Tuning the local electronic structure of a single-site Ni catalyst by co-doping a 3D graphene framework with B/N atoms toward enhanced CO2 electroreduction†
Tianyi
Shao‡
 a,
Delong
Duan‡
a,
Shengkun
Liu
a,
Chao
Gao
a,
Delong
Duan‡
a,
Shengkun
Liu
a,
Chao
Gao
 *a,
Hengxing
Ji
*a,
Hengxing
Ji
 a and
Yujie
Xiong
a and
Yujie
Xiong
 *ab
*ab
aHefei National Laboratory for Physical Sciences at the Microscale, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, Anhui 230026, China
bInstitute of Energy, Hefei Comprehensive National Science Center, 350 Shushanhu Rd., Hefei, Anhui 230031, China. E-mail: yjxiong@ustc.edu.cn; gaoc@ustc.edu.cn
First published on 9th December 2021
Abstract
Various single metal sites supported on N-doped carbon materials have been demonstrated to be effective catalysts for CO2 electroreduction. However, it remains a challenging task to gain comprehensive understanding on how the local electronic structures of single metal catalytic sites are rationally tuned, which eventually holds the key to significantly enhance the electrocatalytic performance. Herein, we implement B–N bonds into an N-doped 3D graphene framework by B doping to further stabilize the supported catalytic Ni single-sites and simultaneously tune their local electronic structure. Moreover, electrochemical in situ Fourier-transform infrared spectroscopy reveals that the B–N bonds can further facilitate the production of pivotal *COOH intermediates in comparison with only N doping. As a result, the Ni single-site catalyst on the B, N co-doped 3D graphene framework achieves excellent catalytic performance with a CO faradaic efficiency (FE) of 98% and a turnover frequency (TOF) value of 20.1 s−1 at −0.8 V (vs. RHE), whereas the FE and TOF for the control sample without B doping are as low as 62% and 6.0 s−1, respectively. This work highlights the superiority of modulating local electronic structures of single-site catalysts toward efficient electrocatalytic CO2 reduction.
Introduction
Great attention has been paid to carbon materials in the past few years due to their good stability and reactivity, especially in the field of supercapacitors1,2 and H2 evolution,3 CO2 reduction,4,5 N2 reduction,6 O2 reduction,7–9 and O2 evolution reactions.10,11 Single-atom/site catalysts dispersed on carbon materials are able to realize the maximal atom utilization efficiency toward high catalytic performance. The common carbon-based single-atom/site catalysts for CO evolution through CO2 electroreduction are porous carbon materials anchored with Fe, Co, and Ni single-atoms/sites. The general strategy for enhancing the catalytic performance is introducing N atoms to coordinate with catalytic metal sites in order to tune their local electronic structure inside the carbon skeleton.12–14 In particular, implanting N atoms into the graphene skeleton results in the creation of more defects and the enrichment of more negative charges, thus regulating the electron density of catalytic metal sites.15 Moreover, the strategy of integration with another metal atom has also been proven to tune the electronic structure of single-atom metal catalytic sites dispersed in the carbon network.16,17 Although a few studies have shown that simultaneously doping two non-metallic elements in the carbon network could enable enhanced electrocatalytic performance, it still remains largely unexplored for tuning the CO2 electroreduction reaction through co-doping two non-metallic heteroatoms in carbon-based single-atom/site catalysts.18,19 To this end, we would like to explore the possibility of the strategy by incorporating another non-metallic heteroatom to tune the local electronic structure of single-atom metal catalytic sites in an N-doped carbon network toward enhanced catalytic performance in CO2 electroreduction.Given the low density, high aspect ratio, good conductivity, tunable channel structure, and abundant C/O functional groups as anchoring sites, 3D reduced graphene oxide (rGO) frameworks are theoretically regarded as the ideal support for various single-atom/site catalysts with a readily tunable coordination environment toward various electrochemical reactions.20,21 Herein, taking the single-site Ni catalyst supported on an N-doped 3D graphene framework as a model, we further incorporate B atoms into the catalyst system to tune the local electronic structure of single Ni catalytic sites in consideration of the distinct electronegativity between B and N.
In the traditional doping methods, single-atom/site catalysts supported on N-doped carbon networks are prepared by flowing ammonia gas, adding nitrogen-containing small organic molecules during the pyrolysis process, or directly pyrolyzing N-containing metal–organic frameworks.22–24 Considering that the Ni single-site are anchored with the N atom, the doped B atom should be located nearby the N atom to form the B–N bond to precisely tune the local electronic structure of the single Ni catalytic sites. To this end, we utilize 1,4-benzenediboronic acid (BDBA) and o-phenylenediamine (oPD) as B and N sources to in situ grow polymer nanofibers along with the pore channel throughout the self-assembled three-dimensional (3D) graphene framework. In this way, the B–N bonds have been formed in advance and then the B and N can be doped simultaneously and adjacently in space during pyrolysis. The presence of B atoms not only facilitates the formation of Ni single-site catalysts, but also tunes their local electronic structure to promote the production of key *COOH intermediates in the CO2 reduction reaction. Moreover, the long and continuous channel in the 3D graphene framework can promote the long-range diffusion and transport of CO2 molecules. Consequently, as compared to the counterparts with only N doping, the single-site Ni catalyst supported on the B, N co-doped 3D graphene framework exhibits significantly enhanced catalytic performance in a wider applied voltage range.
