 Open Access Article
Open Access ArticleFerroelectric bismuth-titanate nanoplatelets and nanowires with a new crystal structure†
Darko
Makovec
 *ab,
Nina
Križaj
ab,
Anton
Meden
c,
Goran
Dražić
*ab,
Nina
Križaj
ab,
Anton
Meden
c,
Goran
Dražić
 d,
Hana
Uršič
d,
Hana
Uršič
 e,
Rok
Kostanjšek
e,
Rok
Kostanjšek
 f,
Martin
Šala
f,
Martin
Šala
 d and
Sašo
Gyergyek
d and
Sašo
Gyergyek
 a
a
aDepartment for Materials Synthesis, Jožef Stefan Institute, Jamova 39, SI-1000 Ljubljana, Slovenia. E-mail: darko.makovec@ijs.si
bJožef Stefan International Postgraduate School, Jamova 39, SI-1000 Ljubljana, Slovenia
cFaculty of Chemistry and Chemical Technology, University of Ljubljana, Večna pot 113, SI-1000 Ljubljana, Slovenia
dNational Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia
eDepartment for Electronic Ceramics, Jožef Stefan Institute, Jamova 39, 1000 Ljubljana, Slovenia
fDepartment of Biology, Biotechnical Faculty, University of Ljubljana, Jamnikarjeva 101, SI-1000 Ljubljana, Slovenia
First published on 14th February 2022
Abstract
Two different morphologies of ferroelectric bismuth titanate (Bi4Ti3O12) nanoparticles, i.e., nanoplatelets and nanowires, were synthesized by changing the concentration of NaOH during a hydrothermal treatment of precipitated Ti4+ and Bi3+ ions. The nanoparticles’ crystal structures were characterized using atomic-resolution imaging with a CS-probe-corrected scanning-transmission electron microscope in combination with X-ray diffractometry and Raman spectroscopy. The nanoplatelets (10 nm thick and from 50 nm to 200 nm wide) exhibit the Aurivillius-type layered-perovskite crystal structure that is characteristic of Bi4Ti3O12, whereas the nanowires (from 15 nm to 35 nm wide and from several hundreds of nm to several μm long) exhibit an entirely new structure with an orthorhombic unit cell (a = 3.804(1) Å, b = 11.816(3) Å, and c = 9.704(1) Å). The nanowire structure is composed of two structural layers alternating along the orthorhombic c-direction: a structural layer composed of two parallel layers of Bi atoms that resembles the (Bi2O2)2+ layer of the Aurivillius structure, and a structural layer composed of two parallel layers of Ti atoms, where every sixth Ti is replaced with Bi. Observations of the ferroelectric domains with transmission electron and piezo-response force microscopy indicated the ferroelectric nature of both nanostructures. The nanowire structure is a metastable polymorph of the bismuth titanate stabilized at the nanoscale. With annealing at temperatures above 500 °C the nanowire structure topotactically transforms into the Aurivillius structure.
Introduction
Bismuth titanate (Bi4Ti3O12, BIT) is the most studied and exploited member of a large family of ferroelectric Aurivillius mixed oxides that are characterized by a layered structure composed of bismuth-oxide layers alternating with perovskite-like layers. The BIT structure can therefore be described in terms of two layers: a (Bi2O2)2+ layer and a perovskite (Bi2Ti3O10)2− layer stacked along the pseudo-tetragonal c-axis. Below the Curie temperature (TC ∼ 675 °C) BIT adopts the monoclinic P1a1 unit cell.1,2 The ferroelectric BIT exhibits a modest spontaneous polarization with the larger component of ∼50 μC cm−2 oriented along the a-direction and the smaller component of ∼4 μC cm−2 along the c-direction, a high coercive field, and an excellent fatigue resistance. As a bulk material, BIT is promising for ferroelectric random-access-memory devices and lead-free, high-temperature piezoelectric and pyroelectric devices.3,4 Also, in its nano form, BIT is becoming increasingly important for different applications, including nanogenerators for biomechanical energy harvesting,5 sensing,6,7 visible-light photocatalysis,8–10 electrocatalysis,7 and piezocatalysis.11,12BIT nanoparticles can be synthesized using a simple hydrothermal treatment of precipitated Bi3+ and Ti4+ ions in an aqueous alkali hydroxide at moderate concentrations.6–9,11–17 Because of their layered structure the bismuth titanate nanoparticles tend to grow predominantly in the a/b plane to form 2-D rectangular platelet crystals, i.e., nanoplatelets and nanosheets, with the large surfaces parallel to the (001) crystal planes.7,8,13,14,16,17 However, numerous other nanostructures including 1-D nanocrystals (e.g., nanowires, nanobelts, nanobundles, nanorods),7,8,15 and 3-D nanostructures assembled from 1-D or 2-D nanoparticles,6,9,11,12,15,16 were synthesized using the hydrothermal method. The morphology of the BIT nanoparticles can be controlled by selecting the synthesis conditions, e.g., source of titania, type of mineralizer, reactant concentrations, reaction temperature and time, and use of surfactants.7,8,15,17
In this investigation we synthesized BIT nanoplatelets (NPLs) and nanowires (NWs) just by changing the concentration of the NaOH during the hydrothermal treatment of precipitated Bi3+ and Ti4+ ions. Their characterization showed that both nanostructures are ferroelectric, but they differ in their crystal structure: the NPLs exhibit the Aurivillius-type structure characteristic for BIT, whereas the NWs exhibit an entirely new layered structure, not described before.
Experimental
Bismuth-titanate nanoparticles were synthesized with a hydrothermal treatment of separately precipitated Ti4+ and Bi3+ ions. In a typical experiment, 1.0 g of titanium butoxide was dissolved in 20 mL of tert-butanol. The Ti4+ ions were precipitated with the addition of 40 mL of water under vigorous stirring. The white precipitate was separated with centrifugation and washed two times with water and finally with NaOH at the same concentration as used for the subsequent hydrothermal treatment (0.5 mol L−1 or 2 mol L−1). In parallel, a stoichiometric amount of Bi(NO3)3 (Bi/Ti = 4/3) was dissolved in diluted HNO3 (0.68 wt%). The Bi3+ ions were precipitated with the addition of aqueous ammonia (0.94 wt%) under vigorous stirring to set the final pH to ∼8. The white precipitate was separated with centrifugation and washed two times with water and finally with NaOH. After washing with NaOH, the colour of the precipitate turned yellow. Finally, both precipitates were quantitatively transferred to a 70 mL Teflon-lined autoclave and filled with a NaOH aqueous solution (0.5 mol L−1 or 2 mol L−1) to a final volume of 45 mL. The autoclave was closed and heated for 38 hours at 200 °C. The white product was thoroughly washed with water and dried at 60 °C. The materials used are listed in the ESI.†The BIT nanostructures were characterized with a transmission electron microscope (TEM; Jeol 2010F) and a CS-probe-corrected scanning-transmission electron microscope (STEM; Jeol ARM 200CF). X-ray powder diffraction (XRD) patterns were recorded with a PANalytical X'Pert Pro MPD diffractometer. The Raman spectra of the samples were recorded with a NT-MDT model Integra Spectra for Materials Science equipped with a confocal microscope. The chemical compositions of the samples were measured with inductively coupled plasma (ICP-OES) using a Varian 715-ES ICP optical emission spectrometer after lithium metaborate fusion digestion. The topography and piezo-response force microscopy (PFM) images were recorded using an atomic force microscope (AFM; Jupiter XR, Asylum Research, CA). Details of the characterization methods are given in the ESI.†
Results
Morpho-structural properties
The hydrothermal treatment of the mixture of Bi and Ti precipitates resulted in the formation of BIT in the form of NPLs at the higher NaOH concentration of 2 mol L−1, whereas the NWs grew at the lower NaOH concentration of 0.5 mol L−1, as evident from the TEM images in Fig. 1(a) and (b). The NPLs were approximately 10 nm thick and from 50 nm to 200 nm wide, whereas the NWs were from 15 nm to 35 nm wide and from several hundreds of nm to several μm long. Quantification of the energy-dispersive X-ray spectra (EDXS) taken from the NPLs and the NWs in the TEM showed similar compositions, with the Bi/Ti atomic ratio being 1.3 ± 0.1 and 1.2 ± 0.1 for the NPLs and the NWs, respectively, matching the composition of the Bi4Ti3O12. Sodium, which can be incorporated into the nanoparticles due to the synthesis in NaOH, was not detected.The compositions of the two morphologies were also confirmed with ICP-OES. The Bi/Ti atomic ratio was close to the theoretical value for Bi4Ti3O12 of 1.33. The measurement showed Bi/Ti = 1.34 for the NPLs and Bi/Ti = 1.40 for the NWs. The concentration of Na in the samples with NPLs and NWs was low, i.e., 0.4 wt% and 0.1 wt%, respectively. Here, it should be noted that we cannot exclude the presence of a small quantity of secondary phases in the analysed samples.
The XRD pattern of the NPLs (Fig. 1(c)) showed broad peaks that match with the Aurivillius (AU) Bi4Ti3O12 phase. However, the XRD of the nanowires is completely different. A comprehensive search showed no match with the XRD data for any known phase, suggesting a new crystal structure. A similar XRD pattern was previously reported for BIT nanowires, which the authors ascribed to a mixture of Bi2O3, TiO2, and K2Ti6O13.17 However, electron diffraction and atomic-resolution imaging with a STEM (discussed below) showed that the material contains just one phase, having an orthorhombic unit cell with the approximate dimensions a ∼ 3.8 Å, b ∼ 12.0 Å, c ∼ 9.5 Å. Based on this information it was possible to find an unit cell that gave a satisfactory LeBail fit to the observed powder pattern (see Fig. SI1 in the ESI†).18 The refined unit-cell parameters were a = 3.804(1) Å, b = 11.816(3) Å, and c = 9.704(1) Å.
The Raman spectroscopy also revealed significant differences in the structure of the two morphologies of bismuth titanate. The bands of internal vibrational modes of the TiO6 octahedron occur for the NWs at significantly higher frequencies than for the NPLs (Fig. SI2†). The difference in the position of bands is most likely related to the difference in the TiO6 octahedra's connectivity, which is corner sharing in the NPLs and edge sharing in the NWs (see the ESI† for details).19,20
Atomic-resolution STEM imaging
Differences in the crystal structures of the two bismuth-titanate morphologies were revealed using direct atomic-resolution STEM imaging. Special care was taken during the STEM imaging to avoid excessive degradation of the nanostructures under the electron beam (see the ESI† for details). However, evaporation of the bismuth could not be entirely avoided. The STEM images clearly show the main characteristics of the two different structures. Fig. 2(a) and (b) show STEM images of the NPLs oriented along the [001]AU direction and the [110]AU direction, respectively.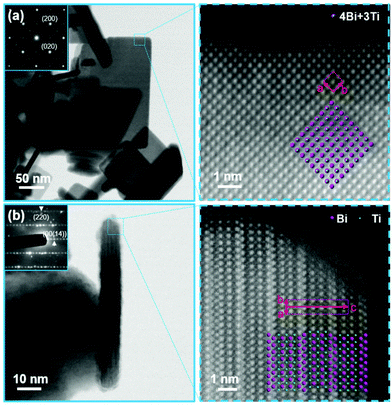 | ||
| Fig. 2 BF STEM images (insets: corresponding electron-diffraction patterns) and HAADF STEM images of bismuth-titanate NPLs oriented along [001]AU direction (a) and [110]AU direction (b). The projected structural models are superimposed over the images to illustrate the positions of the Bi3+ and Ti4+ ions. The unit cell of the BIT Aurivillius structure is marked with red rectangles on the HAADF images.1 | ||
The atomic-resolution high-angle annular dark-field (HAADF) STEM images of the NPLs clearly correspond to the layered, Aurivillius-type (AU) structure of Bi4Ti3O12. Since the intensity of the individual atomic column in the “Z-contrast” HAADF images depends on the column's average atomic number Z (∼Zα with α slightly lower than 2)21 the positions of the various columns in the BIT AU structure can be identified by their brightness. Generally, the columns containing the heavier Bi3+ ions (Z = 83) appear much brighter than the columns containing only the Ti4+ ions (Z = 22), while the O2− (Z = 6) columns are too light to be detected in the HAADF images. Along the [001]AU orientation the brightness of the spots in the HAADF image (Fig. 2(a)) remains constant throughout the image because all the cationic columns have the same composition (4Bi + 3Ti). When the NPL is oriented along the [110]AU direction, we can clearly distinguish the columns that contain the Bi atoms from the much weaker Ti columns (Fig. 2(b)). The two alternating structural layers, i.e., a (Bi2O2)2+ layer and a (Bi2Ti3O10)2− perovskite-like layer, can be clearly resolved (Fig. 2(b)). Interestingly, the NPLs always terminate at the large (001) surfaces with the (Bi2O2)2+ structural layer. The nanoparticles with layered structures, such as the Aurivillius-type structure, normally adopt a specific structure defined by a termination at surfaces with a specific, low-energy atomic layer.22
The structure of the NWs is very different to that of the NPLs. An analysis of the electron-diffraction patterns suggested an orthorhombic unit cell. Along the [001]NW direction, the electron-diffraction pattern (Fig. 3(b)) consists of stronger reflections that match with the pattern of the Aurivillius structure in the [001]AU zone axis (see inset of Fig. 2(a)) and the weaker, superstructural reflections in the direction perpendicular to the NW. The a-direction of the new structure was set along the length of the NW with the a-parameter of the unit cell being ∼3.8 Å. Interestingly, the superstructural reflections are not periodic, as evident from the corresponding intensity profile in Fig. 3(c). They consist of groups composed of the primary reflections (marked with red arrows in Fig. 3(c)) with a set of weaker satellite reflections characteristic of a multiple diffraction (marked with lines of different colours). The superstructural reflections originate from the structural modulation clearly visible in the corresponding HAADF image (Fig. 3(d)). In the thinner part, near the rim of the NW, every sixth row of spots along the [010]NW direction is much stronger (marked on Fig. 3(d) with red arrows), suggesting a larger proportion of Bi atoms. The b-parameter of the unit cell was measured to be ∼12 Å. In the thicker part of the NW, usually two rows have a stronger intensity (marked with blue arrows), or the stronger intensity is distributed over three rows (marked with green arrows). This uneven contrast is a consequence of the stacking faults, i.e., antiphase boundaries, lying perpendicular to the electron beam (visible in the HAADF images taken along the [100]NW direction, see below).
 | ||
| Fig. 3 TEM image (a), electron-diffraction pattern (b) and HAADF STEM image (d) of a bismuth-titanate NW oriented along [001]NW direction. Fig. 3(c) shows an intensity profile across the row of reflections marked on (c) with a yellow rectangle. The projected model proposed for the NW structure is superimposed over the image (d) to illustrate the positions of the Bi3+ and Ti4+ ions. The unit cell of the NW structure is marked with a red rectangle on the HAADF image (d). | ||
The HAADF image of the NW oriented along the [010]NW direction (Fig. 4(c)) shows a layered structure with two cationic layers alternating along the c-direction: a layer composed of two parallel rows of brighter spots (marked in Fig. 4(c) with “B”), which resembles the (Bi2O2)2+ layer from the Aurivillius structure (see Fig. 2(b) for the comparison), and a layer of two parallel rows of weaker spots (marked with “T”). One row of the Ti atoms in the T layer is brighter than the other, suggesting that it contains a proportion of the Bi atoms. The c-parameter of the unit cell was found to be ∼9.5 Å.
For the STEM imaging of the structure along the [100]NW direction (i.e., along the nanowire) the NWs were incorporated into a polymer and cut with an ultramicrotome (see the ESI† for details). Also in the [100]NW zone, the two cationic layers alternating along the c-direction, i.e., the B layer and the T layer, are clearly resolved in the HAADF images (Fig. 5(a) and (b)). At the (001) surfaces the NW structure always terminates at the B layers. In a zig-zag arrangement of the two rows of spots representing the T layer every sixth weak spot is replaced by a much brighter spot, consistent with the exchange of Ti atoms for Bi atoms. As can be expected from the non-periodic weak reflections in the diffraction pattern of the [001]NW zone axis (Fig. 3(c)), stacking faults are frequently observed in the (010) planes. Due to the random and partial insertion of extra layers of Ti4+/Bi3+ ions, the periodicity of the brighter spots in the T layers locally change to 7, i.e., locally, every seventh weaker spot is exchanged with a brighter spot (indicated by the blue arrows in Fig. 5(b) and Fig. 6).
Based on HAADF STEM images taken along the three axes we propose a tentative arrangement of the cations in the orthorhombic unit cell of NW structure (Fig. 6). This tentative model of the cation sublattice was superimposed over the HAADF images to illustrate the positions of Bi3+ and Ti4+ ions along all three zone axes (see Fig. 3(e), 4(c) and (b) for [001]NW, [010]NW, and [100]NW zone axes, respectively).
Topotactic transformation of the nanowire structure to the Aurivillius structure
During annealing at temperatures above 500 °C the bismuth-titanate NWs transform to the Aurivillius structure. XRD did not show any significant changes after annealing of the NWs for 2 hours at 500 °C, whereas after the annealing for 2 hours at 550 °C the NWs completely transformed into the AU structure (XRD patterns are given in the ESI, Fig. SI3†). After the transformation the particles with the AU structure grow in the form of platelets (Fig. SI4†). When the NWs were annealed for 2 hours at 525 °C, they only partially transformed into the AU structure. Fig. 7 shows HAADF STEM images of a particle from the NW sample annealed at 525 °C. The particle is composed of a NW sandwiched between two lamellas with the AU structure (oriented along ([010]NW||[110]AU zone axes). The coherent interface between the two phases ((100)NW||(110)AU, (001)NW||(001)AU), (010)NW||(−110)AU) shows a topotactic NW-to-AU transformation. Projected models are superimposed over the atomic-resolution HAADF image of Fig. 5(b) to illustrate the different arrangements of the Bi3+ and Ti4+ cations in the two structures. Note that no precipitation of any secondary phases during the topotactic transformation of the NWs was detected, strongly suggesting that the NWs have the same composition as the Bi4Ti3O12 Aurivillius phase. | ||
| Fig. 7 HAADF STEM images of a BIT particle from the NW sample annealed for 2 hours at 525 °C. The particle is composed of a NW sandwiched between two lamellas with the AU structure ([010]NW||[110]AU). Projected models of the AU structure and the NW structure are superimposed over Fig. 7(b) to illustrate the positions of the Bi2+ and Ti4+ ions. | ||
Ferroelectric properties of the nanoplatelets and the nanowires
In the 1-D ferroelectric particles the polarization vector P is usually oriented along their longest dimension. For the ferroelectric NWs the polarization is therefore expected along the [100]NW direction. The polarity of the crystal structure can be revealed using dark-field (DF) imaging with TEM based on a failure of Friedel's law in the kinematical diffraction. According to Friedel's law a difference in the contrast should be observed when DF images of a non-centrosymmetric structure are formed with +g and −g, where the diffraction vector g lies in the direction of P.23 Consequently, the contrast of a DF image will reverse across a 180°-domain boundary. Fig. 8 shows BF and DF images of a NW. The DF images were formed with (200) and (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 00) reflections. The contrast in the DF images is alternating along the NW and it is reversed from (200) to (
00) reflections. The contrast in the DF images is alternating along the NW and it is reversed from (200) to (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 00) for the same area of the NW, in accordance with the existence of 180°-ferroelectric domains, from approximately 50 to 100 nm in size. Note that the contrast doesn't change significantly when the DF images are formed with the diffraction vectors g and −g oriented perpendicular to the NW (see Fig. SI6†).
00) for the same area of the NW, in accordance with the existence of 180°-ferroelectric domains, from approximately 50 to 100 nm in size. Note that the contrast doesn't change significantly when the DF images are formed with the diffraction vectors g and −g oriented perpendicular to the NW (see Fig. SI6†).
PFM images of the NW inserted into the polymer are shown in Fig. 9(g)–(l). The boundary of a single nanowire parallel to the scan plane is marked by a dotted red line. Also in this case, the enhancement of the piezoelectric response is observed in the in-plane PFM amplitude image, indicating a strong polarization towards the longer axis of the nanowire.
Discussion
The new structure of the NW can be viewed as a metastable polymorph of BIT stabilized at the nanoscale. According to the empirical Ostwald step rule, crystallization occurs in steps in such a way that a thermodynamically unstable phase (i.e., the new nanowire structure) often occurs first because it has a lower nucleation barrier than the stable phase (i.e., the Aurivillius structure). The metastable polymorph remains stable as long as it stays small, if the surface energy of the metastable phase is lower to that of the stable phase.26 The polymorphism is well known in simple oxides. Many technologically important nanomaterials, e.g., magnetic maghemite (γ-Fe2O3) nanoparticles, photocatalytic anatase nanoparticles, ε-Fe2O3 nanoparticles with a giant coercivity, various iron oxide hydroxide pigments, etc., are all metastable phases stabilized at the nanoscale. Recently, we also demonstrated polymorphism in strontium hexaferrite, a technologically important oxide material widely used for magnets exhibiting a complex, layered structure.27During the topotactic transformation of the NWs to the equilibrium Aurivillius structure no secondary phases were precipitated, strongly suggesting that the two phases have the same Bi4Ti3O12 composition. Both structures consist of two alternating structural layers. The B layer of the NW structure is composed of two parallel rows of Bi atoms in a zig-zag arrangement and resembles the (Bi2O2)2+ layer of the Aurivillius structure. The T layer of the NW structure is much thinner than the perovskite layer of the Aurivillius structure. It contains only two parallel rows of cations, whereas the perovskite layer is composed of 5 rows of cations (3 Ti and 2 Bi). The T layer can be presented as the two rows of Ti ions in a zig-zag arrangement, where every sixth Ti is exchanged with Bi (Fig. 5(b) and 6). The arrangement of the Ti atoms in the T layer is consistent with two layers of edge-sharing (TiO6)2− octahedra, as opposed to the corner-sharing (TiO6)2− octahedra of the perovskite layers in the AU structure, where the Ti4+ ions are stacked one above each other. Such an arrangement of the Ti4+ ions in the two structures is consistent with the results of the Raman spectroscopy.
The proposed cation arrangement in the NW structure predicts a Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio of 7
Ti ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, which is only slightly richer in Bi (58.33 at% of cations) than 4
5, which is only slightly richer in Bi (58.33 at% of cations) than 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (57.14 at% of cations). The excess Bi, predicted by the model, is compensated by a random partial insertion of the Ti layers parallel to the (010) planes, visible along the [100] direction (Fig. 5(b) and explained there).
3 (57.14 at% of cations). The excess Bi, predicted by the model, is compensated by a random partial insertion of the Ti layers parallel to the (010) planes, visible along the [100] direction (Fig. 5(b) and explained there).
Both BIT nanostructures, the NPLs and the NWs, are piezoelectric. An enhancement of the piezoelectric signal in the in-plane direction is observed for both NPLs and NWs, indicating a strong polarization in the longitudinal direction of the particles. Some tens-of-nanometres ferroelectric domains are clearly visible in the NPLs. Moreover, switching and the inducing of new domains were observed. On the other hand, the domain structure of NWs was not visible in the PFM scans, probably because the resolution of the technique is above 20 nanometres. However, the 180°-domains were clearly visible on the DF TEM images of the NWs (Fig. 8).
Conclusions
Bismuth titanate (Bi4Ti3O12) nanowires and nanoplatelets were synthesized with a hydrothermal treatment of the precipitated Ti4+ and Bi3+ ions in aqueous NaOH with the concentration of 0.5 mol L−1 and 2 mol L−1, respectively. The nanoplatelets (10 nm thick and from 50 nm to 200 nm wide) exhibit the Aurivillius-type layered-perovskite crystal structure characteristic of Bi4Ti3O12, whereas the nanowires (from 15 nm to 35 nm wide and from several hundreds of nm to several μm long) exhibit an entirely new structure, not described before. X-ray diffractometry showed an orthorhombic unit cell of the new nanowire structure (a = 3.804(1) Å, b = 11.816(3) Å, and c = 9.704(1) Å). Atomic-resolution, high-angle annular dark-field imaging with a CS-probe-corrected scanning-transmission electron microscope showed the cation arrangement in two structural layers alternating along the orthorhombic c-direction: a structural layer composed of two parallel layers of Bi atoms that resembles the (Bi2O2)2+ layer of the Aurivillius-type structures, and a structural layer composed of two parallel layers of the Ti atoms, where every sixth Ti is replaced by Bi. Such an arrangement of cations in the nanowire structure is consistent with a Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio of 7
Ti ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. This discrepancy in the composition is compensated by a random partial insertion of the Ti layers. The basic arrangement of Ti in the structural layer is consistent with two layers of edge-sharing (TiO6)2− octahedra, also suggested by the results of the Raman spectroscopy. With annealing at temperatures above 500 °C the nanowire structure topotactically transforms into the Aurivillius structure without the precipitation of any secondary phases, proving that the nanowire structure is a metastable polymorph of the Bi4Ti3O12 stabilized at the nanoscale. The observation of ferroelectric domains with transmission electron microscopy and piezo-response force microscopy suggested the ferroelectric nature of both bismuth-titanate polymorphs.
5. This discrepancy in the composition is compensated by a random partial insertion of the Ti layers. The basic arrangement of Ti in the structural layer is consistent with two layers of edge-sharing (TiO6)2− octahedra, also suggested by the results of the Raman spectroscopy. With annealing at temperatures above 500 °C the nanowire structure topotactically transforms into the Aurivillius structure without the precipitation of any secondary phases, proving that the nanowire structure is a metastable polymorph of the Bi4Ti3O12 stabilized at the nanoscale. The observation of ferroelectric domains with transmission electron microscopy and piezo-response force microscopy suggested the ferroelectric nature of both bismuth-titanate polymorphs.
Author contributions
D. M., G. D., A. M. and H. U. designed the experimental work programme, methodology, supervised the research and drafted the manuscript. N. K. synthesized the nanoparticles. S. G. and R. K. prepared TEM samples using ultramicrotome. D. M. carried out electron microscopy, G. D. analysed the STEM results. A. M. conducted XRD structure determination and proposed the tentative structural model. S. G. conducted Raman analysis. H. U. carried out the PFM measurements. M. Š. performed ICP-OES analysis. All authors discussed and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Slovenian Research Agency (ARRS) for research core funding No. P2-0089, J2-3041, P1-0034, P2-0105, and the support of Infrastructural Centre “Microscopy of Biological Samples” at Biotechnical faculty, University of Ljubljana. The authors thank B. Anželak, V. Fišinger and J. Cilenšek for help in the laboratory.References
- B. Aurivillius, Ark. Kemi, 1949, 1, 499–512 CAS.
- D. Urushihara, M. Komabuchi, N. Ishizawa, M. Iwata, K. Fukuda and T. Asaka, J. Appl. Phys., 2016, 120, 142117 CrossRef.
- T. Jardiel, A. C. Caballero and M. Villegas, J. Ceram. Soc. Jpn., 2008, 116, 511–518 CrossRef CAS.
- A. Moure, Appl. Sci., 2018, 8, 62 CrossRef.
- H. Li, L. Su, S. Kuang, Y. Fan, Y. Wu, Z. L. Wang and G. Zhu, Nano Res., 2017, 10, 785–793 CrossRef.
- J. Hu, Y. Yu, H. Guo, Z. Chen, A. Li, X. Feng, B. Xi and G. Hu, J. Mater. Chem., 2011, 21, 5352–5359 RSC.
- Z. Chen, Y. Yu, H. Guo and J. Hu, J. Phys. D: Appl. Phys., 2009, 42, 125307 CrossRef.
- L. Xue, G. Qing-Feng, L. Hai-Bo, L. Hong-Ji, B. Chun-Hua and D. Hai-De, Acta Phys.-Chim. Sin., 2012, 28, 1481–1488 Search PubMed.
- S. Niu, R. Zhang, X. Zhang, J. Xiang and C. Guo, Ceram. Int., 2020, 46, 6782–6786 CrossRef CAS.
- T. Xiao, C. Guo, H. Wanga, R. Zhang, Y. Li, W. Shao, Y. Zhang, X. Wu, J. Tan and W. Ye, Mater. Lett., 2020, 269, 127679 CrossRef CAS.
- S. Tu, H. Huang, T. Zhang and Y. Zhang, Appl. Catal., B, 2017, 219, 550–562 CrossRef CAS.
- J. Wu, N. Qin, E. Lin, B. Yuan, Z. Kang and D. Bao, Nanoscale, 2019, 11, 21128–21136 RSC.
- Q. Yang, Y. Li, Q. Yin, P. Wang and Y.-B. Cheng, J. Eur. Ceram. Soc., 2003, 23, 161–166 CrossRef CAS.
- H. Gu, Z. Hu, Y. Hu, Y. Yuan, J. You and W. Zou, Colloids Surf., A, 2008, 315, 294–298 CrossRef CAS.
- F. Wang, J. Wang, X. Zhong, B. Li, J. Liu, D. Wu, D. Mo, D. Guo, S. Yuan, K. Zhang and Y. Zhou, CrystEngComm, 2013, 15, 1397–1403 RSC.
- F. Wang, J. B. Wang, X. L. Zhong, B. Li, Y. Zhang, C. J. Lu, W. N. Ye and Y. C. Zhou, EPL, 2013, 103, 37002 CrossRef.
- G. Xu, Y. Yang, H. Bai, J. Wang, H. Tian, R. Zhao, X. Wei, X. Yang and G. Han, CrystEngComm, 2016, 18, 2268–2274 RSC.
- S. A. A. Coelho, J. Appl. Crystallogr., 2018, 51, 210–218 CrossRef.
- S. Kojima, R. Imaizumi, S. Hamazaki and M. Takashige, Jpn. J. Appl. Phys., 1994, 33, 5559–5564 CrossRef CAS.
- U. Balachandran and N. G. Eror, J. Solid State Chem., 1982, 42, 276–282 CrossRef CAS.
- A.C.T. Koch, Determination of Core Structure Periodicity and Point Defect Density along Dislocations, Ph.D. Thesis, Arizona State University, 2002 Search PubMed.
- D. Makovec, B. Belec, T. Goršak, D. Lisjak, M. Komelj, G. Dražić and S. Gyergyek, Nanoscale, 2018, 10, 14480–14491 RSC.
- R. Serneels, M. Snykers, P. Delavignette, R. Gevers and S. Amelinckx, Phys. Status Solidi B, 1973, 58, 277–292 CrossRef CAS.
- M. Maček Kržmanc, B. Jančar, H. Uršič, M. Tramšek and D. Suvorov, Cryst. Growth Des., 2017, 17, 3210–3220 CrossRef.
- H. Uršič and U. Prah, Proc. R. Soc. London, Ser. A, 2019, 475, 20180782–1–20180782–25 Search PubMed.
- A. Navrotsky, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12096–12101 CrossRef CAS PubMed.
- D. Makovec, G. Dražić, S. Gyergyek and D. Lisjak, CrystEngComm, 2020, 22, 7113–7122 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: List of materials used and additional information on the characterization methods, LeBail profile fitting of the XRD pattern, Raman spectroscopy, TEM/XRD of annealed nanowires, TEM observation of domain structure, and PFM observation of switching and inducing ferroelectric domains. See DOI: 10.1039/d2nr00307d |
| This journal is © The Royal Society of Chemistry 2022 |






