Open Access Article
Open Access ArticleA simple approach based on transmetalation for the selective and sensitive colorimetric/fluorometric detection of copper(II) ions in drinking water†
Ivan Pietro
Oliveri
 ,
Gabriella
Munzi
,
Gabriella
Munzi
 and
Santo
Di Bella
and
Santo
Di Bella
 *
*
Dipartimento di Scienze Chimiche, Università di Catania, I-95125, Catania, Italy. E-mail: sdibella@unict.it
First published on 23rd August 2022
Abstract
The search for feasible and efficient methods for sensing cations in the environment is a challenge of current scientific interest. Among the colorimetric and fluorometric methods, those that enable the direct and rapid detection of the analytes are highly desirable. Here a simple approach based on the transmetalation of a Zn(II) Schiff-base complex for the selective and sensitive colorimetric and fluorometric detection of Cu2+ ions in aqueous solution is reported. This method offers the advantages of being direct, fast, and not requiring any treatment of the sample. A detection limit down to 0.60 μM is obtained from the spectrophotometric data. The method is demonstrated to be efficient for the quantitation of Cu2+ ions in drinking water.
Introduction
The colorimetric and fluorometric sensing of cations is an area of intense current interest. Various review contributions based on conventional organic chemosensors through metal coordination,1–3 including Schiff-bases,4,5 host–guest supramolecular chemistry,6 including macrocycles as ion-pair receptors7 and calixarenes,8 aggregation-induced emission,9 luminescent coordination polymers,10 metal–organic frameworks,11 metal nanoparticles,12,13 quantum dots,13 and sensing arrays,14 or involving a chemodosimetric approach,15,16 have been reported in the recent literature (regarding the last decade). This huge amount of research activity highlights the breadth and interest of this research field. In this context, sensing of copper(II) ions is doubtless one of the most investigated topics.1–19Besides these methods, a further approach for detecting cations is represented by transmetalation or metal exchange.20,21 Thus, starting from a metal complex, sensing can be achieved via a cation-exchange mechanism exploiting optical spectroscopic changes, often fluorescence enhancement or quenching, upon transmetalation. This can offer further advantages with respect to the above sensing methods in terms of greater selectivity and straightforward application. However, sensing data based on transmetalation are limited compared with the other methods. Regarding the sensing of copper(II) ions, few examples based on transmetalation have been reported in the literature,22–30 and only some of them include quantitative detection data.27–30
Copper plays an important role in biology as an essential trace element that is fundamental to the proper functioning of enzymes, such as superoxide dismutase, cytochrome oxidase, or metallothionein.31 Moreover, it is involved in neurobiology and in neurodegenerative disorders.32–34 Both a deficiency and an excessive copper intake produce adverse health effects.35 Copper is widely used for producing wires, pipes, and various manufactures, as well as in agriculture as a antimycotic agent. Sources for copper contamination include agriculture, industrial waste, mining, and photovoltaics.36–38 Therefore, copper pollution represents an emerging issue for environmental and human health.39,40
As the main sources of human copper intake are food and water, the European Union has established a limit of 2 mg L−1 (corresponding approximatively to 30 μM) for the concentration of copper(II) in drinking water,41 as recommended by the World Health Organization (WHO) guidelines for water quality.42,43
Lewis acidic zinc(II) complexes of tetradentate Schiff-base ligands have recently emerged for their aggregation and sensing properties.44–46 In particular, derivatives of 2,3-diaminomaleonitrile, Zn(salmal), are characterized via their relevant optical spectroscopic changes in relation to the nature of the aggregate species or monomeric adducts.47–53 We have demonstrated that in a solution of weak coordinating solvents a 5-tert-butyl-substituted Zn(salmal) complex (1) forms relatively stable adducts, which easily transmetalate with divalent ions of the first transition series, but at different rates.54 In particular, transmetalation with Cu2+ ions is faster than that with other cations, regardless of the nature of the counteranion. These results stimulated us to further explore the selective and sensitive detection of Cu2+ ions using this simple and straightforward approach.
In this paper we report on the selective, sensitive, colorimetric and fluorometric detection of Cu2+ ions in aqueous solution based on transmetalation of the Zn(II) Schiff-base complex 1, highlighting the simple feasibility of this efficient method.