Experimental
Synthesis of the single-site Ni catalyst supported on a B, N co-doped 3D graphene framework
Graphene oxide (GO) was synthesized using the modified Hummers’ method. The self-assembled 3D graphene framework loaded with the B, N-containing polymer and Ni sites was prepared by a facile hydrothermal method. First, 0.3 mL of 10 mM Ni(NO3)2·6H2O and 25 μL of 30% H2O2 were added into 10 mL of 0.3 wt% GO solution under general sonication for 30 min. Then the solution was immediately transferred to an ultrasonic cell disruption system for another vigorous 45 min sonication (solution A). Second, the two small organic molecules of oPD and BDBA with various ratios were fully dissolved in 20 mL of mixed solvent containing 15 mL of ethanol and 5 mL of H2O (solution B). The two solutions A and B obtained above were mixed together and heated at 180 °C for 12 h by a hydrothermal method. After slowly cooling down to room temperature, the obtained self-assembled 3D graphene framework with a cylinder shape was put into a vacuum oven to remove most of the ethanol, followed by freezing dry with liquid nitrogen to achieve complete dryness. The single-site Ni catalysts supported on the B, N co-doped 3D graphene framework with different B/N ratios were finally obtained via pyrolyzing at 820 °C (heating rate: 5 °C min−1) under a flowing Ar atmosphere for 3 h in a tube furnace. The obtained single-site Ni catalysts supported on the B, N co-doped 3D graphene framework with different B/N ratios (BDBA/oPD, 0/0.6, 0.1/0.5, 0.2/0.4, 0.3/0.3, 0.4/0.2, and 0.6/0, mmol mmol−1) were denoted as Ni–N6, Ni–B1N5, Ni–B2N4, Ni–B3N3, Ni–B4N2, and Ni–B6, respectively.X-ray absorption fine structure (XAFS) characterization
Ni K-edge XAFS characterization was performed at the beamline 1 W2B of Beijing Synchrotron Radiation Facility (BSRF), China. The acquired K-edge extended XAFS (EXAFS) data were treated through the Demeter package, and the processed curve was compared with standard samples of Ni foil, NiO, and nickel(II) phthalocyanine. Ni K-edge X-ray absorption near-edge structure (XANES) simulations based on the self-consistent multiple-scattering theory were performed using FEFF 8.2 code. The N and B K-edge XAFS characterization was performed at the MCD Endstation of the BL12B-a Soft X-Ray beamline at the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China.Evaluation of the catalytic performance for CO2 electroreduction
Electrochemical measurements, including CO2 electroreduction and linear sweep voltammetry (LSV), were carried out on an electrochemical station (CHI660D) with a conventional H-type electrochemical cell, in which the cathode and anode in the CO2-saturated 0.5 M KHCO3 electrolyte (pH = 7.2) were separated by a Nafion117 membrane. The three-electrode system consisted of a platinum plate as the counter electrode, an Ag/AgCl (saturated KCl) as the reference electrode, and carbon paper as the working electrode. In a typical preparation for the working electrode, 5 mg of the sample and 50 μL of Nafion solution (5 wt%) were uniformly dispersed in 1 mL of water–ethanol solution with the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by sonication. The catalyst ink was dropped onto the two sides of a carbon paper electrode with a catalyst loading of 0.5 mg cm−2. The working electrode was dried in a vacuum oven overnight. All potentials in this study were measured against the Ag/AgCl reference electrode and converted to the RHE reference scale using E (vs. RHE) = E (vs. Ag/AgCl) + 0.1989 V + 0.059 × pH. The amounts of CO and H2 were measured using a gas chromatograph (GC, 7890A, Ar carrier, Agilent). H2 was detected with a thermal conductivity detector (TCD), and CO was detected with a flame ionization detector (FID).
3 by sonication. The catalyst ink was dropped onto the two sides of a carbon paper electrode with a catalyst loading of 0.5 mg cm−2. The working electrode was dried in a vacuum oven overnight. All potentials in this study were measured against the Ag/AgCl reference electrode and converted to the RHE reference scale using E (vs. RHE) = E (vs. Ag/AgCl) + 0.1989 V + 0.059 × pH. The amounts of CO and H2 were measured using a gas chromatograph (GC, 7890A, Ar carrier, Agilent). H2 was detected with a thermal conductivity detector (TCD), and CO was detected with a flame ionization detector (FID).
Electrochemical in situ Fourier-transform infrared (FTIR) spectroscopy
Electrochemical in situ FTIR spectra were recorded with a Fourier transform spectrometer (Bruker VERTEX 70v/s) equipped with a KBr beam splitter and various detectors connected with an infrared microscope (Bruker Hyperion 2000, ×50 objective) at the Infrared Spectroscopy and Microspectroscopy Endstation (BL01B) in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. The catalysts were uniformly covered on the glassy carbon electrode equipped in the in situ electrochemical cell, which was filled with CO2-saturated 0.5 M KHCO3 solution. The window of the in situ electrochemical cell was covered by a CaF2 plate. Note: Ensure that the electrolyte existing between the CaF2 plate and glassy carbon electrode is a very thin layer since the infrared signals are easily disturbed by water. The in situ electrochemical cell was connected to an electrochemical station during the process of the electrocatalytic CO2 reduction. The spectrum of the sample before applying with voltage was deducted as the background signal, and the time-dependent measurements were performed under a fixed potential.Results and discussion
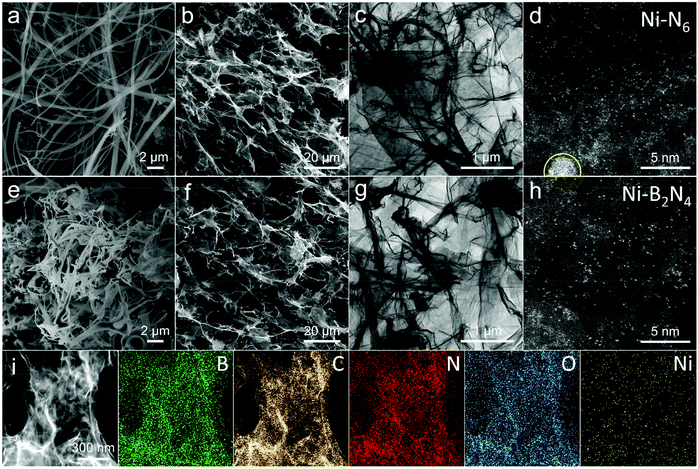
To prepare the model catalyst, a one-step hydrothermal process is primarily developed to obtain the 3D self-assembled rGO framework with the B, N-containing polymer, showing the shape of a cylinder (Fig. S1†). Generally speaking, the N-containing monomer oPD tends to dehydrogenize and self-polymerize to form long and wide nanofibers (Fig. 1a and S2†).25 After integrating the oPD with B-containing BDBA, the unidirectional chain growth is suppressed while the dendronized copolymerization is facilitated (Fig. S3†), producing shorter and more slender nanobelts (Fig. 1e and S4†). Such a morphological change suggests the successful introduction of B–N bonds into the polymer by the copolymerization of oPD and BDBA precursors. In contrast, the single BDBA as the reactant monomer produces the sheet-shape polymer embedded into the 3D graphene network (Fig. S5†).26 The obtained cylinder graphene is further treated under oriented freeze drying from bottom to top so that the tiny ice nucleus would grow along the longitudinal direction through the internal mesopores to form parallel ice columns. Then the crisscrossing and interlaced channels with long-range alignment are created across the whole graphene framework. Finally, the single-site Ni catalysts supported on the B, N co-doped 3D graphene framework are obtained by pyrolyzing the cylinder graphene at 820 °C under a flowing Ar atmosphere for 3 hours to firmly anchor the atomically dispersed Ni sites by the surrounding doped N and B heteroatoms. As shown in Fig. 1b, 1f, and S6† the interlaced channels are kept for the graphene framework after pyrolysis, which would facilitate the diffusion and transport of CO2 molecules. The Brunauer–Emmett–Teller (BET) surface area is obtained by analysis of N2 absorption-desorption isotherms (Fig. S7†). Compared to the sample with only N doping (Ni–N6), the BET surface area of the sample with the B, N co-doped graphene framework (Ni–B2N4) is significantly reduced (322.6 m2 g−1vs. 87.4 m2 g−1), most likely as the interspaces of the graphene framework are partially filled with the carbonized fiber fragments. In addition, transmission electron microscopy (TEM) images (Fig. 1c and g) further show that the graphene maintains a sheet-like structure with crimps and wrinkles after pyrolysis, and no Ni-derived clusters or particles are observed on the nanosheets. To resolve information at an atomic scale, aberration-corrected high-angle annular dark-field scanning TEM (HAADF-STEM) is further used to characterize the dispersion of the Ni sites. For the sample with only N doping (Ni–N6), it clearly exhibits the primary existence of single Ni atoms but simultaneously the presence of a few Ni nanoparticles as highlighted by the yellow circles (Fig. 1d and S8†). In contrast, for the sample with B, N co-doped graphene nanosheets (Ni–B2N4), it demonstrates the uniform distribution of isolated Ni atoms without the presence of Ni nanoclusters or nanoparticles (Fig. 1h and S9†), inferring that the introduction of B can effectively avoid metal agglomeration. The loading amounts of Ni sites are quantified by inductively coupled plasma-atomic emission spectroscopy (ICP-AES), showing that the doped graphene framework samples have similar Ni contents of about 0.5% (Table S1†). To further confirm whether the metal nanoparticles are embedded within the graphene framework during the hydrothermal and pyrolysis process, X-ray diffraction (XRD) is performed and no characteristic peak assigned to Ni nanoparticles (PDF 04-0850) is observed in the obtained XRD patterns (Fig. S10†). The three peaks in the ranges of 20–30°, 40–45°, and 53–58° are related to the (111), (100), and (222) planes of graphene (PDF# 75-2078), respectively. The elemental energy-dispersive X-ray spectroscopy (EDS) mapping analysis illustrates the homogeneous dispersion of B, C, N, O, and Ni elements (Fig. 1i), further confirming that B and N have been uniformly doped into the graphene skeleton. The practical B/N ratios and their doping percentages in the samples are analyzed by X-ray photoelectron spectroscopy (XPS) characterization, revealing that the practical B/N ratios indeed are promoted by increasing BDBA/oPD ratios (Table S2†). In particular, the Ni–B2N4 sample has a doping percentage of 1.73% and 5.09% in weight for B and N, respectively.Upon directly observing the existence of the single Ni atom from the HAADF images above, X-ray absorption spectroscopy (XAS) is implemented to understand the valence states and refined coordination environments. In the XANES spectra (Fig. 2a), the Ni absorption edge of single-site Ni catalysts supported on the B, N co-doped 3D graphene framework with different B/N ratios is located in the range of Ni foil (0) and NiO (+2), indicating that the single Ni site possesses a +δ (0 < δ < 2) valence state in their structural units.27 In the Fourier transform EXAFS (FT-EXAFS) spectra (Fig. 2b), the manifested dominant peaks at about 1.4 Å are assigned to the direct coordination of single Ni sites with N/O/C atoms in the nearest coordination position, while the peaks at about 2.0 Å are assigned to the chemical interactions for single Ni sites with B/C atoms in a bit further distance. The intensity of the peak at about 1.4 Å has a positive correlation with the coordination numbers of Ni sites. Compared with the sample with only N doping (Ni–N6), the introduction of B atoms into the graphene network enhances the peak intensity at about 1.4 Å, indicating the increased coordination numbers of Ni sites that could contribute to stabilizing the single sites. Moreover, the peak at 2.2 Å, attributed to the Ni–Ni bond in Ni foil, is not obviously observed in the profiles of all the samples, which further corroborates the atomic dispersion of Ni sites.
 | ||
| Fig. 2 XAS spectra for the Ni–BxN6−x (x = 0–6) samples. (a) Normalized Ni K-edge XANES spectra. (b) k3-Weighted Ni K-edge FT-EXAFS spectra. (c) B K-edge XANES spectra. (d) N K-edge XANES spectra. | ||
To acquire the precise coordination structures, the EXAFS fitting and optimized configuration (Fig. S11 and Table S3†) are simulated by referring to the fixed standard samples of Ni foil, NiO, and nickel phthalocyanine (NiPc). The fitting results indicate that the Ni sites in the N doped or B, N co-doped samples are coordinated with nearly three neighbouring N/O/C atoms (note that the C/N/O light elements could not be distinguished clearly by EXAFS spectra). Notably, the lowest coordination number of Ni sites for the sample with only B doping (Ni–B6) suggests that the B atoms are not ideal anchoring sites for single Ni sites. Thus the Ni–N/O/C coordination numbers first increase and then decrease with the increasing content of the B precursor, as the introduced B could regulate the local electronic structure to more firmly coordinate with Ni sites while the increased B/N ratio would reduce the number of N anchoring sites to coordinate with Ni sites. In particular, the Ni–B2N4 sample possesses the maximal coordination number, which is in accordance with the peak intensity in EXFAS spectra (Fig. 2b).
The above results demonstrate that the incorporation of the B atom is vital for regulating the coordination numbers and thus the local electronic structure of the single Ni catalytic sites, which may significantly influence the electrocatalytic performance for CO2 reduction. To precisely account for the electronic structure, XANES spectra for B and N are taken into consideration to analyze the doping features in the graphene framework. The B K-edge spectra exhibit sharp peaks at 191.7 eV (1s → π*) and broad bands between 196.7 and 201.3 eV (1s → σ*), which are attributable to the typical resonances of sp2-hybridized B–N bonds (Fig. 2c).28,29 These characteristic signals undoubtedly affirm the formation of B–N moieties inside the graphene network. In addition, the corresponding information of N with π* and σ* features is evaluated by N K-edge spectra (Fig. 2d).29 The results suggest that there are multiple types of N species, including pyridinic N, Ni–N, pyrrolic N, graphitic N, and oxidized N. In particular, the observed peak assigned to the Ni–N bond at about 399.5 eV suggests that the single Ni sites are directly coordinated with N atoms.
To further elucidate the variation of the local coordination environment and electronic structure by B doping, the XPS characterization is analyzed to investigate the various N and B species in the samples (Fig. 3). For N 1s XPS spectra (Fig. 3a–e), the N 1s peak can be fitted to six characteristic peaks, including N–B (397.8 eV), pyridinic N (398.5 eV), Ni–N (399.4 eV), pyrrolic N (400.6 eV), graphitic N (401.3 eV), and oxidized N (402.8 eV).29,30 Remarkably, with the addition of the B precursor (BDBA), the proportions of pyrrolic N and Ni–N increase, while the proportions of pyridinic N and graphitic N decrease. The increased percentage of pyrrolic N originates from the incorporation of B into the graphene lattice network by taking full advantage of the chemical interaction between –NH2 and –B–(OH)2 functional group to form a five-membered ring. Among all the samples, the Ni–B2N4 has the highest ratio of pyrrolic N. For the B 1s XPS spectra (Fig. 3f), the intensities of the peak for B–N (191.6 eV) increase with increasing B/N ratios, further confirming the formation of B–N bonds in the graphene framework.29,31–33 The peak of the B–O bond (193.0 eV) for the Ni–B6 sample is derived from the –B(OH)2 group in BDBA. For the Ni XPS signal of the samples (Fig. S12a†), the binding energy of the Ni 2p3/2 is situated higher than that of Ni0 (582.6 eV) and lower than that of Ni2+ (855.6 eV),27 suggesting that the valence state of Ni sites is between Ni0 and Ni2+. This result is in agreement with the conclusion from the XANES characterization. In addition, the binding energy of the Ni 2p3/2 slightly shifts to a higher binding energy, indicating that the valence state of Ni sites increases with increasing B/N ratios. This result is also confirmed by the Ni Auger electron spectra (Fig. S12b†). The main peak of Ni–B2N4 shifts to a higher binding energy in comparison with Ni–N6, confirming that the valence state of Ni sites increases after B doping. From the above information, the addition of B leads to the formation of B–N bonds that induces the redistribution of charges on Ni sites, thus regulating the local electronic structure of single Ni sites.
Upon acquiring the key information of single-site Ni catalysts supported on the B, N co-doped 3D graphene framework, we are now in a position to evaluate their performance as electrocatalysts for CO2 reduction as well as validate the role of the B-tuned local electronic structure in CO2 electroreduction. As revealed by linear sweep voltammetry (LSV) curves in Fig. 4a, the current densities of Ni–BxN6−x (x = 1–5) are apparently higher than those of the Ni–N6 and Ni–B6 samples and the Ni–B2N4 exhibits the highest current density, demonstrating that the addition of B could significantly enhance the catalytic performance for CO2 electroreduction. The products are CO and H2, without other detectable carbonaceous products in the CO2-saturated 0.5 M KHCO3 electrolyte. Moreover, the faradaic efficiencies (FEs) for CO evolution (Fig. 4b) are recorded at different applied potentials from −0.5 to −1.1 V (vs. RHE). The Ni–B2N4 sample exhibits the optimal CO FE of 98% at −0.8 V with a remarkable CO selectivity over 90%, as well as the widest range of working potentials. In sharp contrast, the Ni–N6 sample demonstrates poor activity toward CO generation. To directly compare the catalytic performance, the turnover frequency (TOF), referring to the number of produced CO per Ni single-site in unit time, is calculated on the basis of total Ni mass loaded on carbon fiber paper (Fig. 4c). The Ni–B2N4 reaches the highest TOF value of 20.1 s−1 at −0.8 V, while the TOF value for Ni–N6 is as low as 6.0 s−1.14,16 Meanwhile, the other counterpart samples with various B contents also manifest significantly higher catalytic performance in comparison with Ni–N6, confirming the tuned local electronic structure by B doping plays a vital role in improving the catalytic performance for CO2 electroreduction. Accordingly, the long-term stability for 20 h continuous measurement confirms that Ni–B2N4 possesses obviously higher steady current density and selectivity toward CO generation (Fig. 4d). The Ni sites coordinated with pyrrolic N have been proven to be closely correlated with the catalytic performance for CO2 electroreduction to CO.34 Considering that Ni–B2N4 has the highest ratio of pyrrolic N and the highest catalytic performance toward CO2 electroreduction, it is supposed that the Ni single-site coordinated with pyrrolic N contributes to the high catalytic performance in CO2 electroreduction. Overall, doping B into the single-site Ni catalyst supported on the N-doped 3D graphene framework enables to modulate the local coordination environment and electronic structure of the Ni catalytic sites, which plays an important role in endowing the remarkable improvement of catalytic performance for CO2 electroreduction.
The overall electrochemical reaction for the reduction of CO2 to CO can be described as the following three elementary steps:35
| CO2(g) + H+ + * + e− → *COOH | (1) |
| *COOH + H+ + e− → *CO + H2O(l) | (2) |
| *CO → CO(g) + * | (3) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, and 1166 cm−1 ascribed to the C–O stretching vibration.31,32 For the in situ CO2 electroreduction on various samples (Fig. 5b–h), there is no observable signal at the beginning of the CO2 electroreduction reactions, and all the peaks related to the intermediate in the spectral data are gradually enhanced with increasing electrolysis time. As the key intermediate in CO2 electroreduction to CO, the *COOH has four characteristic peaks referring to 3550 cm−1 (O–H symmetric stretching), 1680 cm−1 (C
C stretching vibration, and 1166 cm−1 ascribed to the C–O stretching vibration.31,32 For the in situ CO2 electroreduction on various samples (Fig. 5b–h), there is no observable signal at the beginning of the CO2 electroreduction reactions, and all the peaks related to the intermediate in the spectral data are gradually enhanced with increasing electrolysis time. As the key intermediate in CO2 electroreduction to CO, the *COOH has four characteristic peaks referring to 3550 cm−1 (O–H symmetric stretching), 1680 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching), 1360 cm−1 (O–H in-plane bending), and 1160 cm−1 (C–O symmetric stretching). The sample of rGO (Fig. 5b) with no capability of CO2 electroreduction barely shows any signal of the stretching vibration of O–H and C
O symmetric stretching), 1360 cm−1 (O–H in-plane bending), and 1160 cm−1 (C–O symmetric stretching). The sample of rGO (Fig. 5b) with no capability of CO2 electroreduction barely shows any signal of the stretching vibration of O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. In stark contrast, these two prominent peaks for the Ni–BxN6−x (x = 1–5) samples (Fig. 5d–g) are substantially sharper than the Ni–N6 (Fig. 5c) and Ni–B6 (Fig. 5h) counterpart, which is in good accordance with the catalytic performance shown in Fig. 4. The enhanced signal suggests that the production of the *COOH intermediate, the rate-determining step in CO2 electroreduction to CO, is remarkably promoted to give a higher content. In addition, especially for the Ni–B2N4 sample, a strong stretching peak ascribed to the B–N bond is clearly observed at 1410 cm−1 and changes significantly over time, suggesting that the formed B–N bonds after B doping modulate the local electronic structure of Ni active sites and are involved in the CO2 reduction reaction dynamically. To sum up, the coordination environment, or exactly the local electronic structure of Ni sites could alter the adsorption free energy of the intermediates to facilitate the CO2 reduction.36 In our work, the introduction of B modulates the local electronic structure of Ni catalytic sites to facilitate the production of key *COOH intermediates and consequently lowers the energy barrier in the CO2 reduction reaction, thereby boosting the catalytic performance for CO2 electroreduction.
O. In stark contrast, these two prominent peaks for the Ni–BxN6−x (x = 1–5) samples (Fig. 5d–g) are substantially sharper than the Ni–N6 (Fig. 5c) and Ni–B6 (Fig. 5h) counterpart, which is in good accordance with the catalytic performance shown in Fig. 4. The enhanced signal suggests that the production of the *COOH intermediate, the rate-determining step in CO2 electroreduction to CO, is remarkably promoted to give a higher content. In addition, especially for the Ni–B2N4 sample, a strong stretching peak ascribed to the B–N bond is clearly observed at 1410 cm−1 and changes significantly over time, suggesting that the formed B–N bonds after B doping modulate the local electronic structure of Ni active sites and are involved in the CO2 reduction reaction dynamically. To sum up, the coordination environment, or exactly the local electronic structure of Ni sites could alter the adsorption free energy of the intermediates to facilitate the CO2 reduction.36 In our work, the introduction of B modulates the local electronic structure of Ni catalytic sites to facilitate the production of key *COOH intermediates and consequently lowers the energy barrier in the CO2 reduction reaction, thereby boosting the catalytic performance for CO2 electroreduction.
Conclusions
In summary, a synthetic strategy for in situ growing polymer nanofibers along the internal channel of a self-assembled 3D graphene framework has been developed for constructing a B, N co-doped 3D graphene framework coupled with a single-site Ni catalyst. Through the technique of directional freeze drying, the graphene framework treated by pyrolysis possesses continuous channels to favor the diffusion of CO2 to catalytic sites, as well as the release of produced CO molecules. The incorporated B atoms play a crucial role in promoting the atomization of Ni single-sites and significantly impact their electronic structure by modifying the local coordination environment. As a result, the doped B atoms markedly enhance the quantity of Ni single-sites that are coordinated with adjacent pyrrolic N, which are supposed to be responsible for the improved catalytic performance in CO2 electroreduction. Additionally, the formation of B–N bonds detected by in situ FTIR may contribute to facilitate the production of key *COOH intermediates. The appropriate ratio of B and N is also proven to be essential for outstanding catalytic performance. This work not only demonstrates a strategy for tuning the local electronic structure of single metal catalytic sites on 3D graphene frameworks, but also highlights the importance of modulating the local electronic structure of single-site catalysts toward enhanced performance.Author contributions
T. S. designed and performed the experiments, analyzed and discussed the experimental results, and drafted the manuscript. D. D. performed the XAFS characterization and analyzed the results. S. L. joined the discussion of experiments and data. C. G. and Y. X. proposed the research direction, supervised the project, analyzed and discussed the experimental results, and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0406103), NSFC (21725102, 91961106, and 91963108), DNL Cooperation Fund, CAS (DNL201922), and Youth Innovation Promotion Association CAS. Ni K-edge XAFS characterization was performed at the beamline 1W2B of Beijing Synchrotron Radiation Facility (BSRF), China. N and B K-edge XAFS characterization was performed at the MCD Endstation (BL12B) in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. Electrochemical in situ FTIR data were obtained at the Infrared Spectroscopy and Microspectroscopy Endstation (BL01B) in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. We thank the support from USTC Center for Micro- and Nanoscale Research and Fabrication.Notes and references
- S.-C. Li, B.-C. Hu, Y.-W. Ding, H.-W. Liang, C. Li, Z.-Y. Yu, Z.-Y. Wu, W.-S. Chen and S.-H. Yu, Angew. Chem., Int. Ed., 2018, 57, 7085–7090 CrossRef CAS PubMed.
- Y. Zhao, C. Hu, Y. Hu, H. Cheng, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371–11375 CrossRef CAS PubMed.
- L. Cao, Q. Luo, W. Liu, Y. Lin, X. Liu, Y. Cao, W. Zhang, Y. Wu, J. Yang, T. Yao and S. Wei, Nat. Catal., 2019, 2, 134–141 CrossRef CAS.
- L. Ye, Y. Ying, D. Sun, Z. Zhang, L. Fei, Z. Wen, J. Qiao and H. Huang, Angew. Chem., Int. Ed., 2020, 59, 3244–3251 CrossRef CAS PubMed.
- D. Hursan, A. A. Samu, L. Janovak, K. Artyushkova, T. Asset, P. Atanassov and C. Janaky, Joule, 2019, 3, 1719–1733 CrossRef CAS PubMed.
- Y. Liu, Q. Li, X. Guo, X. Kong, J. Ke, M. Chi, Q. Li, Z. Geng and J. Zeng, Adv. Mater., 2020, 32, 1907690 CrossRef CAS PubMed.
- X. Xie, C. He, B. Li, Y. He, D. A. Cullen, E. C. Wegener, A. J. Kropf, U. Martinez, Y. Cheng, M. H. Engelhard, M. E. Bowden, M. Song, T. Lemmon, X. S. Li, Z. Nie, J. Liu, D. J. Myers, P. Zelenay, G. Wang, G. Wu, V. Ramani and Y. Shao, Nat. Catal., 2020, 3, 1044–1054 CrossRef CAS.
- L. Jiao, J. Li, L. L. Richard, Q. Sun, T. Stracensky, E. Liu, M. T. Sougrati, Z. Zhao, F. Yang, S. Zhong, H. Xu, S. Mukerjee, Y. Huang, D. A. Cullen, J. H. Park, M. Ferrandon, D. J. Myers, F. Jaouen and Q. Jia, Nat. Mater., 2021, 20, 1385–1391 CrossRef CAS PubMed.
- Y. Qu, Z. Li, W. Chen, Y. Lin, T. Yuan, Z. Yang, C. Zhao, J. Wang, C. Zhao, X. Wang, F. Zhou, Z. Zhuang, Y. Wu and Y. Li, Nat. Catal., 2018, 1, 781–786 CrossRef CAS.
- H. Fei, J. Dong, Y. Feng, C. S. Allen, C. Wan, B. Volosskiy, M. Li, Z. Zhao, Y. Wang, H. Sun, P. An, W. Chen, Z. Guo, C. Lee, D. Chen, I. Shakir, M. Liu, T. Hu, Y. Li, A. I. Kirkland, X. Duan and Y. Huang, Nat. Catal., 2018, 1, 63–72 CrossRef CAS.
- J. Wang, S.-J. Kim, J. Liu, Y. Gao, S. Choi, J. Han, H. Shin, S. Jo, J. Kim, F. Ciucci, H. Kim, Q. Li, W. Yang, X. Long, S. Yang, S.-P. Cho, K. H. Chae, M. G. Kim, H. Kim and J. Lim, Nat. Catal., 2021, 4, 212–222 CrossRef CAS.
- X. Wang, Z. Chen, X. Zhao, T. Yao, W. Chen, R. You, C. Zhao, G. Wu, J. Wang, W. Huang, J. Yang, X. Hong, S. Wei, Y. Wu and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 1944–1948 CrossRef CAS PubMed.
- J. Gu, C.-S. Hsu, L. Bai, H. Chen and X. Hu, Science, 2019, 364, 1091–1094 CrossRef CAS PubMed.
- T. Zheng, K. Jiang, N. Ta, Y. Hu, J. Zeng, J. Liu and H. Wang, Joule, 2019, 3, 265–278 CrossRef CAS.
- W. Ni, Z. Liu, Y. Zhang, C. Ma, H. Deng, S. Zhang and S. Wang, Adv. Mater., 2021, 33, 2003238 CrossRef CAS PubMed.
- Y.-N. Gong, L. Jiao, Y. Qian, C.-Y. Pan, L. Zheng, X. Cai, B. Liu, S.-H. Yu and H.-L. Jiang, Angew. Chem., Int. Ed., 2020, 59, 2705–2709 CrossRef CAS PubMed.
- J. Pei, T. Wang, R. Sui, X. Zhang, D. Zhou, F. Qin, X. Zhao, Q. Liu, W. Yan, J. Dong, L. Zheng, A. Li, J. Mao, W. Zhu, W. Chen and Z. Zhuang, Energy Environ. Sci., 2021, 14, 3019–3028 RSC.
- S. Wang, L. Zhang, Z. Xia, A. Roy, D. W. Chang, J.-B. Baek and L. Dai, Angew. Chem., Int. Ed., 2012, 51, 4209–4212 CrossRef CAS PubMed.
- J. Zhang, L. Qu, G. Shi, J. Liu, J. Chen and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 2230–2234 CrossRef CAS PubMed.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- K. Pang, X. Song, Z. xu, X. Liu, Y. Liu, L. Zhong, Y. Peng, J. Wang, J. Zhou, F. Meng, W. Jian and C. Gao, Sci. Adv., 2020, 6, eabd4045 CrossRef CAS PubMed.
- X. Zu, X. Li, W. Liu, Y. Sun, J. Xu, T. Yao, W. Yan, S. Gao, C. Wang, S. Wei and Y. Xie, Adv. Mater., 2019, 31, 1808135 CrossRef PubMed.
- J. Yang, Z. Qiu, C. Zhao, W. Wei, W. Chen, Z. Li, Y. Qu, J. Dong, J. Luo, Z. Li and Y. Wu, Angew. Chem., Int. Ed., 2018, 57, 14095–14100 CrossRef CAS PubMed.
- Y. Zhao, H. Zhou, X. Zhu, Y. Qu, C. Xiong, Z. Xue, Q. Zhang, X. Liu, F. Zhou, X. Mou, W. Wang, M. Chen, Y. Xiong, X. Lin, Y. Lin, W. Chen, H.-J. Wang, Z. Jiang, L. Zheng, T. Yao, J. Dong, S. Wei, W. Huang, L. Gu, J. Luo, Y. Li and Y. Wu, Nat. Catal., 2021, 4, 134–143 CrossRef CAS.
- X. Sun, S. Dong and E. Wang, Chem. Commun., 2004, 1182–1183 RSC.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- C. Zhao, X. Dai, T. Yao, W. Chen, X. Wang, J. Wang, J. Yang, S. Wei, Y. Wu and Y. Li, J. Am. Chem. Soc., 2017, 139, 8078–8081 CrossRef CAS PubMed.
- L. Jiao, W. Xu, Y. Zhang, Y. Wu, W. Gu, X. Ge, B. Chen, C. Zhu and S. Guo, Nano Today, 2020, 35, 100971 CrossRef CAS.
- Y. Min, X. Zhou, J.-J. Chen, W. Chen, F. Zhou, Z. Wang, J. Yang, C. Xiong, Y. Wang, F. Li, H.-Q. Yu and Y. Wu, Nat. Commun., 2021, 12, 303 CrossRef CAS PubMed.
- H. Yang, Q. Lin, C. Zhang, X. Yu, Z. Cheng, G. Li, Q. Hu, X. Ren, Q. Zhang, J. Liu and C. He, Nat. Commun., 2020, 11, 593 CrossRef CAS PubMed.
- P. Giusto, H. Arazoe, D. Cruz, P. Lova, T. Heil, T. Aida and M. Antonietti, J. Am. Chem. Soc., 2020, 142, 20883–20891 CrossRef CAS PubMed.
- X. Yu, P. Han, Z. Wei, L. Huang, Z. Gu, S. Peng, J. Ma and G. Zheng, Joule, 2018, 2, 1610–1622 CrossRef CAS.
- Y. Xia, X. Zhao, C. Xia, Z.-Y. Wu, P. Zhu, J. Y. Kim, X. Bai, G. Gao, Y. Hu, J. Zhong, Y. Liu and H. Wang, Nat. Commun., 2021, 12, 4225 CrossRef CAS PubMed.
- D. M. Koshy, S. Chen, D. U. Lee, M. B. Stevens, A. M. Abdellah, S. M. Dull, G. Chen, D. Nordlund, A. Gallo, C. Hahn, D. C. Higgins, Z. Bao and T. F. Jaramillo, Angew. Chem., Int. Ed., 2020, 59, 4043–4050 CrossRef CAS PubMed.
- J. Chen, Z. Wang, H. Lee, J. Mao, C. A. Grimes, C. Liu, M. Zhang, Z. Lu, Y. Chen and S. P. Feng, Mater. Today Phys., 2020, 12, 100176 CrossRef.
- Z. Wang, J. Fan, B. Cheng, J. Yu and J. Xu, Mater. Today Phys., 2020, 15, 100279 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary experimental details, SEM images, HAADF-STEM images, XRD patterns, EXAFS data, XPS spectra and Auger electron spectra. See DOI: 10.1039/d1nr06545a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |