Hot off the Press
Robert A.
Hill
 and
Andrew
Sutherland
and
Andrew
Sutherland

School of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: Bob.Hill@glasgow.ac.uk; Andrew.Sutherland@glasgow.ac.uk
First published on 16th June 2022
Abstract
A personal selection of 32 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as chevalinulin A from Aspergillus chevalieri.
The cytisine alkaloid 1 has been reported from seeds of Sophora alopecuroides and its biosynthesis involving 2,4-di-tert-butylphenol has been suggested.1 Although 2,4-di-tert-butylphenol has previously been reported as a natural product it is probably an environmental pollutant. Bipolarithizole A 2 is a metabolite of the potato-endophytic fungus Bipolaris eleusines and is the first example of a phenylthiazole linked to a sativene sesquiterpenoid.2 Lycophlegmarinine A 3, from Phlegmariurus phlegmaria, is a cleaved Lycodpodium alkaloid with a new ring system.3 Massinidine 4 is an antiplasmodial metabolite of Massilia sp. NR 4-1 with an unusual structure.4 The biosynthetic genes were identified and a biosynthetic pathway to massinidine 4 from phenylalanine, acetate and arginine has been proposed.
Beshanzuamide A 5, a metabolite of the endophytic fungus Aspergillus sp. Y-2 obtained from Abies beshanzuensis, has an unprecedented prenylated indole alkaloid skeleton.5 A biosynthetic pathway to beshanzuamide A 5 has been proposed. The structure of chevalinulin A 6, a metabolite of the deep-sea cold-seep-derived fungus Aspergillus chevalieri CS-122, was established by X-ray analysis.6 It is suggested that the biosynthesis of chevalinulin A 6 involves a Diels–Alder cycloaddition of an indole diketopiperazine with a cyclohexadiene precursor.
The first example of a rearranged picrotoxane sesquiterpenoid with an oxetane ring, dendroterpene E 7, has been isolated from Dendrobium nobile and its structure was confirmed by X-ray analysis.7 Papililone A 8, a metabolite of a Papiliomyces species, has an unprecedented tetracyclic diterpenoid skeleton.8 A biosynthetic pathway to papililone A 8, involving an aldol cyclisation, has been proposed.
Commiphoratone C 9, from the resin Commiphora species, is a sesquiterpene dimer with an unprecedented skeleton.9 The structure of commiphoratone C 9 was established by X-ray analysis and it is suggested that its biosynthesis involves a Diels–Alder cycloaddition between germacrane and cadinane precursors. The dimeric abietane diterpenoid bislangduoid A 10 has been isolated from Euphorbia fischeriana.10 One of the abietane units contains an unusual caged acetal structure.
Schisandra and Kadsura species are a rich source of highly rearranged norcycloartane triterpenoids known as schinortriterpenoids. Two further schinortriterpenoids have been isolated from Schisandra chinensis and their structures have both been confirmed by X-ray analyses. Chinorlactone A 11 has a novel 6/5/8/5 carbocyclic ring system11 and schinensilactone A 12 also has an unprecedented skeleton.12 Biosynthetic pathways to the two new schinortriterpenoids have been proposed.
The novel skeleton of alternatone A 13, a metabolite of the endophyte Alternaria alternata L-10 obtained from Capparis spinosa, was confirmed by X-ray structure analysis.13 A biosynthetic pathway involving a hetero-Diels–Alder cycloaddition between two polyketide precursors has been suggested. A Diels–Alder cycloaddition between two polyketides has also been proposed for the formation of asperosin A 14 in Aspergillus rugulosa.14
An intermolecular Diels–Alder cyclisation is implicated in the biosynthesis of heterocageflavone 15 from Artocarpus heterophyllus.15 Heterocageflavone 15 was isolated as a racemate and its structure was confirmed by X-ray analysis. The pterocarpan phaseollin A 16, from Phaseolus lunatus, has a novel 6/7/5/6/6 pentacyclic system.16
The biosynthesis of phomoxanthone A 17, a homodimer of phenexanthone B has been identified from the marine fungus Diaporthe species SYSU-MS4722.17 As well as revealing that the nonreducing polyketide synthase, phoE is responsible for generation of the backbone, feeding studies demonstrated the role of PhoO, a cytochrome P450 enzyme, which catalyses the para–para coupling of penexanthone B to give phomoxanthone A. The biosynthetic gene cluster of the fungal meroterpenoid, atlantinone B 18 has been identified in Penicillium chrysogenum MT-40.18 Studies revealed the role of the cytochrome P450 enzyme, AtlD in the formation of the lactone-bridged ring and stereoselective 16β-hydroxylation.
A terpene synthase, AcAS from Aspergillus calidoustus has been shown to produce spirocyclic calidoustene 19, which has a novel skeleton and five known sesterterpenes.19 Feeding studies and DFT calculations identified the cyclisation mechanism for all six compounds, as well as the rearrangements that allow formation of 19. A biosynthesis of 2-methyisoborneol 20 has been reported using non-natural substrate analogues.20 Coupling of dimethylallyl diphosphate with (S)- or (R)-2-methylisopentenyl diphosphate using farnesyl diphosphate synthase, followed by terpene cyclisation led to efficient formation of 20. Stereoselectively deuterated 2-methylisopentenyl diphosphate isotopomers were used to study the coupling reaction.
An unprecedented pyridoxal 5′-phosphate-dependent enzyme, NphE has been characterised and shown to catalyse both transamination and two-electron oxidation using oxygen, during the biosynthesis of 8-aminoflaviolin 21.21 Studies showed that NphE transfers the amino group of L-glutamate to mompain, followed by the oxidation of a highly conjugated quinonoid intermediate to produce 8-aminoflaviolin. A new biosynthetic pathway of mansouramycin D 22, an isoquinolinequinone alkaloid from Streptomyces albus Del14 has been reported.22 Gene inactivation and heterologous expression identified the biosynthetic genes and feeding studies revealed that tryptophan is the main biosynthetic precursor.
Novel ergot-type compounds, lentopeptin A and lentopeptin B 23 have been isolated from the pathogenic fungus Aspergillus lentulus.23 Gene disruptions, heterologous gene expression and site-directed mutagenesis have identified the biosynthetic genes for the formation of lentopeptins and led to the discovery of enzymes such as cytochrome P450 LenC, which produces both lentopeptin A and 23via two distinct pathways. By heterologous pathway reconstruction, two mono-modular nonribosomal peptide synthetases (NRPS) have been shown to catalyse aminoacylation during indole diterpene biosynthesis, resulting in compounds such as 14-(N,N-dimethylleucyloxy)paspalinine 24.24 The enzyme AceN was shown to catalyse the condensation of 14-hydroxypaspalinine with leucine, in a rare example of NRPS-like enzymes accepting indole diterpene substrates.
Genome mining of the bacterium Bacillus velezensis FZB42 and subsequent heterologous expression of a cryptic NRPS gene cluster has revealed a family of tri-thiazole natural products, including bacillothiazol A 25.25 Gene deletion and biochemical studies have identified the biosynthetic enzymes of the bacillothiazol pathway, including a flavin mononucleotide-dependent oxidase NrsB, which iteratively oxidises the thiazoline units to thiazoles. Varlaxins 1046A and 1022A 26 have been isolated from Nostoc species UHCC 0870 and been shown to inhibit human trypsin isoenzymes at sub-nanomolar concentrations.26 Genome sequencing and bioinformatic analysis revealed that the varlaxins belong to the aeruginosin family of natural products.
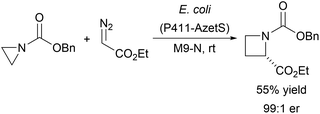
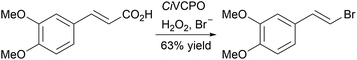
A biocatalytic [1,2]-Stevens rearrangement of aziridines to give azetidines has been developed using an evolved variant of cytochrome P450BM3 (P411-AzetS).27 Reaction of ethyl diazoacetate with carbamate protected aziridines allowed efficient, stereocontrolled one-carbon ring expansion, which could be performed on a preparative scale, generating gram quantities of product (Scheme 1). A chemoselective Hunsdiecker reaction of cinnamic acids using vanadium chloroperoxidase from Curvularia inaequalis (CiVCPO), hydrogen peroxide and bromide ions has been reported (Scheme 2).28 The chloroperoxidase catalyses the hydrogen peroxide-mediated oxidation of bromide to give hypobromite, which then nonenzymatically reacts with the cinnamic acids to give the vinyl bromides.
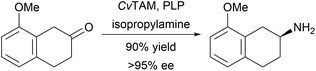
A one-pot, two-step, asymmetric synthesis of N-substituted 1,2-amino alcohols has been developed using enzymatic hydroxymethylation and reductive amination.29 Benzaldehyde lyase-catalysed hydroxymethylation of aldehydes, followed by reductive amination of the resulting α-hydroxymethyl ketones with imine reductases gave 1,2-amino alcohols with excellent enantiomeric excess (Scheme 3). Transaminases have been used for the stereoselective synthesis of (R)- or (S)-8-methoxy-2-aminotetraline from a prochiral β-tetralone (Scheme 4).30 The synthetic utility of this transformation was demonstrated by the conversion of the products of this process to four agonists of the serotonin and melatonin receptors.
The first chemoenzymatic, asymmetric total synthesis of (R)-nodulone C 27, a bioactive polyketide produced by Nodulisporium species has been reported.31 The key step involved an efficient, regio- and stereoselective reduction using tetrahydroxynaphthalene reductase (T4HNR_his) from Magnaporthe grisea (Scheme 5). Re-design of the iron/2-oxoglutarate dependent oxygenase AndA, which catalyses desaturation and isomerisation reactions during the biosynthesis of anditomins, has resulted in a biocatalyst that can perform spiro-ring formation.32 Variants of AndA were shown to convert preandiloid C 28 to unnatural spiro-ring containing compounds via consecutive oxidation reactions (Scheme 6).
References
- Z.-J. Rong, X.-X. Gao, G.-S. Hu, T. Yan, J.-M. Jia and A.-H. Wang, Nat. Prod. Res., 2022, 36, 1864 CrossRef PubMed.
- H.-L. Ai, B.-B. Shi, W. Li, J. He, Z.-H. Li, T. Feng and J.-K. Liu, Org. Chem. Front., 2022, 9, 1814 RSC.
- J.-M. Jiang, D. Xia, X.-L. Zhu, D. Zhu, X.-W. Yang and K. Pan, Tetrahedron, 2022, 114, 132782 CrossRef.
- B. K. Lombe, L. Winand, J. Diettrich, M. Töbermann, W. Hiller, M. Kaiser and M. Nett, Org. Lett., 2022, 24, 2935 CrossRef PubMed.
- Y.-X. Zhu, W. Ding, J.-F. Hu, J. Xiong and J. Li, RSC Adv., 2022, 12, 10534 RSC.
- L.-H. Yan, P.-H. Li, X.-M. Li, S.-Q. Yang, K.-C. Liu, B.-G. Wang and X. Li, Org. Lett., 2022, 24, 2684 CrossRef PubMed.
- P. Wang, X. Chen, C.-H. Cai, F.-D. Kong, S.-Z. Huang, J.-Z. Yuan, X.-L. Xu, W.-L. Mei and H.-F. Dai, Nat. Prod. Res., 2022, 36, 2112 CrossRef PubMed.
- Y. Sun, C.-M. Yuan, S.-Y. Xu, Y. Li, X.-B. Yang, T.-C. Wen and K. Zhou, Tetrahedron Lett., 2022, 93, 153680 CrossRef.
- B.-Y. Hu, Y.-Y. Liu, Y. Liu, Y.-B. Jiao, J.-W. Liu, Y.-M. Yan and Y.-X. Cheng, Org. Chem. Front., 2022, 9, 2549 RSC.
- Z.-L. Yu, M.-R. Zhou, W.-Y. Wang, Y.-B. Chang, C.-P. Sun, X. Lv, C. Wang, W.-Y. Zhao and X.-C. Ma, Bioorg. Chem., 2022, 123, 105759 CrossRef PubMed.
- Y.-C. Yang, T.-R.-G. Bao, S.-Y. Zhu, L.-Q. Liu, G.-Q. Long, Z.-F. Guo, X.-L. Liu, X.-X. Gao, J.-M. Jia and A.-H. Wang, Org. Chem. Front., 2022, 9, 1917 RSC.
- Y. Liu, G.-Z. Liu, X.-M. Li, P. Jiang, Y.-G. Xia, H.-X. Kuang and B.-Y. Yang, Chin. J. Chem., 2022, 40, 1331 CrossRef.
- X. Zhang, X.-X. Liu, Y.-N. Xing, M. Zhang, Y. Zhao, Y.-Y. Wei, B. Zhang and R.-H. Jiao, J. Asian Nat. Prod. Res., 2022, 24, 353 CrossRef PubMed.
- Y. Qiao, X. Tan, Q. Xu, Z. Zhang, Q. Xu, L. Tao, J. Liu, H. Zhu, C. Chen, Y. Ye, Y. Lu, G. Chen, C. Qi and Y. Zhang, Org. Chem. Front., 2022, 9, 2477 RSC.
- G. Ren, L.-S. Gan, L.-Z. Zhu, H.-T. Zeng, F. Xu and T. Yuan, Bioorg. Chem., 2022, 123, 105742 CrossRef PubMed.
- H. Wu, J.-h. Bian, K. Li, Y.-l. Wang, Y.-z. Tan, H.-l. Yan, N. Chen, H.-x. Li, H. Yu, W. Li and G.-h. Lu, Tetrahedron Lett., 2022, 95, 153751 CrossRef.
- S.-W. Yuan, S.-H. Chen, H. Guo, L.-T. Chen, H.-J. Shen, L. Liu and Z.-Z. Gao, Org. Lett., 2022, 24, 3069 CrossRef PubMed.
- B.-w. Qi, N. Li, B.-b. Zhang, Z.-k. Zhang, W.-j. Wang, X. Liu, J. Wang, T. Awakawa, P.-f. Tu, I. Abe, S.-p. Shi and J. Li, Org. Lett., 2022, 24, 2526 CrossRef PubMed.
- Z. Quan, A. Hou, B. Goldfuss and J. S. Dickschat, Angew. Chem., Int. Ed., 2022, 61, e202117273 Search PubMed.
- B. Gu and J. S. Dickschat, Chem. Commun., 2022, 58, 4316 RSC.
- T. Noguchi, S. Isogai, T. Terada, M. Nishiyama and T. Kuzuyama, J. Am. Chem. Soc., 2022, 144, 5435 CrossRef PubMed.
- H. Shuai, M. Myronovskyi, B. Rosenkränzer, C. Paulus, S. Nadmid, M. Stierhof, D. Kolling and A. Luzhetskyy, ACS Chem. Biol., 2022, 17, 598 CrossRef PubMed.
- S. Kishimoto, Y. Matsubara and K. Watanabe, J. Am. Chem. Soc., 2022, 144, 5485 CrossRef PubMed.
- R. M. McLellan, R. C. Cameron, M. J. Nicholson and E. J. Parker, Org. Lett., 2022, 24, 2332 CrossRef PubMed.
- Q. Shen, H. Zhou, G. Dai, G. Zhong, L. Huo, A. Li, Y. Liu, M. Yang, V. Ravichandran, Z. Zheng, Y.-J. Tang, N. Jiao, Y. Zhang and X. Bian, ACS Catal., 2022, 12, 3371 CrossRef.
- L. M. P. Heinilä, J. Jokela, M. N. Ahmed, M. Wahlsten, S. Kumar, P. Hrouzek, P. Permi, H. Koistinen, D. P. Fewer and K. Sivonen, Org. Biomol. Chem., 2022, 20, 2681 RSC.
- D. C. Miller, R. G. Lal, L. A. Marchetti and F. H. Arnold, J. Am. Chem. Soc., 2022, 144, 4739 CrossRef PubMed.
- H. Li, S. H. H. Younes, S. Chen, P. Duan, C. Cui, R. Wever, W. Zhang and F. Hollmann, ACS Catal., 2022, 12, 4554 CrossRef PubMed.
- Y. Li, N. Hu, Z. Xu, Y. Cui, J. Feng, P. Yao, Q. Wu, D. Zhu and Y. Ma, Angew. Chem., Int. Ed., 2022, 61, e202116344 Search PubMed.
- D. Baud, N. Tappertzhofen, T. S. Moody, J. M. Ward and H. C Hailes, Adv. Synth. Catal., 2022, 364, 1564 CrossRef.
- T. Manna, A. Rajput, N. Saha, A. Mondal, S. C. Debnath and S. M. Husain, Org. Biomol. Chem., 2022, 20, 3737 RSC.
- T. Mori, Y. Nakashima, H. Chen, S. Hoshino, T. Mitsuhashi and I. Abe, Chem. Commun., 2022, 58, 5510 RSC.
| This journal is © The Royal Society of Chemistry 2022 |