Results and discussion
The absorption spectrum of 1 in acetonitrile (MeCN) solution in the visible region is characterized by an absorption band centred at 489 nm, followed by a more intense band at 568 nm, both of which are responsible for the violet colour of the solution. Moreover, 1 shows a weak red fluorescence emission (ϕ ≤ 0.01) centred at 645 nm that is independent of the excitation wavelength (Fig. 1). MeCN solutions of 1 are stable over time at room temperature. | ||
| Fig. 1 UV/Vis optical absorption and fluorescence (λexc = 535 nm) spectra of 1 (40 μM solution in MeCN). | ||
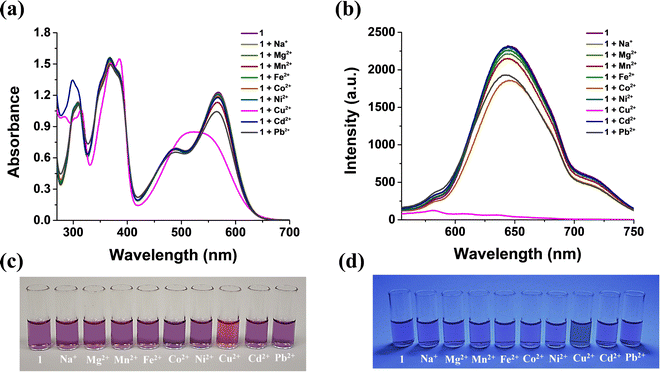
Starting from a 40 μM solution of 1 in MeCN, upon the addition of up to 2.0 equiv. of aqueous solutions of various metal cations, as nitrates or perchlorates, including Na+, Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cd2+, and Pb2+, the optical absorption and fluorescence emission spectra remain almost unchanged. Instead, the addition of 2.0 equiv. of an aqueous solution of Cu2+ salts leads to an immediate and substantial change in the optical absorption spectrum in the visible region, with the disappearance of the band at 568 nm and the formation of a new broad band centred at 530 nm, accompanied by a colour change of the solution from violet to magenta, and a complete quenching of the fluorescence (Fig. 2). It is well known that Cu(II) complexes are characterized by fluorescence turn-off of the organic chromophores, owing to the paramagnetic quenching effect.55
These data are consistent with the fast and complete transmetalation of the Zn2+ ion with the Cu2+ ion (Scheme 1). In fact, identical results, in terms of the optical absorption spectra without any fluorescence emission, are obtained by comparing the spectra of 1 in MeCN upon the addition of 2.0 equiv. of aqueous solutions of Cu2+ salts with those recorded by dissolving the Cu(salmal) complex in MeCN.54
These findings suggest that 1 can be involved in the selective sensing of Cu2+ ions in aqueous solution. To assess the sensing performance, various spectrophotometric titrations were carried out with the concentration of 1 being varied in the range of 10–40 μM. The comparison of three different titrations (Fig. S1, ESI†) shows that the best performance, in terms of providing a larger linear dynamic range, is obtained with a concentration of 40 μM. Therefore, spectrophotometric and spectrofluorometric titrations were performed using this concentration.
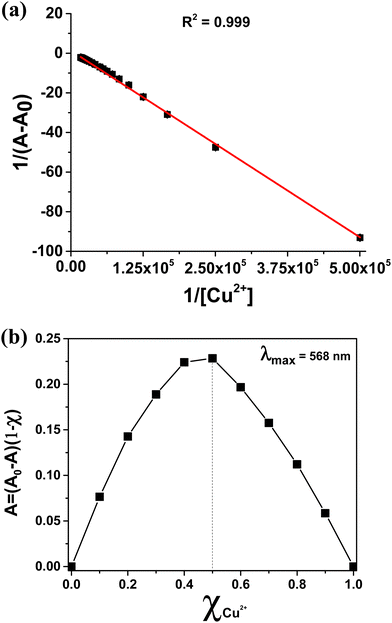
Spectrophotometric titrations indicate that the progressive addition of Cu2+ ions implies a parallel decrease in the optical absorption band intensity centred at 568 nm, and the formation of the new broad band centred at 530 nm, which is associated with the formation of the Cu(salmal) complex (Fig. 3). The formation of multiple isosbestic points further supports the formation of a new species upon transmetalation. Saturation is reached after the addition of 1.5 equiv. of Cu2+. It is noteworthy that the titration data clearly indicate a linear relationship between the absorbance at 568 nm and the concentration of Cu2+ added over the entire 0–60 μM titration range (Fig. S2, ESI†). The binding constant for the formation of the Cu(salmal) complex upon transmetalation, obtained from the Benesi–Hildebrand plot56 of 1/(A − A0) versus 1/[Cu2+], was found to be 6.7 × 103. Moreover, the Benesi–Hildebrand plot (Fig. 4a) shows excellent linearity, supporting a transmetalation ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This was confirmed by the Job plot analysis (Fig. 4b). According to the IUPAC definition,57,58 the limit of detection (LOD) of 1 towards Cu2+ was determined to be 0.60 μM, which is much lower than the allowed maximum level of Cu2+ (30 μM) in drinking water.41–43
1. This was confirmed by the Job plot analysis (Fig. 4b). According to the IUPAC definition,57,58 the limit of detection (LOD) of 1 towards Cu2+ was determined to be 0.60 μM, which is much lower than the allowed maximum level of Cu2+ (30 μM) in drinking water.41–43
Analogously, spectrofluorometric titrations upon the progressive addition of Cu2+ show a decrease in the band emission at 645 nm, until complete quenching of the fluorescence after the addition of 1.5 equiv. of Cu2+, with a linear dependence between the intensity of the fluorescence and the concentration of the Cu2+ added (Fig. 5). Moreover, the related estimated binding constant (K = 2.3 × 103; Fig. S3, ESI†) and LOD (0.90 μM; Fig. S4, ESI†) are comparable to those derived from the spectrophotometric data.
Therefore, overall these data suggest that 1 can potentially be employed for the quantitative detection of Cu2+, via colorimetric/fluorometric dual mode, by transmetalation. These results favourably compare with the few quantitative data reported in the literature for the detection of Cu2+ by transmetalation,27–30 with the advantage that in our case the cation salts are dissolved in water, without any pretreatment of the sample.
The selectivity of 1 towards Cu2+ ions was further proven through competitive experiments (Fig. 6). Specifically, the optical absorption/fluorescence emission response of 1 was evaluated in the presence of other cations. In this regard, some common cations (Na+, Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cd2+, and Pb2+) were considered as potential interferents. Cations that give acidic hydrolysis in aqueous solution (e.g., Al3+, Fe3+) were not considered because these species lead to the demetalation of 1. We made this choice because all colorimetric/fluorometric measurements involving 1 in MeCN solution and aqueous solutions of cations, including Cu2+, were carried out without pH adjustment. The optical absorption/fluorescence response of 1 in the presence of Cu2+ ions is very similar to that obtained after the addition of Cu2+ ions mixed with each potential interfering cation (Fig. 6). This demonstrates the selectivity of 1 for Cu2+ ions, even in the presence of other cations.
Given the good sensing performance of 1, our method was tested using real water samples, obtained as laboratory tap water and supermarket spring water. Blank experiments using known amounts of the water samples added to the MeCN solution of 1 always indicated negligible changes in the absorption/fluorescence. Therefore, 1 was tested for spiking/recovery experiments, adding different concentrations of Cu2+ ions to samples of water. Data were analysed using changes in the absorption spectra and the calibration line from spectrophotometric titrations (Fig. S2, ESI†). As shown in Table 1, every sample shows a good recovery rate and a low relative standard deviation (RSD). Therefore, complex 1 is suitable for practical applications.
| Sample | Cu2+ added (μM) | Found (μM) | Recovered ± RSD (%) |
|---|---|---|---|
| Tap water | 10.0 | 9.20 | 92.0 ± 2.9 |
| 20.0 | 18.6 | 93.0 ± 4.3 | |
| 40.0 | 39.3 | 98.3 ± 4.6 | |
| Spring water | 10.0 | 9.30 | 93.0 ± 3.2 |
| 20.0 | 19.1 | 95.5 ± 1.9 | |
| 40.0 | 38.8 | 97.0 ± 2.1 |
Conclusions
The search for simple and efficient methods for the sensing of cations in the environment is a challenge of current scientific interest. Here, we have presented a straightforward approach, based on transmetalation, for the colorimetric/fluorometric sensing of Cu2+ ions in aqueous solution. This method offers the advantages of being simple, direct, and fast, without the need for any treatment of the sample, for the selective and sensitive detection of Cu2+ ions in drinking water. Moreover, it may represent a general method for the sensing of Cu2+ or other cations in various environments through optimization of the subtle relationships between the structure and stability of Zn(II) salen-type complexes, and transmetalation.Experimental
Materials and general procedures
Complex 1 was synthesized, purified, and characterized as previously reported.54 Chemicals were purchased from Sigma-Aldrich and used as received. Ultrapure water purified using the Milli-Q purification system was used to prepare the aqueous solutions of nitrate or perchlorate salts. Stock solutions of 1 in MeCN were prepared by dissolving a known amount of the complex in the solvent using a volumetric flask. MeCN solutions of 1 used for spectrophotometric/spectrofluorometric measurements were prepared by diluting the stock solution.Measurements
Optical absorption spectra were recorded at room temperature using an Agilent Cary 60 spectrophotometer. Fluorescence spectra were recorded at room temperature using a JASCO FP-8200 spectrofluorometer (JASCO Europe). All measurements were recorded immediately after the addition of the aqueous solutions of the cations to the MeCN solutions of 1. Identical results were obtained even when recording the measurements after some time (10, 20, 30, or 60 min later). Spectrophotometric and fluorometric titrations were performed using a 1 cm path cell. Titrations were performed by adding increasing amounts of a 5.0 mM aqueous solution of copper perchlorate to 3 ml of a 40 μM solution of 1, using Rainin (Mettler Toledo) positive displacement pipettes. The excitation wavelength for fluorometric titrations was chosen to be at an isosbestic point. The addition of defined amounts of distilled water or various samples of tap water or spring water to MeCN solutions of 1 does not involve any change in the optical absorption or 1H NMR spectra, indicating that no demetalation/decomposition occurs. Spiking and recovery experiments were performed by directly adding a known amount of copper perchlorate to the spring water or tap water. The spiked solution was then added to the 40 μM solution of 1 in MeCN and the recovered percentage was calculated using the equation of the calibration line from the spectrophotometric titration data. The fluorescence quantum yield for 1 in MeCN was determined with respect to that of the 4-alkoxy-substituted Zn(salmal) derivative (ϕ = 0.24 in THF).47Calculation of the binding constant and limit of detection
The binding constant for the formation of the Cu(salmal) complex upon transmetalation was calculated via the Benesi–Hildebrand plot56 from spectrophotometric (eqn (1)) or spectrofluorometric (eqn (2)) data:| 1/(A − A0) = 1/{K(A − A0)[Cu2+]} + 1/[A − A0] | (1) |
| 1/(F − F0) = 1/{K(F − F0)[Cu2+]} + 1/[F − F0] | (2) |
The limit of detection was calculated according to the IUPAC recommendation,57,58 from both the optical absorption and fluorescence data (eqn (3)):
| LOD = 3σ/k | (3) |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the University of Catania, PIACERI 2020/2022, Linee di Intervento 2 e 3.References
- N. Kaur and S. Kumar, Tetrahedron, 2011, 67, 9233–9264 CrossRef CAS.
- Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568–1600 RSC.
- N. S. Patil, R. B. Dhake, M. I. Ahamed and U. Fegade, J. Fluoresc., 2020, 30, 1295–1330 CrossRef CAS PubMed.
- A. L. Berhanu, Gaurav, I. Mohiuddin, A. K. Malik, J. S. Aulakh, V. Kumar and K.-H. Kim, TrAC, Trends Anal. Chem., 2019, 116, 74–91 CrossRef CAS.
- S. Khan, X. Chen, A. Almahri, E. S. Allehyani, F. A. Alhumaydhi, M. M. Ibrahim and S. Ali, J. Environ. Chem. Eng., 2021, 9, 106381 CrossRef CAS.
- M. C.-L. Yeung and V. W.-W. Yam, Chem. Soc. Rev., 2015, 44, 4192–4202 RSC.
- Q. He, G. I. Vargas-Zúñiga, S. H. Kim, S. K. Kim and J. L. Sessler, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS PubMed.
- R. Kumar, A. Sharma, H. Singh, P. Suating, H. S. Kim, K. Sunwoo, I. Shim, B. C. Gibb and J. S. Kim, Chem. Rev., 2019, 119, 9657–9721 CrossRef CAS PubMed.
- M. H. Chua, H. Zhou, Q. Zhu, B. Z. Tang and J. W. Xu, Mater. Chem. Front., 2021, 5, 659–708 RSC.
- B. Parmar, K. K. Bisht, Y. Rachuri and E. Suresh, Inorg. Chem. Front., 2020, 7, 1082–1107 RSC.
- J. Jin, J. Xue, Y. Liu, G. Yang and Y.-Y. Wang, Dalton Trans., 2021, 50, 1950–1972 RSC.
- Z. Wang and L. Ma, Coord. Chem. Rev., 2009, 253, 1607–1618 CrossRef CAS.
- B. Liu, J. Zhuang and G. Wei, Environ. Sci.: Nano, 2020, 7, 2195–2213 RSC.
- D. G. Smith, I. L. Topolnicki, V. E. Zwicker, K. A. Jolliffe and E. J. New, Analyst, 2017, 142, 3549–3563 RSC.
- K. Kaur, R. Saini, A. Kumar, V. Luxami, N. Kaur, P. Singh and S. Kumar, Coord. Chem. Rev., 2012, 256, 1992–2028 CrossRef CAS.
- R. AbhijnaKrishna and S. Velmathi, Coord. Chem. Rev., 2022, 459, 214401 CrossRef CAS.
- S. Liu, Y. M. Wang and J. Han, J. Photochem. Photobiol., C, 2017, 32, 78–103 CrossRef CAS.
- S. Sharma and K. S. Ghosh, Spectrochim. Acta, Part A, 2021, 254, 119610 CrossRef CAS PubMed.
- B.-B. Tao, N.-N. Wu, H.-D. Zhang and H.-B. Wang, J. Electrochem. Soc., 2022, 169, 037529 CrossRef.
- J. Cheng, X. Zhou and H. Xiang, Analyst, 2015, 140, 7082–7115 RSC.
- R. Pandey, A. Kumar, Q. Xu and D. S. Pandey, Dalton Trans., 2020, 49, 542–568 RSC.
- M. Royzen, Z. Dai and J. W. Canary, J. Am. Chem. Soc., 2005, 127, 1612–1613 CrossRef CAS.
- S. Khatua, S. H. Choi, J. Lee, J. O. Huh, Y. Do and D. G. Churchill, Inorg. Chem., 2009, 48, 1799–1801 CrossRef CAS.
- R. Pandey, P. Kumar, A. K. Singh, M. Shahid, P.-Z. Li, S. K. Singh, Q. Xu, A. Misra and D. S. Pandey, Inorg. Chem., 2011, 50, 3189–3197 CrossRef CAS PubMed.
- Z. Li, L. Zhang, L. Wang, Y. Guo, L. Cai, M. Yu and L. Wei, Chem. Commun., 2011, 47, 5798–5800 RSC.
- W. Cao, X. J. Zheng, D. C. Fang and L. P. Jin, Dalton Trans., 2014, 43, 7298–7303 RSC.
- L. Tang, J. Zhao, M. Cai, P. Zhou, K. Zhong, S. Hou and Y. Bian, Tetrahedron Lett., 2013, 54, 6105–6109 CrossRef CAS.
- H. G. Lee, K. B. Kim, G. J. Park, Y. J. Na, H. Y. Jo, S. A. Lee and C. Kim, Inorg. Chem. Commun., 2014, 39, 61–65 CrossRef CAS.
- J. Guan, P. Zhang, T. Wei, Q. Lin, H. Yao and Y. Zhang, Chem. Res. Chin. Univ., 2015, 31, 347–351 CrossRef CAS.
