Open Access Article
Open Access ArticleDithiocarbamate transfer reaction from methylene-bis(dithiocarbamates) to molybdenum dithiocarbamates in engine lubricants investigated using laboratory experiments†
Yu Min
Kiw
 ab,
Philippe
Schaeffer
ab,
Philippe
Schaeffer
 a,
Pierre
Adam
a,
Pierre
Adam
 *a,
Benoît
Thiébaut
b and
Chantal
Boyer
b
*a,
Benoît
Thiébaut
b and
Chantal
Boyer
b
aUniversity of Strasbourg, CNRS, Institut de Chimie de Strasbourg UMR 7177, F-67000 Strasbourg, France. E-mail: padam@unistra.fr
bTotalEnergies Solaize Research Center, BP22-69360 Cedex, France
First published on 4th November 2022
Abstract
Elaboration of new engine oil specifications and growing level of environmental awareness have been calling for better fuel efficiency and reduction of carbon emissions. In this context, molybdenum dithiocarbamates (MoDTC) are important lubricant additives used to reduce friction coefficients and hence to improve fuel economy. It has been recently shown that under engine test conditions, the combined use of MoDTC and methylene-bis(dithiocarbamates) (MBDTC) leads to the prolonged existence of MoDTC complexes at useful levels in formulated engine oil due to ligand transfer reactions, resulting in the synergistic enhancement of tribological performances. In order to investigate the process of this ligand transfer at the molecular level in detail, laboratory ageing experiments involving MoDTC, MBDTC and zinc dithiophosphates (ZnDTP) – a combination of additives frequently used in formulated lubricants – in solution in hydrocarbon base oils were performed under thermal and thermo-oxidative conditions. The evolution of the concentrations of the substrates and of their transformation products were followed using HPLC-MS, Probe-MS analyses and NMR spectroscopy. It could be shown that Zn(II) complexes such as ZnDTP are able to induce dithiocarbamate (DTC) transfer reactions from MBDTC to MoDTC through a mechanism involving activation of carbon-sulfur bonds on MBDTC. Zn(II) ions act as a Lewis acid inducing the release of DTC from MBDTC. Oxidative conditions (NO2 in air) were also shown to induce the release of DTC from MBDTC, even in the absence of Zn(II) ions, which then become available for ligand exchange with MoDTC or for re-complexation of the metal core of Mo complexes (Mo2O2S2 or Mo oxysulfides) after partial oxidative degradation of the genuine MoDTC ligands.
1. Introduction
Energy saving, worldwide concerns over CO2 emission and the introduction of new engine oil specifications are among the major driving forces to increase fuel economy in internal combustion engines.1–3 The development of new engine technologies4 and lubricants5 plays a significant role in attempting to achieve this goal. Over the past decades, different families of additives have been widely employed in lubricant oils to protect the engine metallic parts from thermal, chemical and mechanical degradation as well as to improve the overall lubricating performances.5 One of the most general trend to improve fuel efficiency is the use of molybdenum dithiocarbamates (MoDTC) as effective friction modifiers in lubricants since they lower significantly the friction coefficient at the tribological contacts under boundary lubrication conditions.6–10However, MoDTC undergo thermo-oxidative degradation during engine functioning, resulting in the progressive loss of the ability of lubricants to reduce friction.11–14 To date, only a few studies have been carried out to follow at the molecular level the progressive degradation of MoDTC, as well as their chemical transformation pathways. Fourier transform infrared spectroscopy (FT-IR),12–14 mass spectrometry (MS),13–17 UV-vis spectrophotometry,18 and NMR spectroscopy15,19 are among the main analytical techniques reported in the literature to investigate the evolution and chemical behaviour of MoDTC in lubricants. A better understanding of their degradation pathways in engine oil when subjected to thermo-oxidative degradation is still important since it might potentially lead to the design of novel molecules with improved tribological properties and enhanced resistance to alteration.
Despite the excellent efficiency of MoDTC to reduce friction coefficient to relatively low values, the tribological performances of MoDTC in engine oils still highly depend on the nature and chemistry of the other additives present in formulated oils. The occurrence of synergistic or antagonistic interactions between MoDTC and other lubricant additives plays an important role in minimizing the friction in automobile engines. For example, zinc dithiophosphates (ZnDTP), which are amongst the most widely used multifunctional additives in engine oils,20 have been shown to promote the formation of MoS2 and to enhance the persistence of the MoS2 tribofilm when used in combination with MoDTC.21,22 In this respect also, the synergistic interactions between MoDTC and methylene-bis(dithiocarbamates) (MBDTC) have been recently shown to increase the durability of MoDTC and to extend the friction reducing capability of formulated engine lubricants to longer periods under engine test conditions.23 Since the MoDTC remaining after prolonged functioning of the engine were shown to exclusively bear ligands corresponding to DTC moieties from MBDTC – the genuine MoDTC species having been completely degraded –, it was concluded that the prolonged existence of MoDTC was due to the progressive and constant replacement of the degraded DTC ligands from MoDTC educts by DTC released from MBDTC during engine functioning.23
The present study aimed to deepen the knowledge of the interactions between MoDTC and MBDTC and to explore their synergy at the molecular level in the presence of different lubricant additives. For this purpose, laboratory ageing experiments under thermal (non-oxidizing) and thermo-oxidative conditions reproducing those undergone by lubricants in operating engines have been performed on mixtures involving MoDTC, MBDTC and eventually Zn(II) complexes in solution in a hydrocarbon base oil or in fully formulated lubricants. The evolution of the substrates and of their transformation products formed upon alteration was followed using HPLC-MS, Probe-MS analyses and NMR spectroscopy. Focus has been put on the evaluation of the effect of ZnDTP and oxidative conditions on the interactions between MoDTC and MBDTC. Interactions of MBDTC with other lubricant additives have, to our knowledge, not been investigated at a molecular level so far using such an approach, MBDTC being postulated to mainly act as an antioxidant in additivated lubricants.24 A comprehensive understanding of the underlying chemical processes involved in the interaction between MoDTC and MBDTC in lubricants is indeed expected to offer new strategies for molybdenum tribochemistry optimization in real engine systems.
2. Experimental section
2.1. Materials (oils and additives)
The mineral base oil used in this work was provided by TotalEnergies Marketing Services. The two fully formulated oils (referred to as “oil A” and “oil AMBDTC”) were also provided by TotalEnergies Marketing & Services and have globally a similar composition comprising, notably, alkylated aryl amine antioxidants and secondary zinc dithiophosphates 2b (bold numbers refer to the chemical structures shown in Fig. 1). The sole difference between oil A and oil AMBDTC is the additional presence of MBDTC 3a (2 wt%) in the oil AMBDTC. The commercial product Sakuralube S525 (mixture of MoDTC 1a–1c; 10 wt% Mo in hydrocarbon base oil) was purchased from Adeka. The primary ZnDTP complexes 2a with (ethylhexyl) alkyl chains (7.3 wt% P in base oil, trade name: OLOA 269R) and the secondary ZnDTP 2b (mixture of compounds with C3 and C6 alkyl chains; 7.0 wt% P in base oil, trade name: Infineum C9426) were both purchased from Infineum. MBDTC 3a (Vanlube 7723) was obtained from Vanderbilt Chemicals. Unless otherwise indicated, all the purchased additives were used without prior purification. ZnDTC 5a, MBDTC 3b and methylene-bis(dithiophosphate) (MBDTP) 4 were obtained by synthesis (cf. § 2.2, § 2.3 and Kiw et al.15).2.2. Synthesis of methylene-bis(di-n-decyl-dithiocarbamate) 3b
MBDTC 3b was prepared according to a published procedure.25 NaOH (1.6 g, 40 mmol, 2.4 eq.) and n-didecylamine (5.0 g, 16.8 mmol, 1 eq.) were mixed and dissolved in water (15 mL) at 5 °C. Carbon disulfide (2.3 mL, 38 mmol, 2.3 eq.) was added dropwise under stirring into the reaction mixture and the temperature was kept below 15 °C. Stirring was continued for a further 30 min (T < 15 °C). A large excess of methylene chloride (20 mL) was added and the mixture was stirred for 1 h. The crude mixture was then transferred into a separatory funnel and ethyl acetate (100 mL) and water (100 mL) were added. The organic phase was recovered, and the mixture was re-extracted with ethyl acetate (100 mL). The combined organic layers were dried over magnesium sulfate, filtrated and concentrated in vacuo. Purification on silica gel using cyclohexane/methylene chloride (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) as eluent yielded methylene-bis(di-n-decyl-dithiocarbamate) 3b (5.29 g, 83%) as a red-orange oil.
10 v/v) as eluent yielded methylene-bis(di-n-decyl-dithiocarbamate) 3b (5.29 g, 83%) as a red-orange oil.
1H-NMR (500 MHz; CDCl3): 5.40 (2H, s), 3.91 (4H, t, J = 7.8 Hz), 3.60 (4H, t, J = 8.0 Hz), 1.75–1.63 (8H, m), 1.2–1.33 (56H, m), 0.88 (12H, t, J = 6.0 Hz).
13C-NMR (126 MHz; CDCl3): 195.09, 55.78, 53.06, 46.26, 32.03, 29.71, 29.69, 29.66, 29.48, 29.45, 29.37, 27.46, 27.03, 26.98, 26.37, 22.84, 14.28.
Probe-MS (EI, 70 eV): m/z (relative intensity) 418 (100%), 386 (4), 340 (15), 308 (9), 200 (9), 170 (23), 85 (23). See also Fig. S1 and S2 (ESI†) for detailed 1H- and 13C-NMR spectra of 3a and 3b respectively, and Fig. S3 (ESI†) for the mass spectrum of 3b under EI ionization mode (probe MS).
2.3. Synthesis of methylene-bis(dithiophosphate) 4
The synthesis of MBDTP 4 was carried out to obtain a reference compound for identification purposes (cf. § 3.2) because this compound is currently not commercially available. Prior use, ZnDTP 2a was purified by column chromatography on silica gel eluting first with cyclohexane (2 dead volumes) to remove the base oil, and then with methylene chloride (3 dead volumes) to recover pure ZnDTP 2a.Purified ZnDTP 2a (78 mg, 101 μmol), NaI (14 mg, 93 μmol), CH2ClBr (100 μL, 193 mg, 1.52 mmol) and MeOH (250 μL) were mixed in a vial which was closed and heated at 60 °C for 6 h, after which CH2ClBr and MeOH were removed under a flux of argon. NaI (14 mg, 93 μmol), MeOH (250 μL) and toluene (250 μL) were added to the crude mixture which was heated for a further 14.3 h. After cooling to room temperature and addition of water (20 mL), the organic phase was extracted with methylene chloride (20 mL) and washed with water (20 mL), dried over anhydrous Na2SO4 and the solvents were removed under reduced pressure. The crude extract (66 mg) was purified on silica gel by eluting with a mixture of methylene chloride/cyclohexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v; 25 mL), yielding a fraction (50 mg) containing MBDTP 4 as the predominant constituent as shown by MS analysis. For characterization purposes, an aliquot (14 mg) of this fraction was fractionated by thin layer chromatography (TLC) on silica gel (20 cm × 20 cm; 0.5 mm silica gel thickness) using ethyl acetate/cyclohexane (2.5
3 v/v; 25 mL), yielding a fraction (50 mg) containing MBDTP 4 as the predominant constituent as shown by MS analysis. For characterization purposes, an aliquot (14 mg) of this fraction was fractionated by thin layer chromatography (TLC) on silica gel (20 cm × 20 cm; 0.5 mm silica gel thickness) using ethyl acetate/cyclohexane (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97.5 v/v) as eluent. The TLC plate was developed twice and the main fraction (Rf ≈ 0.8) was scrapped off from the plate, yielding ca. 7 mg of pure MBDTP 4.
97.5 v/v) as eluent. The TLC plate was developed twice and the main fraction (Rf ≈ 0.8) was scrapped off from the plate, yielding ca. 7 mg of pure MBDTP 4.
1H-NMR (500 MHz; CDCl3): 4.27 (2H, t, J = 17.3), 4.06 (4H, m), 3.96 (4H, m), 1.62 (4H, m), 1.39 (8H, m), 1.30 (22H, m), 0.91 (26H, m).
13C-NMR (126 MHz; CDCl3): 70.38, 70.36, 70.32, 70.30, 39.90, 39.88, 39.83, 39.81, 37.17, 37.13, 37.10, 30.07, 30.00, 28.92, 28.89, 23.47, 23.40, 22.97, 14.07, 10.99, 10.96.
Note that there are several signals comprised within a small ppm range for a given carbon atom because the synthesized MBDTP 4 corresponds to a mixture of diastereomers due to the occurrence of an asymmetric centre on the ethylhexyl alkyl chains.
31P-NMR (121 MHz; CDCl3): 93.0 (2P, s) HPLC-MS (APPI, positive mode), m/z (relative intensity) 721 (100%; [M + H]+), 609 (62), 497 (44), 385 (12), 273 (7). Probe-MS (EI, 70 eV): m/z (relative intensity) 609 (10%), 497 (48), 385 (92), 273 (100) see also Fig. S4 (ESI†) for 1H- and 13C-NMR spectra of 4, Fig. S5 and S6 (ESI†) for HPLC-MS chromatogram and mass spectra of 4 under APPI and EI ionization mode (probe MS).
2.4. Laboratory oil ageing experiments
Table 1 shows the starting products and experimental conditions used for the different laboratory oil ageing experiments. 50 g of hydrocarbon base oil containing MoDTC and other additives (1 or 2 wt%, wt% refers to weight percent of the commercial additives) were heated under stirring (1500 rpm) to insure a good homogenization of the temperature within the reaction medium at 135 °C in a multi-necked round-bottomed flask with a constant flow of gas (100 mL min−1) bubbling within the reaction medium using argon or NO2 in air (2000 ppm) for non-oxidative and thermo-oxidative experiments, respectively. Additionally, thermo-oxidative ageing experiments (NO2, 2000 ppm in air) were performed on formulated engine oils (oils A and AMBDTC) at 135 °C under stirring (1000 rpm) to insure homogenization of the temperature within the reaction mixture and a good mixing of the oil and the reactive gases during the thermo-oxidative experiments.| Experiment | Starting products | Atmosphere | Temperature (°C) |
|---|---|---|---|
| a In hydrocarbon base oil. b 2000 ppm NO2 in air. | |||
| 1 | MoDTC 1a–1c + MBDTC 3aa | Argon | 135 |
| 2 | MoDTC 1a–1c + MBDTC 3a + ZnDTP 2aa | Argon | 135 |
| 3 | MoDTC 1a–1c + MBDTC 3a + ZnDTP 2ba | Argon | 135 |
| 4 | MoDTC 1a–1c + MBDTC 3b + ZnDTP 2aa | Argon | 135 |
| 5 | MoDTC 1a–1c + MBDTC 3a + ZnDTC 5aa | Argon | 135 |
| 6 | MoDTC 1a–1c + MBDTC 3aa | NO2b | 135 |
| 7 | Formulated oil A | NO2b | 135 |
| 8 | Formulated oil AMBDTC | NO2b | 135 |
In combustion engines, the internal temperature of the lubricating oil is in the range 90–105 °C26 with significantly higher temperatures at certain zones of the engine lubricating system. The temperature of 135 °C selected for the laboratory experiments thus corresponds to a compromise taking into account the occurrence of hot spots in the engine. In this respect, temperatures up to 160 °C are generally reported in the literature for lubricant ageing experiments.14,27,28
For the different experiments, the starting products were dissolved in the hydrocarbon oil base (50 g). Aliquots of oil samples (1 mL) were collected at 1 h intervals through a septum using a syringe for analysis by HPLC-MS and for tribological tests. The first sample was collected when the oil mixture reached 135 °C (defined as T = 0 h). In the case of the experiments carried out under NO2 in air, the oil mixtures were first heated up to 135 °C under argon bubbling, and then argon was replaced by 2000 ppm of NO2 in air.
2.5. Reaction between MBDTC 3a and ZnDTP 2a (neat mixture)
A mixture of MBDTC 3a (275 mg, 0.65 mmol) and purified primary ZnDTP 2a (500 mg, 0.65 mmol; see § 2.3 for the purification of 2a) was vacuum-sealed in a glass tube which was heated at 150 °C for 8 h. An aliquot (5 mg) of the mixture was dissolved in CDCl3 for NMR analysis (1H, 13C, 31P) and another aliquot (ca. 1 mg) of the reaction mixture was dissolved in n-heptane/isopropanol (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v; 1 mL) for HPLC-MS analysis.
5 v/v; 1 mL) for HPLC-MS analysis.
2.6. High pressure liquid chromatography-mass spectrometry (HPLC-MS) analyses
MoDTC involved in oil ageing experiments were analyzed by HPLC-MS using an Agilent HP 1100 series HPLC instrument equipped with an auto-injector and connected to a Bruker Esquire 3000Plus ion trap mass spectrometer with an atmospheric pressure photoionization (APPI) source operating in the positive mode as described in Kiw et al.15 Sample preparation and quantification of MoDTC derivatives were carried out as described in Kiw et al.15 It is worth noting that under the analytical conditions developed by Kiw et al.,15 ZnDTP derivatives could not be detected.3. Results
3.1. Interactions between MoDTC and MBDTC
DTC transfer reactions were investigated by means of laboratory oil ageing experiments involving MBDTC 3a (2 wt%) and MoDTC 1a–1c (1 wt%) in solution in a hydrocarbon base oil under argon bubbling at 135 °C for 18 h (Experiment 1, Table 1). Complementary experiments were performed using primary and secondary ZnDTP (2a and 2b, respectively, 1 wt%; experiments 2 and 3, Table 1) in order to evaluate the role of Zn species in the interactions between MBDTC 3a and MoDTC 1a–1c. An experiment was also performed using the synthesized MBDTC 3b bearing C10 alkyl chains instead of the commercial MBDTC 3a bearing C4 alkyl chains (Experiment 4, Table 1). In addition, DTC exchange reactions between MoDTC 1a–1c and MBDTC 3a were investigated in the presence of ZnDTC 5a (Experiment 5, Table 1) and under NO2 in air (Experiment 6, Table 1). Experiments were also carried out with fully formulated engine oils (oils A and AMBDTC; see § 2.1) under thermo-oxidative conditions (Experiments 7 and 8, Table 1). Identification and monitoring of the evolution of the concentration of the various MoDTC derivatives and of the newly-formed products were performed by HPLC-MS.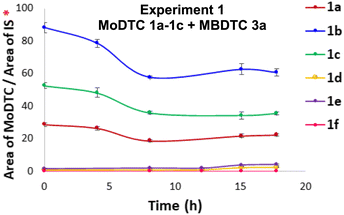 | ||
| Fig. 2 Evolution of the concentrations of MoDTC 1a–1c and of the newly formed MoDTC 1d–1f bearing DTC ligands with C4 chains in an experiment involving MoDTC 1a–1c and MBDTC 3a in hydrocarbon base oil under argon bubbling (Experiment 1, Table 1). IS: internal standard. *Y-axis: arbitrary units. Error bars correspond to triplicate HPLC-MS analyses for each sample. | ||
In contrast to the experiment carried out without ZnDTP (cf. § 3.1.1.), the progressive formation of a yellowish precipitate in the reaction mixture over time was observed in the presence of both primary and secondary ZnDTP (see Fig. S8, ESI† for the visual aspect of the reaction mixture for the experiment with primary ZnDTP 2a). This precipitate was retrieved by filtration from the reaction mixture, dissolved in n-heptane/isopropanol (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v; 1 mL) and analysed using APPI-MS and Probe EI-MS in the case of the reaction with primary ZnDTP 2a (Fig. S9, ESI†). It could be determined that the precipitate corresponded predominantly to MoDTC 1f and MoDTC 1d–1e to a minor extent. The shorter alkyl chains (C4H9) of the DTC ligands as compared to those from the starting MoDTC 1a–1c are likely to be responsible for the lower solubility of the newly-formed MoDTC complexes 1d–1f (notably in the case of 1f) in the hydrocarbon base oil and thereby explaining their progressive precipitation in the hydrocarbon base oil. These complexes (1d–1f) result from DTC exchange reactions between MoDTC 1a–1c and MBDTC 3a. In the present experiment, the formation of a precipitate suggests that the yield of this exchange was obviously significantly increased as compared to the experiments performed without ZnDTP and could be explained by the fact that ZnDTP species is involved in the DTC exchange reaction between MBDTC and MoDTC.
5 v/v; 1 mL) and analysed using APPI-MS and Probe EI-MS in the case of the reaction with primary ZnDTP 2a (Fig. S9, ESI†). It could be determined that the precipitate corresponded predominantly to MoDTC 1f and MoDTC 1d–1e to a minor extent. The shorter alkyl chains (C4H9) of the DTC ligands as compared to those from the starting MoDTC 1a–1c are likely to be responsible for the lower solubility of the newly-formed MoDTC complexes 1d–1f (notably in the case of 1f) in the hydrocarbon base oil and thereby explaining their progressive precipitation in the hydrocarbon base oil. These complexes (1d–1f) result from DTC exchange reactions between MoDTC 1a–1c and MBDTC 3a. In the present experiment, the formation of a precipitate suggests that the yield of this exchange was obviously significantly increased as compared to the experiments performed without ZnDTP and could be explained by the fact that ZnDTP species is involved in the DTC exchange reaction between MBDTC and MoDTC.
In parallel, based on HPLC-MS analysis, the formation of mono- and di-sulfurized analogues of all the MoDTC species present in the reaction mixture was observed (compounds 1S-1a–1f and 2S-1a–1f; see Fig. S10, ESI† for the quantification results of 1S-1d–1f and 2S-1d–1f). They result from the sulfurization of MoDTC induced by ZnDTP, their occurrence and mode of formation being described in Kiw et al.(2022).16 In the following discussion, since DTC exchange reaction is the focus of the present article, the sulfurized analogues of MoDTC will not be considered further, although they can represent up to 47% and 18% of the transformation products of the initial MoDTC in the case of the experiments involving secondary ZnDTP 2b and primary ZnDTP 2a, respectively (see Fig. S11, ESI†).
As a result, the relative concentrations of the MoDTC substrates 1a–1c significantly decreased with time in the presence of both primary and secondary ZnDTP (Fig. 3). Meanwhile, the concentration of the newly formed MoDTC complexes 1d and 1e increased during heating but tended to slowly decrease afterwards (Fig. 3), whereas those of the MoDTC complex 1f increased initially with time and seemed to reach a plateau between 15 and 18 h (Fig. 3).
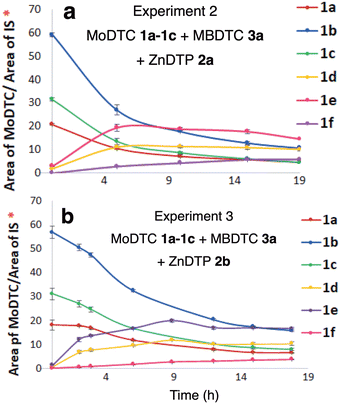 | ||
| Fig. 3 Evolution of the concentrations of MoDTC 1a–1c and of the newly formed MoDTC 1d–1f during the experiments involving MoDTC 1a–1c and MBDTC 3a in the presence of (a) primary ZnDTP 2a (Experiment 2, Table 1) and (b) ZnDTP 2b (Experiment 3, Table 1). IS: internal standard. *Y-axis: arbitrary units. Error bars correspond to triplicate HPLC-MS analyses for each sample. | ||
The progressive decrease of the concentration of MoDTC 1d and 1e can be partly explained by the fact that these species correspond to the intermediate MoDTC species that are ultimately transformed into MoDTC 1f. In addition, it could be shown that the precipitate formed during the experience corresponded to a mixture of MoDTC 1d–1f (see above), which indicates that a noticeable part of the newly-formed MoDTC bearing C4-alkyl chains – and notably MoDTC 1f- escaped from the pool of the samples collected (in solution) for quantitative analysis throughout the experiment.
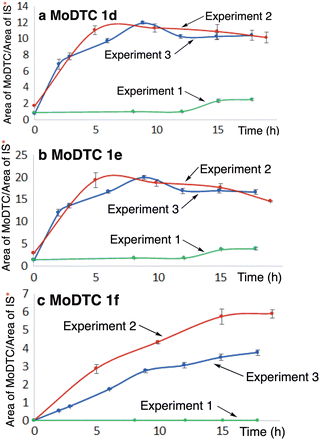 | ||
| Fig. 4 Evolution of the concentrations of the newly formed MoDTC (a) 1d (b) 1e and (c) 1f during the experiments involving: (i) green colour: MoDTC 1a–1c and MBDTC 3a (Experiment 1, Table 1); (ii) blue colour: MoDTC 1a–1c, MBDTC 3a and secondary ZnDTP 2b (Experiment 3, Table 1); (iii) red colour: MoDTC 1a–1c, MBDTC 3a and primary ZnDTP 2a (Experiment 2, Table 1). IS: internal standard. *Y-axis: arbitrary units. Error bars correspond to triplicate HPLC-MS analyses for each sample. The initial concentrations of the substrates for the experiments were: MoDTC: 1 wt%; MBDTC: 2 wt%; ZnDTP: 1 wt% (if present). | ||
However, as pointed out previously, it is worth noting that the formation of a precipitate containing predominantly the MoDTC 1f (cf. Fig. S9, ESI†) during the experiments prevented a precise quantification to be made. By comparison, in the absence of ZnDTP, the MoDTC complexes 1d and 1e were formed at a significantly slower rate (Fig. 4a and b, green colour), and MoDTC 1f was not formed at all (Fig. 4c, green colour). It should be noted that an oil ageing experiment involving MoDTC 1a–1c, MBDTC 3a, and Zn(acac)2 – another Zn(II) species – in a hydrocarbon base oil under argon atmosphere at 135 °C also resulted in the formation of the new MoDTC complexes 1d-1f (results not shown).
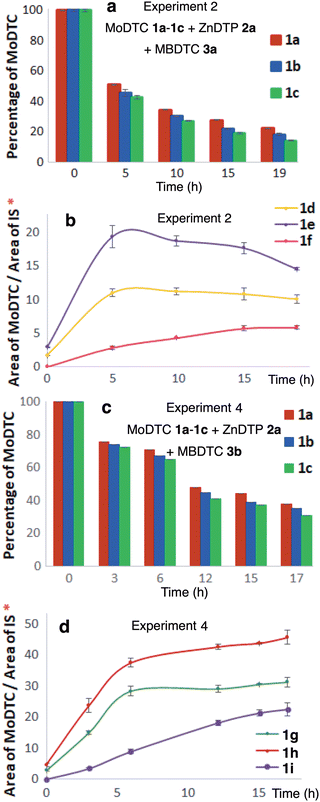 | ||
| Fig. 5 Evolution (a and c) of the relative abundance of MoDTC substrates 1a–1c and (b and d) of the concentrations of the newly formed MoDTC complexes 1d–1i during the experiments involving MoDTC 1a–1c, MBDTC 3a and primary ZnDTP 2a (Experiment 2, Table 1) and MoDTC 1a–1c, MBDTC 3b and primary ZnDTP 2a (Experiment 4, Table 1). IS: internal standard. *Y-axis: arbitrary units. Error bars correspond to the triplicate HPLC-MS analyses of each sample. | ||
As expected, no precipitate was formed during the experiment (Fig. S13, ESI†), and HPLC-MS quantification showed the concomitant decrease of the concentration of the MoDTC substrates 1a–1c (Fig. 5c) together with the progressive increase in concentration of the MoDTC complexes 1g–1i over time (Fig. 5d) as was observed in the case of the experiment with MBDTC 3a (Fig. 5a and b).
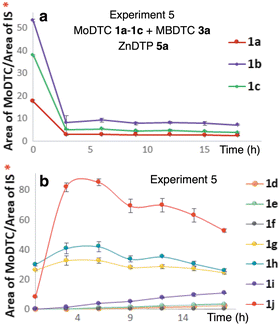 | ||
| Fig. 6 Evolution of the concentrations of (a) MoDTC substrates 1a–1c; (b) newly formed MoDTC complexes 1d–1j during an experiment involving MoDTC 1a–1c, MBDTC 3a and ZnDTC 5a (Experiment 5, Table 1). IS: internal standard. *Y-axis: arbitrary units. Error bars correspond to triplicate HPLC-MS analyses for each sample. | ||
These compounds were formed by DTC exchange reactions between the MoDTC 1a–1c, MBDTC 3a and ZnDTC 5a. Accordingly, during the first 3 h of experiment, a rapid decrease of the concentrations of the MoDTC substrates 1a–1c over time was observed (Fig. 6a) paralleling the progressive formation of the ligand exchange products 1g–1i (Fig. 6b).
3.2. Investigation of the interactions between MBDTC 3a and ZnDTP 2a
In order to get a better understanding of the role of ZnDTP in accelerating the DTC transfer reactions between MBDTC and MoDTC species, the interactions between MBDTC and ZnDTP were investigated by 1H- and 13C-NMR spectroscopy as well as by HPLC-MS. For this purpose, MBDTC 3a was mixed with ZnDTP 2a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio without further dilution, vacuum sealed in a glass tube and heated at 150 °C for a period of 8 h prior analysis.
1 molar ratio without further dilution, vacuum sealed in a glass tube and heated at 150 °C for a period of 8 h prior analysis.
NMR analysis of the reaction mixture after 8 h at 150 °C showed that the 1H chemical shifts of the protons on the methylene groups adjacent to the functional groups (α-OP or α-N) of the substrates ZnDTP 2a and MBDTC 3a, and of the compounds postulated to be formed during the DTC exchange reaction (i.e., ZnDTC 5b, methylene-bis(dithiophosphate) (MBDTP) 4 and the mixed species with DTP/DTC ligands or moieties; Fig. 7) are all in a range comprised between 3.0–5.5 ppm (Fig. 8).
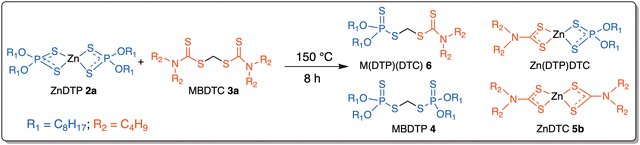 | ||
| Fig. 7 Ligand exchange reactions between ZnDTP 2a and MBDTC 3a potentially leading to the formation of different compounds bearing DTP and DTC moieties or ligands. | ||
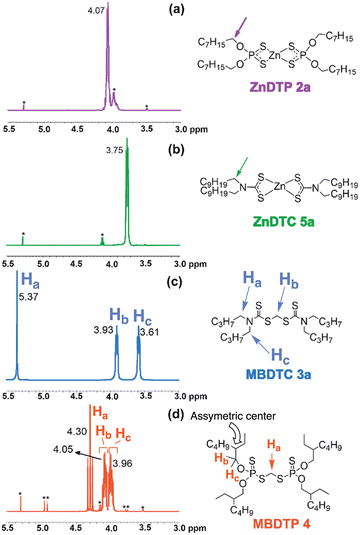 | ||
| Fig. 8 Partial 1H-NMR spectra (3.0–5.5 ppm, 500 MHz, CDCl3) of reference compounds (a) ZnDTP 2a, (b) ZnDTC 5a, (c) MBDTC 3a and (d) MBDTP 4. *: impurities. | ||
Since MBDTP 4 was not commercially available, it was specifically synthesized (cf. § 2.3) in order to obtain reference 1H- and 13C-NMR spectra. The differences in the 1H chemical shifts of the signals of these compounds in the 3.0–5.5 ppm range as well as the interpretation of their integral values allowed the educts and the various products formed by reaction between ZnDTP 2a and MBDTC 3a to be distinguished. The 4 methylene groups adjacent to the functional groups of ZnDTP 2a (resp. ZnDTC 5a) have the same chemical shift due to symmetry reason and only 1 multiplet is observed for these protons at 4.07 ppm for 2a (resp. 3.75 ppm for 5b) (Fig. 8a and b). In the case of MBDTC 3a, the two alkyl chains on the same DTC moiety are not equivalent because of the limited rotation around the C–N bond. As a result, two signals at 3.93 and 3.61 ppm are observed for the methylene groups adjacent to the N–CS2 bonds of 3a. The signal observed at 5.37 ppm (Fig. 8c) corresponds to the protons from the central methylene group.
In the case of MBDTP 4, all the methylene groups located α to the O–P bond are identical. The two protons on these methylenes are however not equivalent because of the presence of an asymmetric center at C-2 (2-ethylhexyl alkyl chains), thus explaining the presence of two multiplet signals (Hb and Hc, Fig. 8d) at 3.96 and 4.05 ppm. The complexity of the coupling pattern can be explained by the occurrence of 3J P–H couplings in addition to the 2J and 3J H–H couplings. Similarly, the signal at 4.30 ppm (Ha, Fig. 8d) which corresponds to the methylene linked to the two dithiophosphate moieties appears as a triplet because of the 3J couplings with both P atoms (see Fig. S15, ESI† for the partial P-decoupled 1H-NMR spectrum of MBDTP 4).
The partial disappearance of the substrates ZnDTP 2a and MBDTC 3a, and the formation of new compounds was observed on the 1H-NMR spectrum of the mixture after 8 h of heating at 150 °C (Fig. 9).
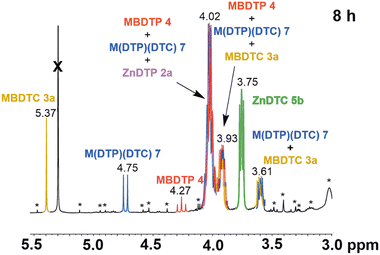 | ||
Fig. 9 Partial 1H-NMR spectrum (3.0–5.5 ppm, 500 MHz, CDCl3) of the mixture obtained after 8 h of heating at 150 °C of MBDTC 3a and ZnDTP 2a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. *: impurities. 1 molar ratio. *: impurities. | ||
The newly formed compounds could be identified based on the comparison of the 1H-NMR spectra of the reaction mixture (Fig. 9) and of reference compounds (Fig. 8 and Kiw et al.15 – Fig. S5, ESI†). Thus, the signal at 3.75 ppm (Fig. 9, green colour) presents chemical shifts and a coupling pattern comparable with that of the methylene groups located on α position to the N–CS2 bonds of ZnDTC 5a bearing n-decyl alkyl chains (Fig. 8b and Kiw et al.15 – Fig. S5, ESI†). It is, therefore, most likely that it corresponds to ZnDTC 5b with DTC ligands bearing C4 alkyl chain originating from MBDTC 3a. The formation of MBDTP 4 was evidenced by the appearance of a triplet at 4.27 ppm (Fig. 9, red colour) which is similar to that of the central methylene group of the reference synthetic compound (Fig. 8d and Fig. S4, ESI†). In addition, the signals at 4.02 and 3.93 ppm are also compatible with those of the methylene groups adjacent to the O–P bonds of MBDTP 4. However, integration of these signals suggests that they are superimposed with the signals of other compounds present in the mixture as discussed below.
The signal at 4.75 ppm (Fig. 9, blue colour), which is absent in the 1H-NMR spectra of reference compounds (Fig. 8), was attributed to the central methylene group of M(DTP)(DTC) 6 bearing mixed DTP/DTC moieties. Indeed, this chemical shift has an intermediate value between that of the methylene group from MBDTC 3a and MBDTP 4. In addition, this signal appears as a doublet due to the coupling of the protons from the central methylene group with one P atom from the dithiophosphate moiety. The chemical shifts of the methylene groups located on α position to the N–CS2 and O–P bonds of M(DTP)(DTC) 6 are expected to be similar or even identical to those of the MBDTC 3a and MBDTP 4, respectively, and most likely contribute to the signals at 4.02, 3.93 and 3.61 ppm (Fig. 9, blue colour). By interpreting the integrated areas of the signals at 5.37, 4.75 and 4.27 ppm in (Fig. 9), around 45% of MBDTC 3a, 38% of M(DTP)(DTC) 6 and 16% of MBDTP 4 were formed after 8 h of reaction between MBDTC 3a and ZnDTP 2a. The mixed Zn(DTC)(DTP) complex was also expected to be formed during the reaction, but was however not detected by NMR at room temperature because the ligand exchange between the different forms of Zn complexes is too rapid on the NMR time scale.16
In addition to this NMR study, HPLC-MS analysis of the mixture recovered after 8 h revealed the presence of a newly- formed compound showing a mass spectrum with [M + H]+ at m/z 721 identified as MBDTP 4 based on the comparison with the mass spectrum and retention time of synthetic MBDTP 4 (see § 2.3). As mentioned earlier (cf. § 2.6), it is worth noting that under the currently used HPLC-MS analytical conditions, ZnDTP, ZnDTC and Zn(DTP)(DTC) species cannot be detected.
By combining the 1H, 13C NMR and HPLC-MS results, it clearly appears that partial DTC/DTP exchange reactions took place between MBDTC 3a and ZnDTP 2a at high temperatures (150 °C), leading to the formation of ZnDTC 5b, MBDTP 4 and mixed M(DTP)(DTC) 6. In this respect, the formation of the intermediate ZnDTC species in this process may be central to the transfer of DTC from MBDTC to MoDTC in the presence of ZnDTP since ZnDTC have been shown to exchange ligands with MoDTC (see above and Kiw et al.15). Possibly due to the relative inertness of MoDTC, this exchange is extremely slow at room temperature. The reaction is faster (although not immediate) at 135 °C, the equilibrium between MoDTC and ZnDTC being reached relatively rapidly.15
3.3. Effect of NO2 on the interactions between MoDTC and MBDTC under laboratory conditions
The interactions between MoDTC and MBDTC in the absence of ZnDTP was also investigated under thermo-oxidative conditions at the laboratory scale. To this aim, an oil ageing experiment was carried out using MoDTC 1a–1c and MBDTC 3a under a flow of NO2 in air (2000 ppm) at 135 °C. Monitoring of the evolution of the reaction mixture with time by HPLC-MS showed that the relative concentrations of the initial MoDTC 1a–1c decreased, mainly due to thermo-oxidative degradation and to DTC transfer reactions with MBDTC 3a (Fig. 10a).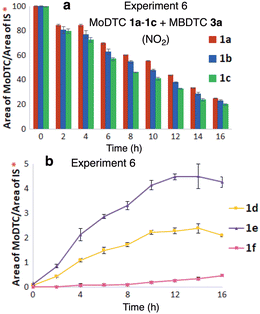 | ||
| Fig. 10 Evolution of the concentrations of (a) MoDTC 1a–1c and of (b) the newly formed MoDTC 1d–1f during the experiment involving MoDTC 1a–1c and MBDTC 3a under thermo-oxidative conditions (Experiment 6, Table 1). IS: internal standard. *Y-axis: arbitrary units. Error bars correspond to triplicate HPLC-MS analyses for each sample. | ||
Indeed, MoDTC 1d–1f bearing C4 DTC ligands originating from MBDTC were formed and their concentrations increased gradually during the oil ageing experiment despite the absence of Zn derivatives (Fig. 10b). As pointed out in Section 3.1.1, when a similar reaction medium was used, but under inert atmosphere (Experiment 1, Table 1), MoDTC 1d–1e were formed in extremely low amounts, MoDTC 1f being even undetectable under these conditions. These results suggest that, in the absence of ZnDTP, the DTC transfer reactions between MBDTC and MoDTC take place at a faster rate under oxidative conditions than under non-oxidative conditions. The former conditions are thus able to induce the release of DTC from MBDTC which then become available for complexing the metal core of Mo species (Mo2O2S2 or Mo oxysulfides) and to replace the ligands from degraded MoDTC upon oxidation.
3.4. Effect of MBDTC on the enhanced persistence of MoDTC in formulated lubricants under thermo-oxidative laboratory conditions
Previous studies have shown that during engine tests, the presence of MBDTC 3a in a formulated oil (oil AMBDTC) resulted in an enhanced persistence of MoDTC and extended significantly the friction reducing capability of this oil as compared to the same oil devoid of MBDTC 3a (oil A).23 The predominant MoDTC remaining at the end of the engine test (after 143 h) comprised mainly MoDTC 1d–1f with C4 alkyl chains formed by the progressive replacement of the genuine DTC ligands from MoDTC educts by DTC released from MBDTC. In order to determine the mechanisms involved in this enhanced persistence of MoDTC in the presence of MBDTC, a simplified – as compared to an engine complex system – laboratory model was set up. To this aim, the thermo-oxidative conditions undergone by lubricant additives from the same two formulated engine oils (oil A et AMBDTC) were simulated using oil ageing experiments under bubbling with a blend of 2000 ppm NO2 in air at 135 °C (Experiments 7 and 8, Table 1).Fig. 11 shows the evolution of the relative concentrations of MoDTC 1a–1c substrates in the oils A and AMBDTC during the laboratory ageing experiments.
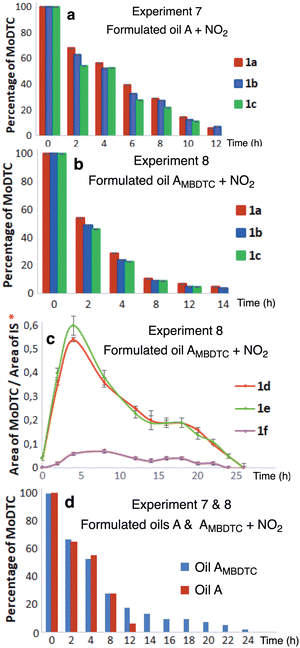 | ||
| Fig. 11 Evolution of the relative abundance of MoDTC species in formulated engine oils during laboratory ageing experiments (Experiments 7 and 8, Table 1) under thermo-oxidative conditions: (a) MoDTC 1a–1c in oil A; (b) MoDTC 1a–1c in oil AMBDTC; (c) newly formed MoDTC complexes 1d–1f in oil AMBDTC; (d) summed MoDTC 1a–1f in oil A (red colour) and AMBDTC (blue colour). IS: Internal standard. *Y-axis: arbitrary units. Error bars correspond to triplicate HPLC-MS analyses for each sample. | ||
Upon oil ageing, the MoDTC substrates 1a–1c decreased progressively over time in both oils, and were completely consumed between 12–14 h in oil A and between 14–16 h in oil AMBDTC. However, in the case of the oil AMBDTC, newly formed MoDTC complexes 1d–1f were detected, their relative concentrations increasing during the first 4 h (Fig. 11c), and then decreasing gradually before being totally consumed between 24–26 h. The formation of the MoDTC complexes 1d–1f in oil AMBDTC ascertained the occurrence of ligand transfer reactions between MBDTC 3a and MoDTC 1a–1c. The whole MoDTC complex pool in oil AMBDTC (summed MoDTC 1a–1f) decreased gradually over time, but MoDTC were completely consumed much later than in the case of the oil A (i.e., between 24–26 h vs. 12–14 h).
These results clearly show that MoDTC complexes persisted for a longer period of time in oil AMBDTC. Since both oils were of similar composition, except for the additional presence of MBDTC in oil AMBDTC, this enhanced persistence can most likely be attributed to DTC transfer reactions from MBDTC 3a to the partially degraded MoDTC substrates 1a–1c or to the metal cores of Mo complexes (Mo2O2S2 or Mo oxysulfides) remaining after thermo-oxidative degradation of the initial DTC ligands. The results of these two laboratory experiments are in good agreement with those obtained previously in the case of engine tests involving oils A and AMBDTC under engine operating conditions.23
4. Discussion
DTC transfer reactions between MBDTC and MoDTC have been unambiguously evidenced by HPLC-MS analysis and NMR spectroscopy, and laboratory experiments have shown that these reactions appear to be induced by the presence of Zn(II) complexes such as ZnDTP and Zn(acac)2. The Lewis acid properties of Zn(II) complexes as well as the capacity of Zn(II) to bind to sulfur atoms and, in particular, to activate dithioacetals, have been previously reported in other contexts.29,30 Therefore, in the case of the experiments involving MBDTC and ZnDTP, Zn(II) ions are likely to act as Lewis acids, being able to activate C–S bonds from MBDTC towards nucleophilic attack (Fig. 12). The dithiophosphate ions from ZnDTP 2a, which are good nucleophilic species, could then attack the central methylene group of MBDTC 3a and substitute their DTC moieties, thus leading to the release of ZnDTC 5b and to the formation of methylene-bis(dithiophosphate) 4 together with the mixed methylene-(dithiophosphate)(dithiocarbamate) species 6 (cf. § 3.2). In the case of the experiments involving other Zn(II) species such as Zn(acac)2 or ZnDTC 5a, nucleophiles other than dithiophosphates available in the reaction medium comprising OH− or DTC could also attack the central methylene group of MBDTC, leading to the release of DTC from MBDTC to form ZnDTC species.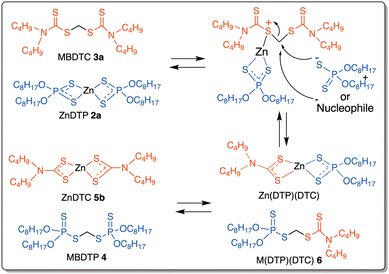 | ||
| Fig. 12 Proposed mechanism of C–S bond activation of MBDTC by ZnDTP complexes, the latter acting as a Lewis acid that promotes the reaction. | ||
If Zn(II) species, and notably ZnDTP, were shown to efficiently promote the release of DTC from MBDTC and most likely play a significant role in this process in formulated engine lubricants, ZnDTP are highly sensitive to oxidation and dithiophosphates were shown to be rapidly degraded during engine tests or laboratory experiments.20,31 Therefore, although Zn(II) ions most likely remain available during ageing of lubricants under thermo-oxidative conditions, dithiophosphates progressively disappear and other species such as, for instance, hydroxide ions, thiolates or amines from other additives might intervene as “nucleophilic species” in the release of DTC from MBDTC in the case of altered lubricants as described in Fig. 12.
In addition, the results of the laboratory ageing experiments involving MoDTC and MBDTC in a base oil at 135 °C under oxidative conditions (2000 ppm of NO2 in air) in the absence of any source of Zn(II) (§ 3.3) also showed the progressive formation of MoDTC with ligands originating from MBDTC with the concomitant decrease of the genuine MoDTC species. It therefore seems that the mere thermo-oxidative conditions are capable of inducing the release of DTC from MBDTC which then become available for complexing the metal core of Mo complexes (Mo2O2S2 or Mo oxysulfides) and to replace the degraded ligands. The release of DTC from MBDTC under such conditions might proceed via a process comprising the oxidation of the sulfur atom from one DTC ligand involved in the dithioacetal bond of MBDTC, followed by the release of the second intact DTC in a process analogous to that described in a different context by Liu and Thayumanavan32 for dithioacetals under oxidative conditions (Fig. 13).
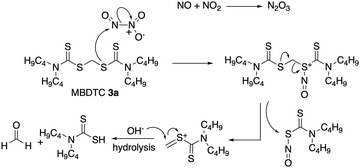 | ||
| Fig. 13 Proposed oxidative cleavage mechanism of MBDTC under NO2 (2000 ppm in air). Adapted from Liu and Thayumanavan.32 | ||
It could thus be demonstrated that the progressive release of DTC from MBDTC induced either by Zn(II) or oxidative conditions insured a progressive supply of “fresh” DTC ligand which is potentially able to replace degraded/oxidized DTC ligands on the metal core of Mo complex (Mo2O2S2 or Mo oxysulfides) and thus to regenerate the initial structures of MoDTC complexes in lubricants. This process might therefore allow the enhanced persistence of MoDTC ensuring friction reduction properties of engine lubricants for extended periods.
In addition to MBDTC, it appears that ZnDTC also easily exchange their DTC ligands towards altered Mo species. However, MBDTC represent better ‘‘provider’’ of DTC ligands than ZnDTC for altered MoDTC in lubricants, since ZnDTC are highly oxidizable and thus decompose easily upon oxidation to form disulfides (thiurams) or other degradation products. Such is not the case with MBDTC, DTC occurring as dithioacetals and being likely more resistant towards oxidative processes. DTC can thus be potentially released in the engine oil following a delayed/controlled process, providing progressively fresh DTC for the regeneration of MoDTC complexes.
5. Conclusion
The extension of friction reducing properties of engine oils for longer periods of time plays an important role in energy saving and in coping with global environmental problems. The control of thermo-oxidative degradation of Mo-based friction modifiers in formulated engine oils upon engine functioning remains a key challenge to achieve this objective. In this context, a preliminary study has shown that the interactions between MoDTC and MBDTC has a beneficial effect on the life time of MoDTC in engine lubricants and on the persistence of their tribological properties. This effect was associated with DTC transfer reactions between MBDTC and MoDTC and it was assumed that the DTC from MBDTC are progressively transferred to the metal core of MoDTC after oxidative degradation of their ligands. As a result, the concentrations of MoDTC could be preserved at a useful level over extended ageing periods, thus maintaining the friction reducing properties of engine oils.Additionally, the role of Zn(II) complexes (e.g., ZnDTP, Zn(acac)2) on ligand transfer reactions between MBDTC and MoDTC could be clearly demonstrated in the present study. Thus, Zn(II) ions are likely to act as a Lewis acid that is able to activate the C–S bond cleavage of MBDTC, thus inducing the release of DTC from MBDTC as ZnDTC. The latter then become available for ligand exchange with MoDTC or complexation of the metal core of Mo complexes after oxidative degradation of their initial ligands. In the absence of Zn(II) ions, the release of DTC from MBDTC can also be induced by oxidative processes under thermo-oxidative conditions. DTC from MBDTC thus represent potentially a ‘‘stock’’ of DTC ligands which can be progressively released during functioning of the engine and replace ligands from MoDTC educts degraded by thermo-oxidative processes following processes promoted by Zn(II) ions and/or oxidative conditions. The MoDTC species remaining after thermo-oxidative degradation of simple mixtures or of fully formulated lubricants containing MoDTC and MBDTC in an oil base consist mainly of newly formed complexes with DTC ligands originating from MBDTC as was also observed during engine tests in a preliminary study.
The results obtained provide insight in future additive design by considering similar delayed DTC transfer mechanisms demonstrated by MBDTC aimed at extending the functional lifetime of MoDTC. One might, notably, envisage to pursue the investigation of potential structures bearing other types of functionalities liable to allow the progressive release of DTC during engine functioning. The present study that investigated the interactions between MoDTC with other additives at the molecular level can also help to predict the friction reduction efficiency of engine oils as well as to provide guidance for the optimization of MoDTC tribological performance for the future development of engine oils.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank TotalEnergies Marketing Services for funding this research work that was carried out at the University of Strasbourg and TotalEnergies Solaize Research Center (CRES), and for supplying the oils and the additives used in this study. The authors also thank E. Motsch for her help in the MS analyses. Y. M. K. thanks TotalEnergies Marketing Services and the French “Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation” for a doctoral fellowship (CIFRE).Notes and references
- S. I. Tseregounis, M. L. McMillan and R. M. Olree, Engine oil effects on fuel economy in GM vehicles – separation of viscosity and friction modifier effects, SAE Technical Paper No. 982502, 1998.
- K. Hoshino, H. Kawai and K. Akiyama, Fuel efficiency of SAE 5W-20 friction modified gasoline engine oil, SAE Technical Paper No. 982506, 1998.
- M. D. Johnson, R. K. Jensen, E. M. Clausing, K. Schriewer and S. Korcek, Effects of aging on frictional properties of fuel efficient engine oils, SAE Technical Paper No. 952532, 1995.
- C. M. Taylor, Automobile engine tribology – design considerations for efficiency and durability, Wear, 1998, 221, 1–8 CrossRef CAS.
- L. R. Rudnick, Lubricant additives: chemistry and applications, CRC Press, Boca Baton, FL, 2nd edn, 2009 Search PubMed.
- J. Graham, H. Spikes and S. Korcek, The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part I – factors influencing friction reduction, Tribol. Trans., 2001, 44, 626–636 CrossRef CAS.
- J. Graham, H. Spikes and R. Jensen, The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part II – durability of friction reducing capability, Tribol. Trans., 2001, 44, 637–647 CrossRef CAS.
- G. Spengler and A. Webber, On the lubricating performance of organic molybdenum compounds, Chem. Ber., 1939, 92, 2163–2171 CrossRef.
- C. Grossiord, K. Varlot, J. M. Martin, T. L. Mogne, C. Esnouf and K. Inoue, MoS2 single sheet lubrication by molybdenum dithiocarbamate, Tribol. Int., 1998, 31, 737–743 CrossRef CAS.
- K. Kubo, N. Mitsuhiro, T. Shitamichi and K. Motoyama, The effect of ageing during engine running on the friction reduction performance of oil soluble molybdenum compounds, Proceeding of the International Tribology Conference, Yokohama, 1995.
- M. Shekarriz, An investigation of antioxidant properties of zinc and molybdenum dithiocarbamates in hydrocarbons, Pet. Sci. Technol., 2016, 6, 109–113 Search PubMed.
- M. De Feo, C. Minfray, M. I. De Barros Bouchet, B. Thiebaut, T. L. Mogne, B. Vacher and J. M. Martin, Ageing impact on tribological properties of MoDTC-containing base oil, Tribol. Int., 2015, 92, 126–135 CrossRef CAS.
- M. De Feo, Impact of thermo-oxidative degradation of MoDTC additive on its tribological performances for steel-steel and DLC-steel contacts, PhD thesis, Université de Lyon, 2015 Search PubMed.
- M. De Feo, C. Minfray, M. I. De Barros Bouchet, B. Thiebaut and J. M. Martin, MoDTC friction modifier additive degradation: Correlation between tribological performance and chemical changes, RSC Adv., 2015, 5, 93786–93796 RSC.
- Y. M. Kiw, P. Schaeffer, P. Adam, B. Thiébaut, C. Boyer and G. Papin, Ligand exchange processes between molybdenum and zinc additives in lubricants: evidence from NMR (1H,13C,31P) and HPLC-MS analysis, RSC Adv., 2020, 10, 37962–37973 RSC.
- Y. M. Kiw, P. Schaeffer, P. Adam, B. Thiébaut and C. Boyer, Molecular evidence for sulfurization of molybdenum dithiocarbamates (MoDTC) by zinc dithiophosphates: a key process in their synergetic interactions and the enhanced preservation of MoDTC in formulated lubricants?, RSC Adv., 2022, 12, 3545–3553 Search PubMed.
- Y. Sogawa, N. Yoshimura and O. Iwasaki, R&D on new friction modifier for lubricant for fuel economy improvement, Japan Petroleum Energy Centre Report, 1999, E1.1.5.
- T. M. Shea and A. J. Stipanovic, Solution phase reactions of organomolybdenum friction modifier additives for energy conserving engine oils, Tribol. Lett., 2002, 12, 13–22 CrossRef CAS.
- R. K. Jensen, M. D. Johnson, S. Korcek and M. J. Rokosz, Friction-reducing and antioxidant capabilities of engine oil additive systems under oxidative conditions. I. Effects of ligand exchange between molybdenum dialkyldithiocarbamate and zinc dialkyldithiophosphate in hexadecane, Lubr. Sci., 1998, 10, 99–120 CrossRef CAS.
- H. Spikes, The history and mechanisms of ZDDP, Tribol. Lett., 2004, 17, 469–489 CrossRef CAS.
- A. Morina, A. Neville, M. Priest and J. H. Green, ZDDP and MoDTC interactions in boundary lubrication – The effect of temperature and ZDDP/MoDTC ratio, Tribol. Int., 2006, 39, 1545–1557 CrossRef CAS.
- A. Neville, A. Morina, T. Haque and M. Voong, Compatibility between tribological surfaces and lubricant additives – How friction and wear reduction can be controlled by surface/lube synergies, Tribol. Int., 2007, 40, 1680–1695 CrossRef CAS.
- Y. M. Kiw, P. Adam, P. Schaeffer, B. Thiébaut, C. Boyer and N. Obrecht, Molecular evidence for improved tribological performances of MoDTC induced by methylene-(bisdithiocarbamates) in engine lubricants, RSC Adv., 2022, 12, 23083–23090 RSC.
- A. Somayaji and P. B. Aswath, The role of antioxidants on the oxidation stability of oils with F-ZDDP and ZDDP, and chemical structure of tribofilms using XANES, Tribol. Trans., 2011, 52, 511–525 CrossRef.
- B. Jover, J. Forstner, J. Petro, S. Szoboszlay, I. Fekete, K. Csergo, G. Sztreharszki, G. Raksi, J. Kiss, F. Miko, J. Baladincz and J. Toth, Process for manufacturing methylene-bis(dibutyl-dithiocarbamate) with ASTM colour less than 2, US Pat., 5744629A, 1998.
- A. F. A. Rasid, T. I. Mohamad, M. J. Ghazali and W. M. F. W. Mahmood, Effect of lubricant temperature in a motorized engine, World Appl. Sci. J., 2012, 20, 927–930 CAS.
- R. K. Jensen, S. Korcek and M. D. Johnson, Friction-reducing and antioxidant capabilities of engine oil additive systems under oxidative conditions. II. Understanding ligand exchange in a molybdenum dialkyldithiocarbamate zinc dialkyldithiophosphate additive system in various base oils, Lubr. Sci., 2001, 14, 25–42 CrossRef CAS.
- A. Ponjavic, T. Lemaigre, M. Southby and H. A. Spikes, Influence of NOx and air on the ageing behaviour of MoDTC, Tribol. Lett., 2017, 65, 52, DOI:10.1007/s11249-017-0836-6.
- M. H. Salter, J. H. Reibenspies, S. B. Jones and R. D. Hancock, Lewis acid properties of Zinc(II) in its cyclen complex. The structure of [Zn(Cyclen)(SC(NH2)2](ClO4)2 and the bonding of thiourea to metal ions. Some implications for zinc metalloenzymes, Inorg. Chem., 2005, 44, 2791–2797 CrossRef CAS PubMed.
- L. R. Sutton, W. A. Donaubauer, F. Hampel and A. Hirsch, Tris(thioacetals) from benzene hexathiol: towards covalent self-assembly, Chem. Commun., 2004, 1758–1759 RSC.
- J. J. Dickert Jr. and C. N. Rowe, Thermal decomposition of metal O,O-dialkyl phosphorodithioates, J. Org. Chem., 1967, 32, 647–653 CrossRef.
- B. Liu and S. Thayumanavan, Mechanistic investigation on oxidative degradation of ROS-responsive thioacetal/thioketal moieties and their implications, Cell Rep. Phys. Sci., 2020, 1, 100271 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj04123e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |