Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids
Yong
Shen
abcdef,
Wen-Juan
Liang
g,
Ya-Na
Shi
ah,
Edward J.
Kennelly
*ij and
Da-Ke
Zhao
*bcde
aCollege of Agronomy and Biotechnology, Yunnan Agricultural University, Kunming, 650201, P. R. China
bBiocontrol Engineering Research Center of Plant Disease and Pest, Yunnan University, Kunming, 650504, P. R. China. E-mail: zhaodk2012@ynu.edu.cn
cBiocontrol Engineering Research Center of Crop Disease and Pest, Yunnan University, Kunming, 650504, P. R. China
dSchool of Life Science, Yunnan University, Kunming, 650504, P. R. China
eKunming Kangren Biotechnology Co., Ltd., Kunming, 650033, P. R. China
fResearch & Development Center for Functional Products, Yunnan Agricultural University, Kunming, 650201, P. R. China
gCollege of Food Science and Technology, Yunnan Agricultural University, Kunming, 650201, P. R. China
hInstitute of Medicinal Plants, Yunnan Academy of Agricultural Sciences, Kunming, 530103, P. R. China
iDepartment of Biological Sciences, Lehman College, City University of New York, Bronx, New York, 10468, USA. E-mail: Edward.kennelly@lehman.cuny.edu
jPhD Programs in Biochemistry, Biology, and Chemistry, The Graduate Center, City University of New York, New York, 10016, USA
First published on 2nd December 2022
Abstract
Correction for ‘Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids’ by Yong Shen et al., Nat. Prod. Rep., 2020, 37, 763–796, https://doi.org/10.1039/D0NP00002G.
The authors regret that there are errors within the article, and the corrections are listed as follows:
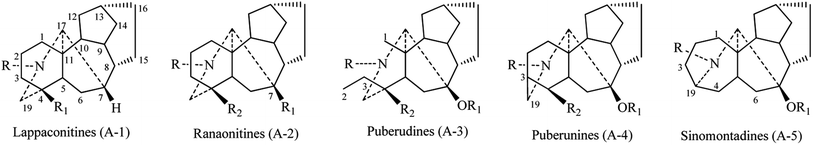
(1) The inclusion of 4β-H for structural types A-2–A-4 in Fig. 1 is not clear, since the alkaloids with 4β-OR (R = H, alkyl, or acyl) groups were not taken into consideration. 4β-R (R = H, OH, alkyl, or acyl) groups, instead of 4β-H, is a better way to represent structural types A-2–A-4 in Fig. 1. The corrected figure is shown below:
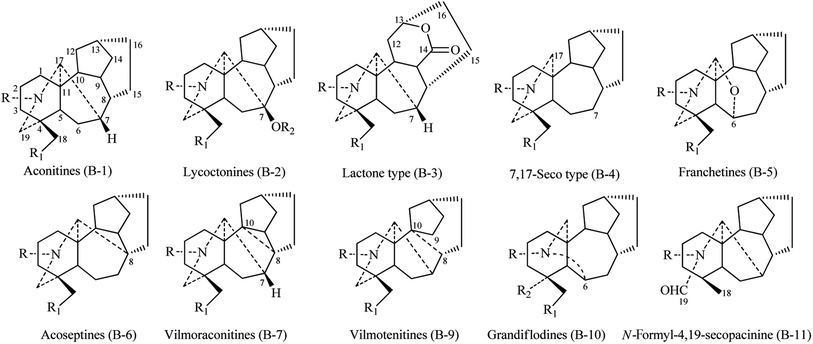
(2) After reviewing the two original references and corresponding NMR data, the alkaloids referred to as hemsleyaconitins and vimotenitins have the same chemical structure. The structure of skeleton B-8 (hemsleyaconitines) in Fig. 2 is incorrect, and should instead be B-9. Therefore, the skeleton B-8 has been deleted. The corrected Fig. 2 is shown below:
(3) The structures of compounds 79, 80, 82, and 83 in Fig. 9 have been redrawn in the 8β-orientation instead of the 8α-orientation. The 8β-OR (R = OAc, OEt, OAs) groups in C18- or C19-DAs are presented according to the Dreiding molecular model. The corrected figure is shown below:
(4) The structures of compounds 245 and 246 in Fig. 16 should be revised to 247 and 248, respectively. The original articles for compounds 245 and 246 (ref. 106 in the original article, ref. 1 here) and 247 and 248 (ref. 2) reported the same NMR data but different structures. The incorrect structures, compounds 245 and 246 in Fig. 16, have been removed. The corrected figure is shown below:
(5) Ref. 57 should be removed from this sentence “Moreover, 19R-acetonyl-talatisamine (49) and hemaconitine D (50) share the same skeleton both bearing an additional-CH2COCH3 group at C-19, which this substituent may be an artifact of isolation and/or purification procedures. 47, 48, 57” on p. 770.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- Y. Shen, A. X. Zuo, Z. Y. Jiang, X. M. Zhang, H. L. Wang and J. J. Chen, Helv. Chim. Acta, 2011, 94, 268 CrossRef CAS.
- L. Cai, H. X. Fang, T. P. Yin, J. Yu, Z. J. Li, J. W. Dong and Z. T. Ding, Phyt. Lett., 2015, 14, 106 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |