Enabling practical nanoparticle electrodeposition from aqueous nanodroplets†
Abstract
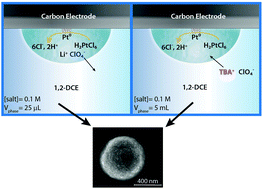
The rapid rise of technology in the modern world has led to an increased demand for energy. Consequently, it is essential to increase the efficiency of current energy-producing systems due to the poor activity of their catalysts. Nanoparticles play a significant role in energy storage and conversion; however, electrodeposition of nanoparticles is difficult to achieve due to surface heterogeneities, nanoparticle diffusion layer overlap, and the inability to electrodeposit multi-metallic nanoparticles with stoichiometric control. These problems can be solved through nanodroplet-mediated electrodeposition, a technique where water nanodroplets are filled with metal salt precursors that form stable nanoparticles when they collide with a negatively-biased electrode. Further, this method has demonstrated control over nanoparticle size and morphology, displaying a wide variety of applications for the generation of materials with excellent catalytic properties. Historically, the cost of nanodroplet-mediated electrodeposition experimentation is prohibitive because practitioners use 0.1 M to 0.5 M tetrabutylammonium perchlorate (TBAP) dissolved in the oil phase (∼10 mL). Such high concentrations of electrolytes have been used to lower ohmic drop and provide ions to maintain charge balance during electrodeposition. Here, we show that supporting electrolyte is not necessary for the oil phase. In fact, one can use a suitable salt (such as lithium perchlorate) in the aqueous phase to achieve nanoparticle electrodeposition. This simple change, grounded in an understanding of ion transfer, drives down the cost per experiment by nearly three orders of magnitude, representing a necessary step forward in enabling practical nanoparticle electrodeposition from water nanodroplets. This approach is a promising procedure for future cost-effective energy conversion systems relying on electrocatalytic nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...