- H. Sharma, A. Tamrakar, T. Maddeshiya, P. Raj Shakya, K. Kant Tiwari, M. D. Pandey and R. Pandey, Luminescence, 2022, 1–7 Search PubMed.
- M. C. Linder and M. Hazegh-Azam, Am. J. Clin. Nutr., 1996, 63, 797S–811S CAS.
- R. Dringen, I. F. Scheiber and J. F. B. Mercer, Front. Aging Neurosci., 2013, 5, 9 CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed.
- V. Desai and S. G. Kaler, Am. J. Clin. Nutr., 2008, 88, 855S–858S CrossRef CAS.
- R. Uauy, M. Olivares and M. Gonzalez, Am. J. Clin. Nutr., 1998, 67, 952S–959S CrossRef CAS.
- M. Rafique, S. Hajra, M. B. Tahir, S. S. A. Gillani and M. Irshad, Environ. Sci. Pollut. Res. Int., 2022, 29, 16772–16781 CrossRef CAS PubMed.
- P. Nain and A. Kumar, J. Cleaner Prod., 2022, 343, 130978 CrossRef CAS.
- G. Izydorczyk, K. Mikula, D. Skrzypczak, K. Moustakas, A. Witek-Krowiak and K. Chojnacka, Environ. Res., 2021, 197, 111050 CrossRef CAS PubMed.
- M. Rehman, L. Liu, Q. Wang, M. H. Saleem, S. Bashir, S. Ullah and D. Peng, Environ. Sci. Pollut. Res. Int., 2019, 26, 18003–18016 CrossRef CAS.
- P. P. Leal, C. L. Hurd, S. G. Sander, E. Armstrong, P. A. Fernández, T. J. Suhrhoff and M. Y. Roleda, Sci. Rep., 2018, 8, 14763 CrossRef PubMed.
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. (https://data.europa.eu/eli/dir/2020/2184/oj).
- Guidelines for drinking-water quality, World Health Organization, 4th edn, 2011 Search PubMed.
- Copper in drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality, World Health Organization, 2004 Search PubMed.
- S. Di Bella, Dalton Trans., 2021, 50, 6050–6063 RSC.
- G. Consiglio, I. P. Oliveri, S. Failla and S. Di Bella, Molecules, 2019, 24, 2514 CrossRef CAS PubMed.
- L. Leoni and A. Dalla Cort, Inorganics, 2018, 6, 42 CrossRef.
- G. Consiglio, S. Failla, P. Finocchiaro, I. P. Oliveri, R. Purrello and S. Di Bella, Inorg. Chem., 2010, 49, 5134–5142 CrossRef CAS.
- I. P. Oliveri, S. Failla, G. Malandrino and S. Di Bella, New J. Chem., 2011, 35, 2826–2831 RSC.
- I. P. Oliveri and S. Di Bella, Tetrahedron, 2011, 67, 9446–9449 CrossRef CAS.
- G. Consiglio, I. P. Oliveri, C. Cacciola, G. Maccarrone, S. Failla and S. Di Bella, Dalton Trans., 2020, 49, 5121–5133 RSC.
- M. Strianese, D. Guarnieri, M. Lamberti, A. Landi, A. Peluso and C. Pellecchia, Inorg. Chem., 2020, 59, 15977–15986 CrossRef CAS PubMed.
- G. Munzi, S. Failla and S. Di Bella, Analyst, 2021, 146, 2144–2151 RSC.
- G. Munzi, G. Consiglio, S. Failla and S. Di Bella, Inorganics, 2021, 9, 49 CrossRef CAS.
- I. P. Oliveri, G. Consiglio, G. Munzi, S. Failla and S. Di Bella, Dalton Trans., 2022, 51, 11859–11867 RSC.
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- L. A. Currie, Anal. Chim. Acta, 1999, 391, 127–134 CrossRef CAS.
- Analytical Methods Committee, Analyst, 1987, 112, 199–204 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional UV/vis and fluorescence spectroscopic data. See DOI: https://doi.org/10.1039/d2nj03695a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |