers of the spherical structures developed (cf.Fig. 6D). The calculated lateral diameter of the spherical LTA aggregates increased from 46 ± 10 nm at 25 °C to 77 ± 20 nm at 40 °C. These values were considerably higher than those obtained by the Cryo-TEM measurements (cf.Fig. 2). Thus, the large aggregates adsorbed at the interface were attributed to clustering of LTA micelles consistent with the SANS analysis. Higher resolution scans (cf.Fig. 6B and C) further revealed the “raspberry” structure of the surface aggregates, showing clusters by 3–6 nascent LTA micelles, thus, pointing to ∼10–102 micelles per cluster, presumably formed due to stronger inter-aggregate interactions due to a combination of inter-micelle H-bonding and Ca2+ bridging. Fig. S2(c) in the ESI section† shows the raspberry structure observed from three different sizes. AFM imaging at 40 °C (cf. Fig. S2(d) and (e)†) showed the presence of elongated structures and the presence of larger spherical structures, which indicated inhomogeneous clustering of micelles due to a decrease in the hydration around LTA aggregates and an increased chain fluidity of the hydrocarbon chain that results in larger area occupied by the LTA molecules. The 2 mg mL−1 LTA solution contained a large number of LTA micelles existing at smaller inter-micellar distances. By comparison, at 0.2 mg mL−1 LTA concentration, aggregates were less susceptible to clustering due to considerably lower micellar density and larger inter micellar distances.
These results are broadly in agreement with the SANS data that showed an increase in the size of the LTA aggregates on increasing the temperature to 40 °C. Fig. 6E shows an example of the presence of a considerably larger non-spherical aggregate (cf. also Fig. S2(d) and (e)†). Fig. 6F shows the presence of clusters although the individual LTA micelles constituting the clusters were not resolved, possibly due to enhanced fluidity of the micelle–water interface at the elevated temperature. These observations are consistent with the SANS analysis indicating the presence of two correlation lengths describing the presence of clusters in the low-q region.
QCM-D investigations on LTA aggregates adsorbed on SiO2 substrate
Fig. 7 shows the resonant frequency shift Δf and the dissipation shift ΔD as a function of deposition time of 0.2 mg mL−1 LTA in Milli-Q water and in 10 mM CaCl2 aqueous solution. The frequency change Δf (cf.Fig. 7A) reflects the amount of LTA adhering to the SiO2 surface, and the dissipation ΔD (cf.Fig. 7B) informs on the morphology of the adsorbed layer. In pure water, the Δf and ΔD values changed only slightly, suggesting negligible adsorption, consistent with the AFM observations above. Upon rinsing with 10 mM CaCl2, a decrease in Δf and an increase in ΔD was observed due to the adsorption of divalent Ca2+ ions on the negatively charged SiO2 crystals sites. Furthermore, it is evident that the addition of 10 mM CaCl2 imparted a significant effect on the adsorption behaviour of LTA aggregates. The frequency decreased initially as a result of LTA adsorption, and then increased towards a plateau value, indicative of LTA desorption upon rinsing with the pure water. By applying the Voigt viscoelastic model, the final mass (mfin) and the final thickness (tfin) were determined. The final frequency change (Δffin = −24.0 Hz) corresponds to an adsorbed mfin of 420.9 ng cm−2 and the tfin of the adsorbed LTA layer was estimated to be 3.2 nm assuming the adsorbed LTA layers had a density of 1.3 g cm−3. These results are in good agreement with the AFM images (cf.Fig. 6), indicating Ca2+ promoted the adsorption of the LTA aggregates at the SiO2–water interface. The estimated layer thickness ∼3.2 nm points to deformed or compacted LTA aggregates due to the shrinking of the polar chains caused by the salt-bridge formation that also facilitates the adsorption at the charged SiO2 surface. It is conceivable that LTA adsorbed as isolated clusters rather than a homogenous film, which would affect the accuracy of QCM analyses. Nonetheless, the QCM results concurred with the AFM imaging that the Ca2+ addition promoted LTA adsorption on the negatively charged SiO2 surface.Conclusions
Self-assembly and adsorption behaviour of lipoteichoic acid (LTA), a polymeric amphiphile extracted from Bacillus subtilis, has been investigated in the presence of CaCl2 (2, 5 and 10 mM) and at different temperatures (25, 40, and 60 °C). The nanostructures from self-assembly and hierarchical clustering of the simple model LTA systems on different length scales are complex. There is not a single definitive technique that could probe unequivocally the structures on all length scales. This serves to demonstrate the value in undertaking fundamental studies of LTA self-assembly using complementary physicochemical methods. The Cryo-TEM images revealed formation of spherical micelles (D ∼ 12 nm) that decreased in size upon addition of CaCl2. The electrostatic charge neutralisation at the phosphate-decorated surface of LTA micelles and the formation of inter-LTA salt-bridges resulted in the shrinking of the micelle shell. The analysis of the SANS data using the two-Lorentzian model indicated the presence of two correlation lengths for, respectively, the clusters (ξ1, low-q) and the local structures due to intra- and inter-LTA correlations (ξ2, high-q). These two correlations gave insights into the LTA aggregate structure which depended sensitively on the interplays of the polyglycerophosphate chain flexibility and hydration (or H-bonding) capacity, both affected by both temperature and the presence of divalent Ca2+. The decrease in ξ1 on the addition of CaCl2 can be attributed to the formation of stable and long-lived salt-bridges between the negatively charged phosphate groups (both intra- and inter-chain). The corresponding increase in the Lorentzian exponent n (low-q) points to the decreased interactions between water and LTA. On the other hand, the ξ2 values increased with Ca2+ addition, which indicates an increase in the intra-LTA correlations. With an increase in the temperature, the LTA clusters grew in size due to the enhanced hydrophobic interactions from dehydration between nascent LTA micelles, possibly overcompensating for the steric interactions arising from enhanced LTA chain fluidity. Our results on the effect of the Ca2+ addition suggest that the hydrated polyglycerophopshate chains likely adopt a relatively extended conformation in the LTA micellar shell in water. Loose aggregates may form due to interpenetration of the chains between neighbouring micelles. With the addition of Ca2+, the resultant neutralization or bridging leads to more compact micellar structures.AFM imaging revealed an increase in the surface LTA coverage at the SiO2–water interface at 40 °C and particularly in the presence of Ca2+ which acts to bridge the negatively charged LTA and SiO2. The LTA adsorption coverage and morphology depended on the concentration of both LTA and Ca2+. In 2 mg mL−1 LTA, further adsorption of the LTA aggregates atop the underlying layer was evident and the raspberry-like clusters with ∼10–100 LTA micelles were also observed in 10 mM Ca2+ at 25 °C. The QCM-D results were consistent with the AFM results, showing a decrease in the resonant frequency Δf and an increase in the dissipation ΔD in the presence of 10 mM CaCl2, which indicates a significant adsorption at the SiO2–water interface. These unprecedented results point to the intricate and complex interplays between LTA and water molecules, sensitively dependent on divalent cations and solution temperature, that underpin self-assembly of Bacillus subtilis LTA in solution and at the solid–water interface. Such fundamental insights are relevant to our understanding of LTA-mediated bacterial adhesion, colonisation, and sepsis. In a further publication, we will discuss how the structure of the LTA monolayer at the air–water interface can be affected by the presence of mono-/di-/tri-valent cations at different temperatures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the Institut Laue-Langevin (ILL) for the awarded beamtime under experiment number: 9-13-912 [https://doi.ill.fr/10.5291/ILL-DATA.9-13-912]. We thank Lauren Matthews and Xueying Guo for their help with the SANS measurements and useful discussions. We also acknowledge the Wolfson Foundation for establishing the Wolfson Bioimaging Facility, University of Bristol. This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement SoftNanoHybrid no. 797038 (Bhavesh Bharatiya) and grant agreement HYDRA no. 793574 (Gang Wang). WHB would like to acknowledge funding from Engineering and Physical Science Research Council (EPSRC; EP/H034862/1 and EP/H014861/1). MW and PW acknowledge support from the Ministerstwo Edukacji i Nauki via the statutory subsidy for Jerzy Haber Institute of Catalysis and Surface Chemistry PAS.References
- I. Ginsburg, Lancet Infect. Dis., 2002, 2(3), 171–179 CrossRef CAS PubMed.
- I. Fedtke, D. Mader, T. Kohler, H. Moll, G. Nicholson, R. Biswas, K. Henseler, F. Götz, U. Zähringer and A. Peschel, Mol. Microbiol., 2007, 65(4), 1078–1091 CrossRef CAS PubMed.
- M. Gross, S. E. Cramton, F. Götz and A. Peschel, Infect. Immun., 2001, 69(5), 3423–3426 CrossRef CAS PubMed.
- C. Weidenmaier, J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond and A. Peschel, Nat. Med., 2004, 10(3), 243–245 CrossRef CAS PubMed.
- A. J. Wicken, J. D. Evans and K. W. Knox, J. Bacteriol., 1986, 166(1), 72–77 CrossRef CAS PubMed.
- H. S. Courtney, W. A. Simpson and E. H. Beachey, Infect. Immun., 1986, 51(2), 414–418 CrossRef CAS PubMed.
- A. Helenius and K. Simons, Biochim. Biophys. Acta, Biomembr., 1975, 415(1), 29–79 CrossRef CAS.
- C. Tanford, Science, 1978, 200(4345), 1012–1018 CrossRef CAS PubMed.
- J. L. Markham, K. W. Knox, A. J. Wicken and M. Hewett, Infect. Immun., 1975, 12(2), 378–386 CrossRef CAS PubMed.
- P. Harrop, R. O'Grady, K. W. Knox and A. J. Wicken, J. Periodontal Res., 1980, 15(5), 492–501 CrossRef CAS PubMed.
- H. Labischinski, D. Naumann and W. Fischer, Eur. J. Biochem., 1991, 202(3), 1269–1274 CrossRef CAS PubMed.
- W. Fischer, S. Markwitz and H. Labischinski, Eur. J. Biochem., 1997, 244(3), 913–917 CrossRef CAS PubMed.
- B. Bharatiya, G. Wang, S. E. Rogers, J. S. Pedersen, S. Mann and W. H. Briscoe, Colloids Surf., B, 2021, 199, 111551 CrossRef CAS PubMed.
- A. Tripathy, P. Sen, B. Su and W. H. Briscoe, Adv. Colloid Interface Sci., 2017, 248, 85–104 CrossRef CAS PubMed.
- C. Redeker and W. H. Briscoe, Langmuir, 2019, 35(48), 15739–15750 CrossRef CAS PubMed.
- G. Sauerbrey, Z. Phys., 1959, 155(2), 206–222 CrossRef CAS.
- F. Höök, M. Rodahl, P. Brzezinski and B. Kasemo, Langmuir, 1998, 14(4), 729–734 CrossRef.
- M. Rodahl, F. Höök, A. Krozer, P. Brzezinski and B. Kasemo, Rev. Sci. Instrum., 1995, 66(7), 3924–3930 CrossRef CAS.
- M. V. Voinova, M. Rodahl, M. Jonson and B. Kasemo, Phys. Scr., 1999, 59(5), 391 CrossRef CAS.
- K. Lieutenant, P. Lindner and R. Gahler, J. Appl. Crystallogr., 2007, 40(6), 1056–1063 CrossRef CAS.
- https://www.ill.eu/users/instruments/instruments-list/d11/description/instrument-layout .
- B. Bharatiya, G. Wang and W. H. Briscoe, et al., Mixed liposomes containing bacterial lipoteichoic acid (LTA), Institut Laue-Langevin (ILL), 2020, DOI:10.5291/ill-data.9-13-912.
- https://www.sasview.org/docs/user/models/two_lorentzian.html .
- M. Sammalkorpi, M. Karttunen and M. Haataja, J. Phys. Chem. B, 2009, 113(17), 5863–5870 CrossRef CAS PubMed.
- J. H. Pollack, A. S. Ntamere and F. C. Neuhaus, Microbiology, 1992, 138(5), 849–859 CrossRef CAS PubMed.
- F. Horkay and B. Hammouda, Colloid Polym. Sci., 2008, 286(6), 611–620 CrossRef CAS.
- G. Yuan, X. Wang, D. Wu and B. Hammouda, Polymer, 2016, 100, 119–125 CrossRef CAS.
- M. A. Da Silva, S. Lenton, M. Hughes, D. J. Brockwell and L. Dougan, Biomacromolecules, 2017, 18(2), 636–646 CrossRef CAS PubMed.
- B. Hammouda, D. L. Ho and S. Kline, Macromolecules, 2004, 37(18), 6932–6937 CrossRef CAS.
- B. Hammouda, D. Ho and S. Kline, Macromolecules, 2002, 35(22), 8578–8585 CrossRef.
- J. Pleštil, M. Ilavský, H. Pospíšil, D. Hlavatá, Y. M. Ostanevich, G. Degovics, M. Kriechbaum and P. Laggner, Polymer, 1993, 34(23), 4846–4851 CrossRef.
- K. J. Lee and S. I. Yun, Polymer, 2018, 139, 86–97 CrossRef CAS.
- N. T. Reichmann and A. Gründling, FEMS Microbiol. Lett., 2011, 319(2), 97–105 CrossRef CAS PubMed.
- J. N. Israelachvili and G. E. Adams, J. Chem. Soc., Faraday Trans., 1978, 74, 975–1001 RSC.
- J. N. Israelachvili, Intermolecular and surface forces, Academic Press, London, 2nd edn, 1992 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nr00595f |
| This journal is © The Royal Society of Chemistry 2022 |