Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible-emitting Cu(I) complexes with N-functionalized benzotriazole-based ligands†
Jesús
Castro
 a,
Valentina
Ferraro
a,
Valentina
Ferraro
 *b and
Marco
Bortoluzzi
*b and
Marco
Bortoluzzi
 *bc
*bc
aDepartamento de Química Inorgánica, Universidade de Vigo, Facultade de Química, Edificio de Ciencias Experimentais, 36310 Vigo, Galicia, Spain
bDipartimento di Scienze Molecolari e Nanosistemi, Università Ca’ Foscari Venezia, Via Torino 155, I-30172 Mestre (VE), Italy. E-mail: valentina.ferraro@unive.it; markos@unive.it
cConsorzio Interuniversitario Reattività Chimica e Catalisi (CIRCC), via Celso Ulpiani 27, 70126 Bari, Italy
First published on 8th September 2022
Abstract
Luminescent mono- and dinuclear cationic heteroleptic Cu(I) complexes [Cu(N∧N′)(P)2]+, [Cu(N∧N′)(P∧P)]+ or [Cu2(N∧N′)2(μ-P∧P)2]2+ containing bidentate N-donor ligands (N∧N′) with benzotriazole, pyridine, pyrimidine or substituted triazine moieties in combination with mono- (P) and bidentate (P∧P) phosphines were synthesized and characterized. Eight single-crystal X-ray diffraction structures were obtained and showed marked distortions from the ideal tetrahedral geometry around Cu(I). Cyclic voltammetry on selected complexes showed reduction processes around −2 V vs. ferrocene/ferrocenium and irreversible oxidation close to 1 V. The long-wavelength absorptions were observed in the range of 350 to 450 nm and attributed to MLCT transitions. Upon excitation with near-UV and violet light, the complexes exhibited emissions from bright yellow (max 538 nm) to red (max 637 nm). Emission maxima, luminescence lifetimes and photoluminescence quantum yields that reach up to 0.92 on powder samples resulted in strong dependence on the choice of the coordinated ligands, the acceptor character of the N∧N′ ligands in particular. DFT calculations confirmed the electrochemical and photophysical outcomes and strongly suggested that the emission has a metal-to-ligand charge transfer (MLCT) nature, with intersystem crossing affording triplet emitting states.
Introduction
Luminescent first-row transition metal complexes are viable alternatives to second- and third-row transition elements and trivalent lanthanide derivatives for advanced applications such as organic light-emitting diodes (OLEDs), light-emitting electrochemical cells (LECs), solar cells, and sustainable photoactivated reactions.1–13 In particular, d-block metals such as Ir(III) and Pt(II) are commonly exploited in photoluminescent dyes for OLEDs,14–18 but their high cost, scarce abundance and toxicity determined growing interest for cheaper and greener alternatives. Moreover, the employment of precious and rare-earth metals is geopolitically problematic because of the presence of only a few mining countries. The extraction and separation processes are highly energy- and water-consuming and sometimes require toxic chemicals.In this context, Cu(I) complexes are considered a valid choice thanks to their photophysical properties, including the possibility of tuning the colour of the emission. The closed-shell d10 ground-state configuration circumvents the problem of efficient non-radiative deactivation of excited states via low-lying metal-centred states, as generally observed for several other 3d metal ions with a partially filled d-shell. Moreover, Cu(I) complexes can exhibit peculiar features ascribable to thermally activated delayed fluorescence (TADF), appealing for OLED technology thanks to the possibility of harvesting both singlet and triplet excitons.19–24 Consequently, a maximum of 100% efficiency can be expected from exciton spin statistics, similar to precious metal-based phosphorescent OLEDs. In most of the cases luminescence is attributed to metal-to-ligand charge transfers (MLCT), where the excited state formally contains an oxidized metal centre and a reduced ligand. Small modifications on the skeleton of the ligands can determine appreciable changes in photoluminescence, since the lowest unoccupied molecular orbital (LUMO) usually corresponds to a π* orbital of the coordinated ligands.25,26 Common N-donor ligands exploited for the preparation of luminescent Cu(I) complexes are polypyridines such as 1,10-phenanthroline and its derivatives, often combined with phosphines in a metal coordination sphere. Particular interest was devoted to the effects on the luminescence features of the extension of the π-delocalization in the N-donor chelates by the introduction of well-designed substituents. Moreover, the correlation existing between the bite angle of chelating phosphines and the energy of the MLCT absorption was investigated. Also, the intermolecular π-interactions at the ground state were revealed to influence the photoluminescence quantum yields.27–34
Recently, azoles proved to be promising building blocks for the synthesis of suitable ligands in the field of Cu(I) luminescent compounds. For instance, benzimidazole and its functionalized derivatives were extensively used.35,36 The functionalization of azoles with other heterocycles or π-delocalized donor moieties is a common approach to simultaneously obtain rigid bidentate structures and remove high energy oscillators. An example is the straightforward isolation of a luminescent binuclear Cu(I) complex with a conjugate base of 4-tert-butylphenyl(pyrrole-2yl-methylene)amine starting from precursor [Cu(NCCH3)4][BF4].37 A wide variety of further examples involving azoles from pyrazoles to tetrazoles was reported.9,38–53 The four coordinated complex [Cu(bis(2-(diphenylphosphino)phenyl)ether)(5-(2-pyridyl)tetrazolate)] was deeply studied also from a computational point of view, focusing the attention on the ISC process.54
Despite its renowned versatility, benzotriazole is not commonly used as a ligand for the preparation of luminescent transition metal complexes.55–57 Our research group recently exploited benzotriazole and the related heterocycle indazole as coordinating moieties in polydentate ligands applied for the preparation of luminescent Cu(I) complexes.58–60
So far, the conjugation of benzotriazole in combination with different aromatic N-donor heterocycles was not considered. Herein, we report the synthesis and characterization of bright yellow- and reddish orange-emitting Cu(I) complexes with bidentate ligands obtained by functionalization of the benzotriazole NH moiety with other heterocycles, in particular pyridine, pyrimidine and alkoxy-substituted triazines. The ligands and their acronyms are reported in Scheme 1 for clarity.
Results and discussion
Synthesis and characterization of the ligands
1-(Pyridin-2-yl)benzotriazole (py-btz) and 1-(pyrimidin-2-yl)benzotriazole (pym-btz) were obtained from methods previously reported by Katritzky and co-workers, based on the aromatic nucleophilic substitution of C-bonded halides by benzotriazole.61,62 The triazinyl-benzotriazoles trzOMe-btz, trzOEt-btz and trzOPh-btz were obtained by reacting the related 2-chloro-triazines with benzotriazole. The synthetic procedures, summarized in Scheme 1, are detailed in the Experimental section together with characterization data.Mono- and bidimensional NMR spectra are collected in the ESI† (Fig. S1–S6). In the cases of 1-(4,6-dimethoxy-1,3,5-triazin-2-yl)benzotriazole (trzOMe-btz) and 1-(4,6-diphenoxy-1,3,5-triazin-2-yl)benzotriazole (trzOPh-btz), the formulae were further confirmed by single crystal X-ray diffraction obtained from slow evaporation of CH2Cl2 (trzOMe-btz) or acetone (trzOPh-btz) solutions. The description of the structures of trzOMe-btz and trzOPh-btz is reported in the ESI† (see also Fig. S7–S11 and Table S1).
Synthesis and characterization of the complexes
To obtain heteroleptic Cu(I) complexes with PPh3 in the coordination sphere, all the ligands in Scheme 1 were coordinated by reaction of their conjugated acids (obtained in situ by adding HBF4·Et2O) with [Cu(κ2-BH4)(PPh3)2]. The same synthetic approach using Cu(I) borohydride precursors with other phosphines63 afforded multiple products; therefore, the synthesis of the other complexes prosecuted using [Cu(NCCH3)4][BF4] as a precursor. The acetonitrile complex was reacted with the proper phosphine, triisopropylphosphine PiPr3, 1,2-bis(diphenylphosphino)ethane dppe or bis[(2-diphenylphosphino)phenyl]ether DPEphos, and subsequently with the chosen chelating N-donor ligand. The procedures followed, sketched in Scheme 2 together with the numbering of the complexes and the yields, are detailed in the Experimental Section with the corresponding characterization data. 1H and 31P{1H} NMR spectra are collected in the ESI† (Fig. S12–S30).In all the cases, elemental analyses agree with the proposed formulae and conductivity measurements in acetone indicate that the complexes behave as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolytes, with the exception of the dinuclear dppe derivatives 1c–5c (vide infra), acting as 1
1 electrolytes, with the exception of the dinuclear dppe derivatives 1c–5c (vide infra), acting as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytes. The IR spectra confirmed the disappearances of both coordinated borohydride and acetonitrile. The observed bands are essentially ascribable to the stretching of coordinated N- and P-donor ligands and to the BF4 counterion (νBF4 1150–950 cm−1).64
2 electrolytes. The IR spectra confirmed the disappearances of both coordinated borohydride and acetonitrile. The observed bands are essentially ascribable to the stretching of coordinated N- and P-donor ligands and to the BF4 counterion (νBF4 1150–950 cm−1).64
The high-frequency region of the 1H NMR spectra shows, besides the signals related to aromatic phosphines and pyridine, pyrimidine or phenoxytriazine substituents, a set of resonances for the benzotriazole heterocycle. In the aliphatic region, the P-bonded –CH(CH3)2 or –CH2CH2– resonances are detectable for the 1b–4b and 1c–5c complexes. Two distinct groups of resonances for the O-bonded substituents were observed at low temperatures for selected trzOMe-btz and trzOEt-btz complexes, depending upon the choice of the phosphine and the bulk of the alkoxy groups. In particular, two separate –OR groups were detected for the PPh3 and dppe derivatives 3a, 3c and 4a, 4c. On the other hand, in the 1H NMR spectra of the PiPr3 complexes 3b and 4b, only one set of resonances is present. Finally, the trzOMe-btz DPEphos complex 3d shows only one singlet for the two –OMe groups at 243 K, while two distinct –OEt fragments are recognizable in the 1H NMR spectrum of the related trzOEt-btz species 4d under the same experimental conditions. The effects of coordination on the chemical equivalence are present also for some pym-btz derivatives, 2c and 2d in particular. The solutions of the complexes in CDCl3 maintained the same 1H and 31P{1H} NMR resonances after more than two weeks, so arrangement processes of fast ligands can be excluded.
Crystals suitable for X-ray diffraction were isolated from CH2Cl2/Et2O (1a, 2b, 2c, 3b and 5d), CH2Cl2/EtOH (2a, 1d) and from the slow evaporation of CH2Cl2 solutions (4d).
Crystal data and structure refinement of complexes 1a and 2a are reported in Table S2 (ESI†). The structure of [1a]+ is shown in Fig. 1 (see Fig. S31, ESI† for the ellipsoid plot of both the cations). The py-btz and pym-btz ligands behave as chelate N-donors and the Cu(I) coordination sphere is completed by two PPh3. Although the complexes differ only for one nitrogen atom, they exhibit important differences in the packing, and consequently, the molecules are not superimposable (see Fig. S32, ESI†). The reason may rely on the conformation of the N-donor ligand and the supramolecular network, in part generated in the case of 2a by the π,π-stacking between the pyrimidine ring and the benzene fragment of a neighbour btz ring with symmetry operation 1 − x, 1 − y, and 2 − z (see Fig. S33, ESI†). The distance between the centroids of these planes is 3.6026(16) Å, with a slippage of 1.344 Å, and the dihedral angle between them is 1.89(13)°. A comparable π,π-stacking interaction does not exist in the crystal structure of 1a.
Crystal data and structure refinement for the complexes 2b, 1d, 2c, 3b, 4d and 5d are reported in Tables S3 and S4 (ESI†). The structures of [2c]2+ and [4d]+ are shown in Fig. 3. Ellipsoid plots of all the complexes are reported in Fig. S34 (ESI†). Selected bond distances and angles are set out in Tables S5–S8 (ESI†).
The Cu–P bond lengths depend upon the phosphine, being shorter for PPh3 and DPEphos derivatives and longer for PiPr3 complexes. The Cu–N bond lengths are also influenced by the choice of the phosphine, since longer Cu–P bonds correspond to shorter Cu–N bonds. Noticeable differences can be detected by comparing the angles in the environment of Cu atoms. Only in the PiPr3 complexes 2b and 3b, the P–Cu–P angle is the biggest, with values of 132.95(5)° and 130.51(1)°, respectively, and represents the main source of distortion from tetrahedral geometry. In the case of the cations [1d]+, [4d]+ and [5d]+ several N–Cu–P angles are more obtuse than the P–Cu–P angle, probably because of the bidentate character of the DPEphos ligand (see Tables S6 and S8, ESI†). The same situation was previously observed in similar compounds.65–68 The P–Cu–P angle is also the most important difference between compounds 2a and 2b, as detectable in the superimposition reported in Fig. S35 (ESI†). Although the value for 2b is surprisingly large, it is far from the angle of 149.02(6)° found in a similar compound having 2-(pyridin-2-yl)quinoxaline as a ligand.47 Moreover, as previously observed in complex 1a, also for 2b, π,π-stacking interaction is not observed, probably because of the steric hindrance of the isopropyl groups.
 | ||
| Fig. 1 Ellipsoid plot of the cations [1a]+, [2c]2+ and [4d]+. Hydrogen atoms are omitted for clarity. | ||
In complex 3b, trzOMe-btz maintains the same conformation and planarity observed in the free ligand. The root-mean square deviation from the best-fitted plane (calculated for 15 atoms) is equal to 0.057 Å, while in the free ligand, it was 0.070 or 0.029 Å. The –OMe carbons are instead one 0.282(2) Å below and the other one 0.251(2) Å above the plane. Cu atoms lie mainly in the plane, being displaced by only 0.024(1) Å. As for trzOMe-btz, the aliphatic substituents of trzOEt-btz maintain the same disposition in the corresponding complex 4d, i.e. one in syn and the other one in anti. The aromatic groups of trzOPh-btz in 5d are instead situated both in syn, in opposition to what was found in the free ligand, where they were both in anti. This outcome is understandable for one of the phenoxy-groups, otherwise, N(11) would be too sterically hindered to act as a ligand. The superimposition of complex 5d with the free ligand is reported in Fig. S36 (ESI†). The planarity of the N-donors is reduced by coordination, in such a way that the root-mean-square deviation for the 15 atoms forming the central body is 0.1149 Å for 4d and 0.0834 Å for 5d (0.0062 Å in the free species). In complex 5d, the dihedral angle between this plane and each phenyl ring is 62.10(5) and 64.35(4)°. Instead, Cu(I) atom is out of the plane, being deviated by 0.344(3) Å and 0.087(1) Å respectively, in 4d and 5d. One phenoxy oxygen atom is deviated up to 0.373(1) Å from the plane. In other words, the five-membered metallacycle is puckered into an envelope conformation with coordinates Q(2) = 0.104(4) Å and ϕ(2) = 4(2)° for 4d, and Q(2) = 0.0955(11) Å and ϕ(2) = 151.4(7)° for 5d.69
The two bidentate phosphines considered afforded different results. DPEphos behaves as a chelating P-donor, as observed for the majority of the complexes reported in CCDC,70 while dppe acts as a bridging ligand. The P⋯P distance in free DPEphos is between 4.876 Å and 4.881 Å.71,72 Once coordinated to Cu(I), such a distance is reduced to values between 3.7054(5) (5d) and 3.8755(8) Å (1d). For comparison, in the tricoordinated cation [Cu(DPEphos)2]+, one of the phosphorus atoms is not coordinated and the P⋯P distance increases up to 5.08 Å, while for the chelating DPEphos, this value is around 3.8 Å.73 Similar values were found also in other complexes with this κ2-ligand.39,48–53 On the other hand, the distance P⋯P in free dppe is between 4.437 Å and 4.463 Å,74,75 that is maintained also in the corresponding complex 2c, where it is equal to 4.445(2) Å.
The environment around the Cu(I) ions could be described as tetrahedral in all the complexes, but it is highly distorted. Continuous Shape Measures calculation [SHAPE V2.1]76 gives the vacant trigonal bipyramid as the best geometry approximation for these compounds (C3v symmetry, axial vacant). The output data for all the complexes are collected in Table S9 (ESI†) together with the parameters used to evaluate the distortion of a tetrahedron.77–80 The values range from 0.77 to 0.88 (τ4) or from 0.75 to 0.85  , suggesting a large deviation from the perfect tetrahedral geometry. It is worth noting that for both the parameters, 1.0 corresponds to the tetrahedron, 0.0 to the square planar and 0.85 to the trigonal prism. The cation of compound 5d is best described as a vacant trigonal bipyramid, being τ4 and
, suggesting a large deviation from the perfect tetrahedral geometry. It is worth noting that for both the parameters, 1.0 corresponds to the tetrahedron, 0.0 to the square planar and 0.85 to the trigonal prism. The cation of compound 5d is best described as a vacant trigonal bipyramid, being τ4 and  equal to 0.85.
equal to 0.85.
Electrochemical, absorption and emission measurements
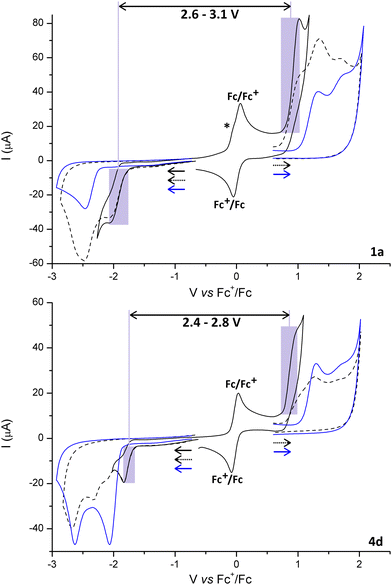
Electrochemical measurements on 1a and 4d and on the free py-btz and trzOEt-btz ligands show irreversible processes related to the oxidation of the {CuP2} fragment starting around 0.75 V vs. Fc/Fc+ (Fig. 2). Several other oxidation processes occur at higher potential, closely comparable to those observed for the free N-donor ligands. The first reduction process is observable for potentials lower than −1.75 V in the case of 1a and lower than −1.65 V for 4d. The reduction processes of the free ligands start at lower potentials, in particular in the case of py-btz. This outcome can be ascribed to the σ-donation of electron density to the Cu(I) centre, which makes the N-donor heterocycles more electron deficient and therefore more oxidant. The reduction processes are irreversible in all the cases. The potential gap between first oxidation and first reduction is in the 2.6–3.1 V range for 1a, while it is slightly lower for 4d (2.4–2.8 V range).The complexes are characterized by absorptions for wavelengths below 500 nm in diluted CH2Cl2 solutions. The superimposition of the UV-vis spectra of complexes 2a–2d is reported in Fig. 3 and for all the other complexes in Fig. S37 (ESI†). The maximum molar extinction coefficients are comprised between 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 60
000 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. The tails in the visible range account for the yellow colour observed for concentrated solutions and powder samples. The comparison with the free ligands allows us to point out that the absorption spectra are almost in part related to the π* ← π transitions of the aromatic fragments.
000 M−1 cm−1. The tails in the visible range account for the yellow colour observed for concentrated solutions and powder samples. The comparison with the free ligands allows us to point out that the absorption spectra are almost in part related to the π* ← π transitions of the aromatic fragments.
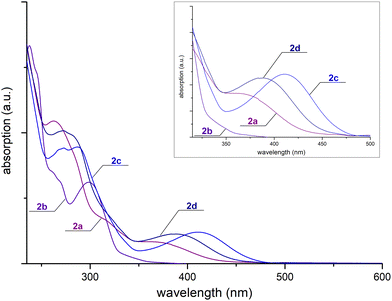 | ||
| Fig. 3 Absorption spectra of complexes 10−5 M in CH2Cl2 (2a, purple line; 2b, violet line; 2c, navy blue line; 2d, blue line). | ||
In the case of aromatic phosphine derivatives, it is also possible to notice a band tentatively attributed to MLCT transitions around 400 nm. The same band is present, but with a much lower extinction coefficient, also for the PiPr3 complexes 1b–4b. The onsets of the lowest energy absorptions of 1a, about 400 nm, and of 4d, about 450 nm, are perfectly in line with the potential gaps between the first oxidation and the first reduction of the complexes, supporting the MLCT assignment.
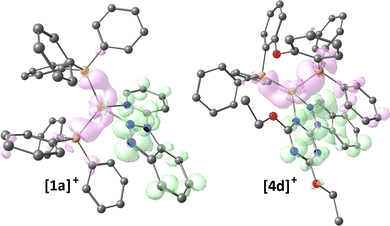
DFT calculations on [1a]+ and [4d]+ as representative compounds confirmed the MLCT nature of the lowest energy absorption. The computed ground-state stationary points are in good agreement with the X-ray data (RMSD of 0.353 Å and 0.480 Å for [1a]+ and [4d]+, respectively) and the computed wavelength of the lowest energy singlet ← singlet transitions are in line with the absorption spectra (341 nm for [1a]+ and 376 nm for [4d]+). The hole and electron distributions associated with the singlet–singlet transition, depicted in Fig. 4, clearly indicate that the electron moves from an occupied orbital centred on the {CuP2} fragment to an unoccupied π* orbital localized on the N-donor heterocycle. In both cases, the absorption is related to a transition involving the frontier orbitals, and the computed distance between the centres of hole and electron distributions is around 2.9 Å.
Excitation of powder samples with near-UV and violet light affords emissions from yellow to reddish-orange centred between 538 and 637 nm. The luminescence is not maintained in the solution. The normalized PL and PLE spectra are shown in Fig. 5. On comparing complexes with the same N-donor chelate, the emission maxima fall at longer wavelengths when PiPr3 is used as a P-donor ligand. The choice of the N-donor ligands also influences the emission maximum, with longer wavelengths generally observed for the trzOPh-btz derivatives. Photophysical data for all the complexes are summarized in Table 1.
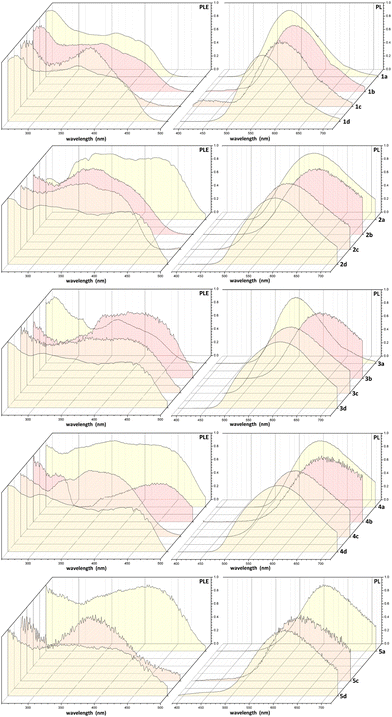 | ||
| Fig. 5 PL and PLE spectra of the complexes (solid state, r.t.). See Table 1 for experimental details. | ||
| UV-visa nm | PL (FWHM)b nm (cm−1) | PLEc nm | Stokes shiftd cm−1 | τ μs | Φ (%) | |
|---|---|---|---|---|---|---|
| a CH2Cl2 solution, 298 K. b Powder samples, r.t., λexcitation = 350 nm (1b, 1c, 1d, 2a, 2b, 2c, 3a, 3d, 4a, 4b, 4c, 4d, 5a); 365 nm (1d, 5c, 5d); 375 nm (1a); 415 nm (3b, 3c). c Powder samples, r.t., λemission = 525 nm (4d); 535 nm (1a); 555 nm (3a); 570 nm (1c, 1d, 2a, 2c, 2d); 575 nm (1b, 4a, 4c); 590 nm (2b, 3c); 610 nm (4b); 620 nm (5a); 630 (3b, 3d, 5c, 5d). d Not determined for the PiPr3 derivatives because of the difficult assignment of the maximum of MLCT absorptions. e Powder samples, r.t., λexcitation = 290 nm (1a, 1b, 1c, 1d, 3a, 4a, 4b, 4c, 4d, 5c, 5d); 445 nm (2a, 2b, 2d, 3b, 3c, 3d, 5a). f Powder samples, r.t., λexcitation = 365 nm. | ||||||
| 1a | <430, 262 (ε = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1), 270 (sh), 285 (sh), 307 (sh), 365 (sh) 500 M−1 cm−1), 270 (sh), 285 (sh), 307 (sh), 365 (sh) |
538 (FWHM = 4100) | <440 | 8800 | 164 | 26 |
| 1b | <350, 238, 247 (sh), 272 (ε = 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1), 282 (sh) 900 M−1 cm−1), 282 (sh) |
575 (FWHM = 3800) | <470 | — | 34 | 10 |
| 1c | <475, 266 (sh), 272, 289 (ε = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1), 404 200 M−1 cm−1), 404 |
580 (FWHM = 3800) | <450 | 7500 | 47 | 4 |
| 1d | <475, 264 (sh), 272 (sh), 288 (ε = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1), 382 200 M−1 cm−1), 382 |
561 (FWHM = 3700) | <470 | 8400 | 14 | 13 |
| 2a | <450, 263 (ε = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1), 310 (sh), 370 (sh) 800 M−1 cm−1), 310 (sh), 370 (sh) |
558 (FWHM = 4100) | <500 | 9100 | 20 | 35 |
| 2b | <380, 238 (ε = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1), 245 (sh), 261 (sh), 268 (sh), 298 300 M−1 cm−1), 245 (sh), 261 (sh), 268 (sh), 298 |
588 (FWHM = 3600) | <475 | — | 23 | 7 |
| 2c | <475, 267 (sh), 273, 287 (ε = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1), 412 800 M−1 cm−1), 412 |
561 (FWHM = 3500) | <460 | 6500 | 23 | 6 |
| 2d | <475, 273 (ε = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1), 282 (sh), 388 300 M−1 cm−1), 282 (sh), 388 |
557 (FWHM = 4200) | <500 | 7800 | 37 | 36 |
| 3a | <450, 260 (ε = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1), 387 800 M−1 cm−1), 387 |
557 (FWHM = 3600) | <460 | 7900 | 32 | 7 |
| 3b | <350, 237 (ε = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1), 263 (sh), 270 (sh), 299 400 M−1 cm−1), 263 (sh), 270 (sh), 299 |
637 (FWHM = 4000) | <500 | — | 21 | 2 |
| 3c | <500, 267 (sh), 273, 290 (ε = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1), 433 700 M−1 cm−1), 433 |
598 (FWHM = 4500) | <500 | 6400 | 11 | 6 |
| 3d | <490, 265 (sh), 273 (ε = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1), 407 300 M−1 cm−1), 407 |
604 (FWHM = 5000) | <500 | 8000 | 65 | 6 |
| 4a | <450, 259 (ε = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1), 314 (sh), 380 800 M−1 cm−1), 314 (sh), 380 |
577 (FWHM = 3400) | <520 | 9000 | 5 | 17 |
| 4b | <350, 261, 270 (sh), 297 (ε = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1), 328 (sh) 300 M−1 cm−1), 328 (sh) |
600 (FWHM = 3600) | <515 | — | 43 | 14 |
| 4c | <500, 267 (ε = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1), 272 (sh), 289, 429 400 M−1 cm−1), 272 (sh), 289, 429 |
578 (FWHM = 3300) | <475 | 6600 | 11 | 17 |
| 4d | <460, 265 (sh), 272 (ε = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1), 404 600 M−1 cm−1), 404 |
550 (FWHM = 4200) | <475 | 6600 | 43 | 92 |
| 5a | <470, 259 (ε = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1), 319 (sh), 390 000 M−1 cm−1), 319 (sh), 390 |
615 (FWHM = 3700) | <500 | 9400 | 23 | 2 |
| 5c | <530, 268, 273, 293 (ε = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1), 442 600 M−1 cm−1), 442 |
571 (FWHM = 4800) | <470 | 5100 | 14 | 3 |
| 5d | <500, 274 (ε = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1), 318 (sh), 415 000 M−1 cm−1), 318 (sh), 415 |
598 (FWHM = 4000) | <510 | 7400 | 4 | 7 |
Despite the roughly comparable PL and PLE spectra, the photoluminescence quantum yield values (Φ) are markedly different. Table 1 indicates that weaker emission was in general observed for the mononuclear PiPr3 complexes and the dinuclear dppe derivatives. The flexibility of the isopropyl substituents and of the ethylene bridges probably favours non-radiative decay routes. Moreover, in the PiPr3 derivatives, there is no possibility of extra-stabilization offered by π–π stacking with the N-donor chelates, differently from the other complexes here considered.
For what concerns the N-donor ligands, the worst results were achieved using trzOPh-btz and trzOMe-btz. On the other hand, higher Φ values were surprisingly measured using the comparable trzOEt-btz ligand. The quantum yield values do not however show any evident trend related to the choice of the P- or N-donor ligands. For instance, higher Φ was obtained with py-btz in combination with PPh3 (1a) with respect to DPEphos (1d), while the related pym-btz complex 2a and 2d have very similar quantum yields. The measured Φ of the trzOEt-btz PPh3 derivative 4a is 17%, while the value increases up to the astonishing value of 92% by replacing the two PPh3 ligands with DPEphos (4d). It is to conclude that the combined electronic and steric features of the coordinated ligands play a hardly predictable role in the stabilization of ground and excited states. As an example, for what concerns the ground state geometry the methoxy-substituted complex 3d revealed fluxional behaviour in solution, differently from the more rigid ethoxy-substituted derivative 4d under the same experimental conditions.
The Stokes shifts (not reported for the PiPr3 derivatives because of the difficult assignment of the maximum of MLCT absorptions) are comprised between 5100 and 9400 cm−1. The highest values are generally related to the PPh3 complexes, while the lowest is to the dppe derivatives. The high Stokes shift range and the wide emissions (FWHM between 3500 and 5000 cm−1) suggest that triplet excited states are involved in the luminescence. Such an outcome is confirmed by luminescent lifetime (τ) values in the microseconds range.
Table 1 reveals that lifetime values depend upon the Cu(I) coordination sphere, but there is no linear correlation with the photoluminescence quantum yields. For instance, the longest τ, 164 μs, is that of complex 1a, having Φ equal to 26%, while for the most efficient 4d complex, the τ value is 43 μs (see Fig. 6 for the luminescence decay curves).
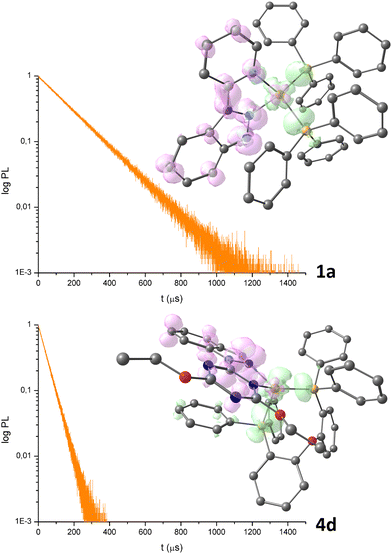 | ||
| Fig. 6 Luminescence decay curves of 1a and 4d (see Table 1 for experimental details) and DFT-optimized structures of [1a]+ and [4d]+ (triplet states) with hole (pink) and electron (green) distributions related to the lowest energy triplet-singlet transitions (surface isovalue = 0.003 a.u.). Colour map: Cu, orange; P, yellowish orange; O, red; N, blue; C; grey. Hydrogen atoms are omitted for clarity. | ||
The radiative (kr) and non-radiative (knr) constants for 4d, estimated on the basis of the equation Φ = kr/(kr + knr) = τ kr are kr = 2.1 × 104 s−1 and knr = 1.8 × 103 s−1. For comparison, the same constants for 1a are kr = 1.6 × 103 s−1 and knr = 4.6 × 103 s−1.21
The computationally optimized structures of [1a]+ and [4d]+ in triplet state (Fig. 6) are comparable to the relative singlet ground states (RMSD of 0.563 Å for [1a]+ and 0.525 Å for [4d]+). The analysis of the hole and electron distributions related to the lowest energy triplet-singlet transitions gives a picture in line with the previously discussed MLCT absorptions, with the electron moving from a π* orbital localized on the N-donor heterocycle to the {CuP2} fragment.
On the basis of experimental and computational outcomes, the emissions are therefore attributed to 3MLCT transitions. The computed energy gaps between singlet and triplet MLCT states, collected in Table 2, are all in the thousands of cm−1 range and do not support the possibility of the TADF mechanism, usually invoked for energy gaps below 1000 cm−1.22
| ΔE(1MLCT–3MLCT), cm−1 | ||
|---|---|---|
| Singlet geometry | Triplet geometry | |
| [1a]+ | 4360 | 4000 |
| [4d]+ | 3130 | 2750 |
Conclusions
Cu(I) complexes with π-delocalized N-donor ligands represent a viable alternative to heavy transition metal derivatives for advanced technology since their photophysical properties can be largely tuned by modifying the coordinated ligands. In this context, here we reported the successful synthesis and characterization of several photoluminescent complexes with N-donor chelates containing the btz fragment bonded to a six-membered heterocycle. Noticeable luminescence in the yellow-reddish orange range is associated with excitation in the near-UV and violet transition, with processes related to MLCT transitions and intersystem crossing. The data provided highlight strong and quite unexpected dependence of the emission features upon the introduction of small variations in the N-donor chelate, in particular the photoluminescence quantum yield. Moreover, the best performances are achieved only when the bidentate heterocycles are combined with the proper phosphine in the Cu(I) coordination sphere. The cationic mononuclear complex with coordinated trzOEt-btz and DPEphos appears to be particularly appropriate for advanced devices thanks to its high quantum yield, wide excitation range, and lifetime in the microseconds range. The inexpensive benzotriazole heterocycle confirms to be a well-suited building block for the preparation of luminescent Cu(I) derivatives, and the strong variability of the results here provided suggests that further improvements are possible.Experimental section
Materials and methods
Commercial solvents (Merck) were purified following reported procedures in order to be used inside the glove-box.81 All the other reagents were Sigma Aldrich products used as received. [Cu(κ2-BH4)(PPh3)2] and [Cu(NCCH3)4][BF4] were obtained from reported syntheses.82,83 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT), 2-chloro-4,6-ethoxy-1,3,5-triazine (CDET) and 2-chloro-4,6-phenoxy-1,3,5-triazine (CDPhT) were prepared following previously described methods.84,85The syntheses of the complexes starting from [Cu(NCCH3)4][BF4] were carried out in an inert atmosphere in a glove-box (MBraun Labstar with MB 10 G gas purifier) filled with N2 and equipped for inorganic syntheses. The reactions starting from [Cu(κ2-BH4)(PPh3)2] were carried out using standard Schlenk techniques.
Elemental analyses (C, H, N) were carried out using an Elementar Unicube micro analyzer. Conductivity measurements in acetone were performed using a Radiometer Copenhagen CDM83 instrument. Melting point measurements were carried out using a modified Falc 360 D apparatus equipped with a video recording device. IR spectra (KBr pellets) were collected in the range of 4000–400 cm−1 using a PerkinElmer Spectrum One spectrophotometer. Heteronuclear magnetic resonance (NMR) spectra were recorded at variable temperatures employing Bruker Avance 300 and Avance 400 instruments operating, respectively, at 300.13 MHz and 400.13 MHz of 1H resonance. 1H and 13C{1H} NMR spectra are referred to as the partially non-deuterated fraction of the solvent, itself quoted to tetramethylsilane. 31P{1H} NMR spectra are referred to as 85% H3PO4 in water. Cyclic voltammetry measurements were performed using an eDAQ ET014-199 instrument in acetone containing 0.1 M LiClO4. The solvent was purified following common techniques81 and all the measurements were carried out under argon at room temperature. The working electrode was a 1 mm glassy carbon disk, while the auxiliary electrode was a Pt-coated titanium rod. The electrodes were provided by eDAQ. Ferrocene was introduced as an internal standard and a Pt wire was used as a pseudo-reference electrode.
Crystal structure determination
Crystallographic data were collected at CACTI (University of Vigo) at 100 K (CryoStream 800) using a Bruker D8 Venture Photon II CMOS detector with Mo-Kα radiation (λ = 0.71073 Å) generated by an Incoatec Microfocus Source IμS. The software APEX3 was used for collecting frames of data, indexing reflections, and the determination of lattice parameters, SAINT for integration of intensity of reflections, and SADABS for scaling and empirical absorption correction.86 The crystallographic treatment was performed with the Oscail program,87 solved using the SHELXT program.88 The structure was subsequently refined by a full-matrix least-squares based on F2 using the SHELXL program.89 The reflections affected by the beam stop were systematically removed from the final refinement. Non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were included in idealized positions and refined with isotropic displacement parameters. In a number of cases, special procedures were used. In the case of the trzOMe-btz crystal, twinning was solved by means of the twin instruction on the classical SHELXL process. Instead, crystal twinning for trzOPh-btz was corrected by the TwinRotMat subroutine in PLATON, and the structure was refined as a 2-component perfect twin.90 Further details concerning crystal data and structural refinement are given in Table S1 (ESI†). In the case of the compounds 1a and 2a disordered solvent molecules, probably MeOH in the former and EtOH in the latter, were found in the final density map, and their contribution was eliminated by using the SQUEEZE procedure on PLATON.90 A disorder on the tetrafluoroborate anion is also present in complex 2a, which was not resolved. Crystal data and structural refinement for complexes 1a and 2a are collected in Table S2 (ESI†). It should be also noted that compound 1d crystallizes in the monoclinic C2/c space group with two molecules in the asymmetric unit. One of them is characterized by some disorder, with an occupancy factor of 0.57(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.43(1). For the dimeric compound 2c, the SQUEEZE procedure was also used. Once again, the tetrafluoroborate anion appeared so disordered that the anisotropic displacement parameters of the fluorine atoms were fixed by using the sadi instruction on SHELXL. Crystal data and structural refinement for complexes 2b, 2c and 1d are given in Table S3 (ESI†). In the case of compound 4d, one of the ethoxy groups and the tetrafluoroborate anion resulted to be so disordered that the anisotropic displacement parameters of some atoms were fixed by using the sadi and eadp instructions on SHELXL. The quality of the crystal data did not allow to fix all the problems, and important residual electronic densities remained after the last refinement. They are probably part of the disordered molecule, not modelled. Crystal data and structural refinement for complexes 3b, 4d and 5d are given in Table S4 (ESI†). PLATON (version 60720) was used to obtain some geometrical parameters from the cif files.90
0.43(1). For the dimeric compound 2c, the SQUEEZE procedure was also used. Once again, the tetrafluoroborate anion appeared so disordered that the anisotropic displacement parameters of the fluorine atoms were fixed by using the sadi instruction on SHELXL. Crystal data and structural refinement for complexes 2b, 2c and 1d are given in Table S3 (ESI†). In the case of compound 4d, one of the ethoxy groups and the tetrafluoroborate anion resulted to be so disordered that the anisotropic displacement parameters of some atoms were fixed by using the sadi and eadp instructions on SHELXL. The quality of the crystal data did not allow to fix all the problems, and important residual electronic densities remained after the last refinement. They are probably part of the disordered molecule, not modelled. Crystal data and structural refinement for complexes 3b, 4d and 5d are given in Table S4 (ESI†). PLATON (version 60720) was used to obtain some geometrical parameters from the cif files.90
Photophysical measurements
Absorption spectra in CH2Cl2 solutions were collected using a PerkinElmer Lambda 40 spectrophotometer. Photoluminescence measurements on solid samples were carried out using air-tight quartz sample holders, filled in glove-box to avoid interactions of the complexes with moisture. Photoluminescence emission (PL) and excitation (PLE) measurements were carried out at room temperature on solid samples using a Horiba Jobin Yvon Fluorolog-3 spectrofluorometer. A continuous-wave xenon arc lamp was used as a source selecting the excitation wavelength using a double Czerny–Turner monochromator. A single grating monochromator coupled to a photomultiplier tube was used as a detection system for optical emission measurements. Excitation and emission spectra were corrected for the instrumental functions. Time-resolved analyses were performed in multi-channel scaling modality (MCS) by using a pulsed UV-led source (Horiba SpectraLEDs) centred at 290 or 445 nm. Photoluminescence quantum yield Φ of the complexes (solid state, r.t.) was measured using an OceanOptics HR4000CG UV-NIR detector, fiber-coupled to an integrating sphere connected to an OceanOptics LED source centred at 365 nm. The values were reported as the average of three measurements.Computational details
Ground and excited state structures of the complexes [1a]+ and [4d]+ were optimized using the global-hybrid meta-NGA functional MN15 DFT functional and the Ahlrichs and Weigend's def2 split-valence polarized basis set.91,92 The C-PCM implicit solvation model was added to MN15 calculations, considering CH2Cl2 as a continuum medium.93,94 The relative energies of the excited states were obtained by carrying out TD-DFT (time-dependent DFT) calculations at the same theoretical level, starting from singlet and triplet state geometries.95 Calculations were carried out using Gaussian 16 and the output files were analysed with Multiwfn, version 3.5.96,97 Cartesian coordinates of the DFT-optimized structures are provided in a separated .xyz file.Synthesis and characterization of the ligands
The syntheses of 1-(pyridine-2-yl)benzotriazole (py-btz) and 1-(pyrimidin-2-yl)benzotriazole (pym-btz) were carried out following methods previously reported by Katritzky et al.61,62Synthesis of 1-(4,6-alkoxy-1,3,5-triazin-2-yl)benzotriazole (trzOR-btz, R = Me, Et, Ph)
The ligands were prepared by reacting 15.0 mmol of 4,6-alkoxy-substituted 2-chloro-triazine and 3.574 g of benzotriazole (30.0 mmol) in 20 mL of dry CH2Cl2 for CDMT or toluene for CDET and CDPhT. To prepare trzOMe-btz, the reaction mixture was stirred at room temperature overnight and then heated to reflux for 3 hours. For trzOEt-btz and trzOPh-btz, the reaction mixture was refluxed for 18 hours in an N2 atmosphere. After cooling down to room temperature, trzOMe-btz and trzOPh-btz were extracted with CH2Cl2, while trzOEt-btz was treated with Et2O. The so-obtained mixture was washed with 2 × 50 mL of cold NaOH 10% and 2 × 50 mL of water. The organic fractions were collected and dried with Na2SO4. The solvents were evaporated under reduced pressure to afford the products as white solids. Yield >80% in all the cases.Synthesis and characterization of complexes 1a–5a
HBF4·Et2O (163 μL, 1.2 mmol) was added to 1.5 mmol of the proper N-donor ligand (py-btz, pym-btz, trzOMe-btz, trzOEt-btz or trzOPh-btz) dissolved in 15 mL of CH2Cl2. The volatiles were evaporated under reduced pressure and 1.0 mmol (0.602 g) of [Cu(κ2-BH4)(PPh3)2] was added. The reagents were kept in an inert atmosphere and cooled at 77 K with a liquid nitrogen bath. CH2Cl2 (20 mL) was then slowly added through a syringe and the reaction mixture was allowed to slowly warm up to room temperature. After stirring overnight, the solution was purified by filtration and CH2Cl2 was evaporated under reduced pressure. The addition of Et2O (20 mL) caused the separation of a solid, that was filtered, washed with 5 mL of Et2O and dried in vacuo. Yield > 75% in all the cases.Synthesis and characterization of complexes 1b–4b, 1c–5c and 1d–5d
To a solution containing 0.472 g of [Cu(NCCH3)4][BF4] (1.5 mmol) in 20 mL of dry CH2Cl2, 3.0 mmol of PiPr3 (4.81 g of 10% solution in hexane) or 1.5 mmol of dppe (0.598 g) or DPEphos (0.808 g) were added. After 4 hours, 1.5 mmol of the proper ligand was added. After stirring overnight, the solvent was evaporated under reduced pressure. The product was precipitated by the addition of Et2O and the solid was filtered, washed and dried in vacuo. Yield > 75% in all the cases.Characterization of [Cu(py-btz)(μ-dppe)]2[BF4]2(1c)
Anal. calcd for C74H64B2Cu2F8N8P4 (1489.98 g mol−1, %): C, 59.65; H, 4.33; N, 7.52. Found (%): C, 59.42; H, 4.35; N, 7.49. M.p. 115 °C (dec.). ΛM (acetone, 298 K): 316 Ohm−1 mol−1 cm2. 1H NMR (CDCl3, 298 K) δ 8.76 (d, 1H, 3JHH = 8.4 Hz, py), 8.70–8.55 (m, 2H, py and btz), 8.37 (d, 1H, 3JHH = 8.2 Hz, btz), 8.05 (d, 1H, 3JHH = 5.0 Hz, py), 8.00 (t, 1H, 3JHH = 8.1 Hz, btz), 7.73 (t, 1H, 3JHH = 7.7 Hz, btz), 7.67–7.13 (m, 21H, btz and Ph), 2.66 (m, 2H, dppe), 2.50 (m, 2H, dppe). 31P{1H} NMR (CDCl3, 213 K) δ −4.55 (FWHM = 30 Hz). IR (KBr, cm−1): 3070–2850 m/w (aromatic νC–H and νC–H), 1640–1560 m (aromatic νC–C and νC–N), 1140–1020 m (νBF4).Author contributions
Jesús Castro: formal analysis, investigation, supervision, validation, writing – original draft, writing – review & editing. Valentina Ferraro: formal analysis, investigation, validation, writing – original draft, writing – review & editing. Marco Bortoluzzi: conceptualization, formal analysis, funding acquisition, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Università Ca’ Foscari Venezia is gratefully acknowledged for financial support (Bando Spin 2018, D. R. 1065/2018 prot. 67416). CACTI (University of Vigo) and Cineca (Bologna) are gratefully acknowledged for X-ray data collection and availability of high-performance computer resources (class C project COLUMN21). We sincerely thank Dr Andrea Morandini for the advice on triazine chemistry.Notes and references
- O. S. Wenger, J. Am. Chem. Soc., 2018, 140, 13522–13533, DOI:10.1021/jacs.8b08822.
- Q.-C. Zhang, H. Xiao, X. Zhang, L.-J. Xu and Z.-N. Chen, Coord. Chem. Rev., 2019, 378, 121–133, DOI:10.1016/j.ccr.2018.01.017.
- R. D. Costa, E. Ortí, H. J. Bolink, F. Monti, G. Accorsi and N. Armaroli, Angew. Chem., Int. Ed., 2012, 51, 8178–8211, DOI:10.1002/anie.201201471.
- C. Bizzarri, E. Spuling, D. M. Knoll, D. Volz and S. Bräse, Coord. Chem. Rev., 2018, 373, 49–82, DOI:10.1016/j.ccr.2017.09.011.
- M. Freitag, J. Teuscher, Y. Saygili, X. Zhang, F. Giordano, P. Liska, J. Hua, S. M. Zakeeruddin, J.-E. Moser, M. Grätzel and A. Hagfeldt, Nature Photon, 2017, 11, 372–378, DOI:10.1038/nphoton.2017.60.
- C. Wegeberg and O. S. Wenger, JACS Au, 2021, 1, 1860–1876, DOI:10.1021/jacsau.1c00353.
- X. Li, Y. Xie and Z. Li, Chem. – Asian J., 2021, 16, 2817–2829, DOI:10.1002/asia.202100784.
- L. M. Cavinato, S. Wölfl, A. Pöthig, E. Fresta, C. Garino, J. Fer-nandez-Cestau, C. Barolo and R. D. Costa, Adv. Mater., 2022, 34, 2109228, DOI:10.1002/adma.202109228.
- Y. Zhang, M. Schulz, M. Wächtler, M. Karnahl and B. Dietzek, Coord. Chem. Rev., 2018, 356, 127–146, DOI:10.1016/j.ccr.2017.10.016.
- P. A. Forero Cortés, M. Marx, M. Trose and M. Beller, Chem. Catalysis, 2021, 1, 298–338, DOI:10.1016/j.checat.2021.05.005.
- C. Bizzarri, Eur. J. Org. Chem., 2022, e202200185, DOI:10.1002/ejoc.202200185.
- C. Förster and K. Heize, Chem. Soc. Rev., 2020, 49, 1057–1070, 10.1039/c9cs00573k.
- E. Mejía, S.-P. Luo, M. Karnahl, A. Friedrich, S. Tschierlei, A.-E. Surkus, H. Junge, S. Gladiali, S. Lochbrunner and M. Beller, Chem. – Eur. J., 2013, 19, 15972–15978, DOI:10.1002/chem.201302091.
- P.-T. Chou and Y. Chi, Chem. – Eur. J., 2007, 13, 380–395, DOI:10.1002/chem.200601272.
- W.-P. To, Q. Wan, G. S. Ming Tong and C.-M. Che, Trends Chem., 2020, 2, 796–812, DOI:10.1016/j.trechm.2020.06.004.
- H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259–3302, 10.1039/C3CS60449G.
- K.-H. Kim and J.-J. Kim, Adv. Mater., 2018, 30, 1705600, DOI:10.1002/adma.201705600.
- W. C. H. Choy, W.-K. Chan and Y. Yuan, Adv. Mater., 2014, 26, 5368–5399, DOI:10.1002/adma.201306133.
- K. Li, Y. Chen, J. Wang and C. Yang, Coord. Chem. Rev., 2021, 433, 213755, DOI:10.1016/j.ccr.2020.213755.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 26, 7931–7958, DOI:10.1002/adma.201402532.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622–2652, DOI:10.1016/j.ccr.2011.01.042.
- H. Yersin, Highly Efficient OLEDs: Materials Based on Ther-mally Activated Delayed Fluorescence, Wiley-VCH, Weinheim, 2018 Search PubMed.
- G. U. Mahoro, J. Fernandez-Cestau, J.-C. Renaud, P. B. Coto, R. D. Costa and S. Gaillard, Adv. Optical Mater, 2020, 8, 2000260, DOI:10.1002/adom.202000260.
- C. Sandoval-Pauker, M. Santander-Nelli and P. Dreyse, RSC Adv., 2022, 12, 10653–10674, 10.1039/D1RA08082B.
- Y. Liu, S.-C. You, C.-L. Ho and W.-Y. Wong, Chem. Coord. Rev., 2018, 375, 514–557, DOI:10.1016/j.ccr.2018.05.010.
- M. S. Lazorski and F. N. Castellano, Polyhedron, 2014, 82, 57–70, DOI:10.1016/j.poly.2014.04.060.
- D. V. Scaltrito, D. W. Thompson, J. A. O'Callaghan and G. J. Meyer, Coord. Chem. Rev., 2000, 208, 243–266, DOI:10.1016/S0010-8545(00)00309-X.
- A. Lavie-Cambot, M. Cantuel, Y. Leydet, G. Jonusauskas, D. M. Bassani and N. D. McClenaghan, Coord. Chem. Rev., 2008, 252, 2572–2584, DOI:10.1016/j.ccr.2008.03.013.
- T. Tsubomura, K. Kimura, M. Nishikawa and T. Tsukuda, Dalton Trans., 2015, 44, 7554–7562, 10.1039/C5DT00835B.
- E. Leoni, J. Mohanraj, M. Holler, M. Mohankumar, I. Nierengarten, F. Monti, A. Sournia-Saquet, B. Delavaux-Nicot, J.-F. Nierengarten and N. Armaroli, Inorg. Chem., 2018, 57, 15537–15549, DOI:10.1021/acs.inorgchem.8b02879.
- S. Saeedi, C. Xue, B. J. McCullough, S. E. Roe, B. J. Neyhouse and T. White, ACS Appl. Energy Mater., 2019, 2, 131–143, DOI:10.1021/acsaem.8b02098.
- S. Keller, A. Pertegás, G. Longo, L. Martínez, J. Cerdá, J. M. Junquera-Hernández, A. Prescimone, E. C. Constable, C. E. Housecroft, E. Ortí and H. J. Bolink, J. Mater. Chem. C, 2016, 4, 3857–3871, 10.1039/C5TC03725E.
- L.-X. Xu, T.-Q. Wang, X.-F. Liu, H. Chen, C.-J. Wei, D. D. Xu, F. Chen, Y. Li and S.-P. Luo, Eur. J. Inorg. Chem., 2020, 4278–4283, DOI:10.1002/ejic.202000648.
- F. Doettinger, Y. Yang, M.-A. Schmid, W. Frey, M. Karnahl and S. Tschierlei, Inorg. Chem., 2021, 60, 5391–5401, DOI:10.1021/acs.inorgchem.1c00416.
- Z. Si, J. Li, B. Li, S. Liu and W. Li, J. Lumin., 2009, 129, 181–186, DOI:10.1016/j.jlumin.2008.09.014.
- J. Min, Q. Zhang, W. Sun, Y. Cheng and L. Wang, Dalton Trans., 2011, 40, 686–693, 10.1039/C0DT01031F.
- O. Back, J. Leppin, C. Förster and K. Heinze, Inorg. Chem., 2016, 55, 9653–9662, DOI:10.1021/acs.inorgchem.6b01400.
- C. Femoni, S. Muzzioli, A. Palazzi, S. Stagni, S. Zacchini, F. Monti, G. Accorsi, M. Bolognesi, N. Armaroli, M. Massi, G. Valenti and M. Marcaccio, Dalton Trans., 2013, 42, 997–1010, 10.1039/C2DT32056H.
- L. Bergmann, C. Braun, M. Nieger and S. Bräse, Dalton Trans., 2018, 47, 608–621, 10.1039/C7DT03682E.
- J.-L. Chen, P. Song, J. Liao, H.-R. Wen, R. Hong, Z.-N. Chen and Y. Chic, Inorg. Chem. Commun., 2010, 13, 1057–1060, DOI:10.1016/j.inoche.2010.06.010.
- J.-L. Chen, Z.-H. Guo, H.-G. Yu, L.-H. He, S.-J. Liu, H.-R. Wen and J.-Y. Wang, Dalton Trans., 2016, 45, 696–705, 10.1039/C5DT03451E.
- A. Alconchel, O. Crespo, P. García-Orduña and M. Concepción Gimeno, Inorg. Chem., 2021, 60, 18521–18528, DOI:10.1021/acs.inorgchem.1c03092.
- K. F. Baranova, A. A. Titov, A. F. Smol’yakov, A. Y. Chernyadyev, O. A. Filippov and E. S. Shubina, Molecules, 2021, 26, 6869, DOI:10.3390/molecules26226869.
- M. Grupe, P. Boden, P. Di Martino-Fumo, X. Gui, C. Bruschi, R. Israil, M. Schmitt, M. Nieger, M. Gerhards, W. Klopper, C. Riehn, C. Bizzarri and R. Diller, Chem. – Eur. J., 2021, 27, 15252–15271, DOI:10.1002/chem.202102760.
- Y. Wu, X. Han, Y. Qu, K. Zhao, C. Wang, G. Huang and H. Wu, J. Mol. Struct., 2019, 1191, 95–100, DOI:10.1016/j.molstruc.2019.04.108.
- B. Hupp, C. Schiller, C. Lenczyk, M. Stanoppi, K. Edkins, A. Lorbach and A. Steffen, Inorg. Chem., 2017, 56, 8996–9008, DOI:10.1021/acs.inorgchem.7b00958.
- L. Pathaw, D. Maheshwaran, T. Nagendraraj, T. Khamrang, M. Velusamy and R. Mayilmurugan, Inorg. Chim. Acta, 2021, 514, 119999, DOI:10.1016/j.ica.2020.119999.
- C. Bizzarri, A. P. Arndt, S. Kohaut, K. Fink and M. Nieger, J. Organomet. Chem., 2018, 871, 140–149, DOI:10.1016/j.jorganchem.2018.07.013.
- G. Li, R. S. Nobuyasu, B. Zhang, Y. Geng, B. Yao, Z. Xie, D. Zhu, G. Shan, W. Che, L. Yan, Z. Su, F. B. Dias and M. R. Bryce, Chem. – Eur. J., 2017, 23, 11761–11766, DOI:10.1002/chem.201701862.
- Y. Sun, V. Lemaur, J. I. Beltrán, J. Cornil, J. Huang, J. Zhu, Y. Wang, R. Fröhlich, H. Wang, L. Jiang and G. Zou, Inorg. Chem., 2016, 55, 5845–5852, DOI:10.1021/acs.inorgchem.6b00101.
- Q. Zhang, X.-L. Chen, J. Chen, X.-Y. Wu, R. Yu and C.-Z. Lu, Dalton Trans., 2015, 44, 10022–10029, 10.1039/C5DT01108F.
- L. Bergmann, J. Friedrichs, M. Mydlak, T. Baumann, M. Nieger and S. Bräse, Chem. Commun., 2013, 49, 6501–6503, 10.1039/C3CC42280A.
- C.-W. Hsu, C.-C. Lin, M.-W. Chung, Y. Chi, G.-H. Lee, P.-T. Chou, C.-H. Chang and P.-Y. Chen, J. Am. Chem. Soc., 2011, 133, 12085–12099, DOI:10.1021/ja2026568.
- N. Lüdtke, J. Föller and C. M. Marian, Phys. Chem. Chem. Phys., 2020, 22, 23530–23544, 10.1039/d0cp04654j.
- E. Loukopoulos and G. E. Kostakis, Coord. Chem. Rev., 2019, 395, 193–229, DOI:10.1016/j.ccr.2019.06.003.
- R. Gérardy and J.-C. M. Monbaliu, Top. Heterocycl. Chem., 2015, 43, 1–66, DOI:10.1007/7081_2015_179.
- A. R. Katritzky and B. V. Rogovoy, Chem. – Eur. J., 2003, 9, 4586–4593, DOI:10.1002/chem.200304990.
- M. Bortoluzzi, J. Castro, M. Girotto, F. Enrichi and A. Vomiero, Inorg. Chem. Commun., 2019, 102, 141–146, DOI:10.1016/j.inoche.2019.02.016.
- V. Ferraro, M. Bortoluzzi, J. Castro, A. Vomiero and S. You, Inorg. Chem. Commun., 2020, 116, 107894, DOI:10.1016/j.inoche.2020.107894.
- V. Ferraro, J. Castro, L. Agostinis and M. Bortoluzzi, Transit. Met. Chem, 2021, 46, 391–402, DOI:10.1007/s11243-021-00458-4.
- A. R. Katritzky and J. Wu, Synthesis, 1994, 597–600, DOI:10.1055/s-1994-25530.
- A. R. Katritzky, A. V. Ignatchenko and H. Lang, Heterocycles, 1995, 4, 131–146, DOI:10.3987/COM-94-6918.
- V. Ferraro, J. Castro, E. Trave and M. Bortoluzzi, J. Organomet. Chem., 2022, 957, 122171, DOI:10.1016/j.jorganchem.2021.122171.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, Wiley-VHC, Hoboken, 2009 Search PubMed.
- C. Li, C. F. R. Mackenzie, S. A. Said, A. K. Pal, M. A. Haghighatbin, A. Babaei, M. Sessolo, D. B. Cordes, A. M. Z. Slawin, P. C. J. Kamer, H. J. Bolink, C. F. Hogan and E. Zysman-Colman, Inorg. Chem., 2021, 60, 10323–10339, DOI:10.1021/acs.inorgchem.1c00804.
- F. Mazzeo, F. Brunner, A. Prescimone, E. C. Constable and C. E. Housecroft, Crystals, 2020, 10, 1, DOI:10.3390/cryst10010001.
- Y. Ma, Y. F. Dong, P. F. She, S. J. Liu, M. J. Xie, Y. X. Yu, Y. H. Li, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1801065, DOI:10.1002/adom.201801065.
- G. U. Mahoro, E. Fresta, M. Elie, D. di Nasso, Q. Zhang, J.-F. Lohier, J.-L. Renaud, M. Linares, R. Wannemacher, J. Cabanil-las-Gonzalez, R. D. Costa and S. Gaillard, Dalton Trans., 2021, 50, 11049–11060, 10.1039/D1DT01689J.
- D. Cremer and J. A. Pople, J. Am. Chem. Soc., 1975, 97, 1354–1358, DOI:10.1021/ja00839a011.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Cryst, 2016, B72, 171–179, DOI:10.1107/S2052520616003954.
- A. Pintado-Alba, H. de la Riva, M. Nieuwhuyzen, D. Bautista, P. R. Raithby, H. A. Sparkes, S. J. Teat, J. M. López-de-Luzuriaga and M. C. Lagunas, Dalton Trans., 2004, 3459–3467, 10.1039/B410619A.
- B. Deb, P. P. Sarmah and D. K. Dutta, Eur. J. Inorg. Chem., 2010, 1710–1716, DOI:10.1002/ejic.200901101.
- J. Yuasa, M. Dan and T. Kawai, Dalton Trans., 2013, 42, 16096–16101, 10.1039/C3DT51390D.
- C. Pelizzi and G. Pelizzi, Acta Cryst, 1979, B35, 1785–1790, DOI:10.1107/S0567740879007779.
- E. R. T. Tiekink, Z. Kristallogr. NCS, 2001, 216, 69–70, DOI:10.1524/ncrs.2001.216.14.69.
- M. Llunell, D. Casanova, J. Cirera, P. Alemany and S. Alvarez, SHAPE V. 2.1, Universitat de Barcelona and University of Jerusalem, Barcelona, 2013 Search PubMed.
- J. Cirera, P. Alemany and S. Alvarez, Chem. – Eur. J., 2004, 10, 190–207, DOI:10.1002/chem.200305074.
- S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell and D. Avnir, Coord. Chem. Rev., 2005, 249, 1693–1708, DOI:10.1016/j.ccr.2005.03.031.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964, 10.1039/B617136B.
- A. Okuniewski, D. Rosiak, J. Chojnacki and B. Becker, Polyhedron, 2015, 90, 47–57, DOI:10.1016/j.poly.2015.01.035.
- W. L. F. Armarego and D. D. Perrin, Purification of Laboratory Chemicals, Butterworth-Heinemann, Oxford, 1996 Search PubMed.
- C. Bianchini, C. A. Ghilardi, A. Meli, S. Midollini and A. Orlandini, Inorg. Chem., 1985, 24, 924–931, DOI:10.1021/ic00200a025.
- G. J. Kubas, B. Monzyk and A. L. Crumblis, Inorg. Synth., 1990, 28, 68–70, DOI:10.1002/9780470132593.ch15.
- S. Xie, Z. Yan, Z. Li, Q. Song and M. Ma, J. Org. Chem., 2018, 83, 10916–10921, DOI:10.1021/acs.joc.8b01587.
- X.-K. Liu, C.-J. Zheng, M.-F. Lo, J. Xiao, Z. Chen, C.-L. Liu, C.-S. Lee, M.-K. Fung and X.-H. Zhang, Chem. Mat, 2013, 25, 4454–4459, DOI:10.1021/cm403318r.
- Bruker, APEX3, SMART, SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2015.
- P. McArdle, J. Appl. Crystallogr., 2017, 50, 320–326, DOI:10.1107/S1600576716018446.
- G. M. Sheldrick, Acta Crystallogr., 2015, A71, 3–8, DOI:10.1107/S2053273314026370.
- G. M. Sheldrick, Acta Crystallogr., 2015, C71, 3–8, DOI:10.1107/S2053229614024218.
- A. L. Spek, Acta Crystallogr., 2020, E76, 1–11, DOI:10.1107/S2056989019016244.
- H. S. Yu, X. He, S. L. Li and D. G. Truhlar, Chem. Sci., 2016, 7, 5032–5051, 10.1039/C6SC00705H.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305, 10.1039/B508541A.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681, DOI:10.1002/jcc.10189.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001, DOI:10.1021/jp9716997.
- C. A. Ullrich, Time-dependent density functional theory, Ox-ford University Press, Oxford, 2012 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592, DOI:10.1002/jcc.22885.
Footnote |
| † Electronic supplementary information (ESI) available: Heteronuclear NMR spectra of the ligands, Fig. S1–S6; description of the X-ray structures of trzOMe-btz and trzOPh-btz; asymmetric units of trzOMe-btz and trzOPh-btz and superimposition, Fig. S7–S9; intermolecular interactions in trzOMe-btz and trzOPh-btz, Fig. S10 and S11; crystal data and structure refinement for trzOMe-btz and trzOPh-btz, Table S1; heteronuclear NMR spectra of the complexes, Fig. S12–S30; crystal data and structure refinement for complexes 1a and 2a, Table S2; ellipsoid plots of [1a]+ and [2a]+, best superimposition and π,π-stacking interaction in complex 2a, Fig. S31–S33; crystal data and structure refinement for the complexes, Tables S3 and S4; ellipsoid plot of cations [2b]+, [1d]+, [2c]2+, [3b]+, [4d]+ and [5d]+, Fig. S34; selected bond lengths [Å] and angles [°] for the complexes, Tables S5–S8; best superimposition of complexes 2a and 2b, Fig. S35; superimposition between free and coordinated ligands trzOPh-btz in complex 5d, Fig. S36; continuous shape measure calculation and other related parameters, Table S9; absorption spectra of complexes in 10−5 M CH2Cl2, Fig. S37. Cartesian coordinates of the DFT-optimized structures as separated .xyz file. CCDC 2100419–2100423, 2111460–2111464. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2nj03165e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |