Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced catalytic activity of perovskite La1−xSrxMnO3+δ for the oxygen reduction reaction†
Wencong
Wang
,
Wei
Liu
 ,
Masao
Kamiko
and
Shunsuke
Yagi
,
Masao
Kamiko
and
Shunsuke
Yagi
 *
*
Institute of Industrial Science, The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo, 153-8505, Japan. E-mail: syagi@iis.u-tokyo.ac.jp
First published on 9th June 2022
Abstract
Fuel cells are considered promising next-generation energy conversion devices because of their high efficiency and environment-friendly attributes. However, a large overpotential for the oxygen reduction reaction (ORR) at the cathode minimizes the energy efficiency of these devices. In this context, ORR catalysts based on 3d transition metal oxides have attracted significant attention as affordable and highly active catalysts with potential to replace expensive noble metal catalysts (e.g., Pt). In this study, the ORR catalytic activities of ‘oxygen-excess’ perovskite La1−xSrxMnO3+δ (x = 0, 0.1, 0.2, 0.3, and 0.4), with both higher activity and stability compared to a regular perovskite, i.e., La0.8Sr0.2MnO3, were investigated and the performances were evaluated with respect to changes in the Mn valence number, amount of cation vacancies and Mn–O bond length. Sr substitution can affect the formation of Mn4+ ions and modulate the cation vacancies, thus changing the length of the Mn–O bond. The lowest overpotential was observed for La0.8Sr0.2MnO3+δ, where the Mn–O bond length was the shortest, resulting in the highest activity. Overall, this work provides guidelines to improve the ORR catalytic activity of Mn-based perovskites.
1. Introduction
The rapidly increasing global environmental pollution caused by the massive use of fossil fuels necessitates the development and use of clean and renewable energy resources.1,2 Accordingly, various energy storage and conversion devices have been proposed in the last few decades.3–7 Among them, fuel cells have attracted considerable attention as next-generation clean energy conversion devices because of their high energy conversion efficiency (60–70%) and environment-friendly attributes.8 In fuel cells, the chemical energy stored in fuels, such as hydrogen, methanol, and ethanol, is directly converted into electrical energy at the anode, while a simultaneous oxygen reduction reaction (ORR) occurs at the cathode. Both the fuel oxidation reaction and ORR must be carried out in the presence of a catalyst that significantly lowers the overpotential, leading to an efficient energy conversion.9,10 However, ORR catalysts must be carefully designed as the ORR is a kinetically slow process;11,12 thus, catalysts that exhibit both high activity and stability are generally expensive, and those with good attributes yet low costs are yet to be found.For the ORR in alkaline solutions, two pathways are possible as shown below.13
4-Electron pathway (eqn (1)):
| O2 + 2H2O + 4e− ↔ 4OH− | (1) |
2-Electron pathway (eqn (2)):
| O2 + H2O + 2e− → HO2− + OH− | (2) |
Noble metals, such as Pt and Pd, have been widely used as ORR catalysts with excellent catalytic activity.14,15 However, these are expensive and constrained by limited resources. Alternative catalysts, which are abundant and thus cheap yet similarly effective to or more effective than the precious metals in catalyzing the ORR, can solve the cost issue associated with ORR catalysts.16,17 Transition metal oxides are considered as the most promising alternative catalysts.18,19 In particular, perovskites with a general formula of ABO3 are of great interest because of their high catalytic activity, significantly low cost, and compositional flexibility.20,21 Hyodo et al. reported the effects of A- and B-site cations on the ORR catalytic activity using various types of perovskites.22 For the A-site cations, La-based perovskites showed a higher ORR catalytic activity than Pr-, Nd-, and Sm-based perovskites. Regarding the B-site cations, Mn, Fe, Co, and Ni with partially filled 3d orbitals showed high ORR catalytic activities. Therefore, LaMnO3 has attracted considerable attention as an ORR catalyst. Notably, the perovskite structure allows the formation of non-stoichiometric compounds, such as oxygen-deficient LaMnO3−δ and ‘oxygen-excess’ LaMnO3+δ. It is worth noting that ‘oxygen-excess’ does not mean the existence of interstitial oxygen ions. Roosmalen et al. proposed that the ‘oxygen-excess’ originates from the existence of cation vacancies at La and Mn sites, and the amounts of La and Mn vacancies should be equal.23 The real chemical formula of LaMnO3+δ should be La1−γMnO1−γO3(γ = δ/(3 + δ)), but for simplicity the former expression is used as the name of the sample. Elemental substitution is a method frequently used to modulate the defect density and electronic structure of LaMnO3 to improve its ORR catalytic activity.24,25 Commonly used elements for replacing La at the A-site are alkaline earth metals, such as Ca and Sr, and those for replacing Mn at the B-site are 3d transition metals, such as Ni, Fe, and Co. Furthermore, the partial replacement of La with Sr has been proven effective in improving the ORR catalytic activity via modulation of the Mn valence state and O vacancy concentration.26,27 Most current studies on LaMnO3 have been concerned with either stoichiometric LaMnO3 or oxygen-deficient LaMnO3−δ; however, the exploration of ‘oxygen-excess’ LaMnO3+δ and its electronic structure modulation induced by Sr replacement is scarce.
In this study, we investigated the effects of Sr substitution in nonstoichiometric LaMnO3+δ on the ORR catalytic activity in an oxygen-saturated 0.1 M KOH aqueous solution. We found that the Sr-substituted and ‘oxygen-excess’ La1−xSrxMnO3+δ compounds (x = 0, 0.1, 0.2, 0.3, and 0.4) showed higher catalytic activity and stability than the normal Sr-substituted perovskite (i.e., La0.8Sr0.2MnO3). Through a combination of spectroscopic and electrochemical studies, we specifically examined the intrinsic reasons for the ORR catalytic activity difference of the La1−xSrxMnO3+δ series. The catalytic activity dependence on the Mn valence state, cation vacancies and Mn–O bond length is discussed.
2. Experimental
2.1. Synthesis of La1−xSrxMnO3+δ
The La1−xSrxMnO3+δ (x = 0, 0.1, 0.2, 0.3, and 0.4) compounds were synthesized by a sol–gel process. First, La(NO3)3·6H2O (Nacalai Tesque, Inc. 99.9%), Sr(NO3)2 (Sigma-Aldrich, 99.0%), and Mn(NO3)2·6H2O (Nacalai Tesque, Inc. 98.0%) were weighed according to their stoichiometric ratios and dissolved in 30 mL deionized water. After stirring the solutions for 2 h, 4.61 g of citric acid (Nacalai Tesque, Inc., 99.5%) was added to each solution, which were then stirred for another 2 h. Subsequently, the solutions were heated to 80 °C and maintained at that temperature for 12 h to obtain yellow gel precursors. The final products were obtained after the calcination of the gel precursors at 750 °C for 5 h. For comparison, stoichiometric La0.8Sr0.2MnO3 (99%) powder was bought from Sigma-Aldrich.2.2. Characterization
The crystal structure was examined by XRD measurements (Ultima IV, Rigaku Co., Ltd) of the powder samples using a molybdenum X-ray tube (λ = 0.7107 Å). Morphologies were observed by scanning electron microscopy (SEM, JEOL JSM-6010 LA) and high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2100). The valence state of Mn was analyzed by XPS using a ULVAC-PHI Quantera instrument. ICP-AES was conducted to determine the ratios of La, Sr, and Mn using a sample dissolved in a 1 M HCl aqueous solution. The sum of 2[Mn3+] and [Mn4+] was determined by iodometry using a titration device (COM-A19S, Hiranuma Co., Ltd). In detail, 10 mg of the sample was mixed with 40 mL of 0.1 g mL−1 KI solution. Then, 3 mL of 6 mol L−1 HCl was added to dissolve the sample. All the Mn3+ and Mn4+ ions will be reduced to Mn2+ by I− as shown below:28| 2Mn3+ + 3I− → 2Mn2+ + I3− | (3) |
| Mn4+ + 3I− → Mn2+ + I3− | (4) |
Then titration was performed using 10 mmol L−1 Na2S2O3 solution assuming the following reaction:
| I3− + 2S2O32− → 3I− + S4O62− | (5) |
The numbers of La3+, Sr2+, Mn3+, Mn4+, La and Mn vacancies (VA-site and VB-site) were estimated by considering the results of ICP, iodometry, and charge balance. XAS was performed in the transmission mode to obtain Mn K-edge absorption spectra at SPring-8 in Japan with a Si(111) double-crystal monochromator operated at 8 GeV. The Athena program was used for the background subtraction and normalization of the XAS data, and the Artemis program integrated with the IFEFFIT package was used for shell fitting of the XAS spectra in the R space.29
2.3. Electrochemical measurements
The catalyst ink used for testing was prepared as follows. First, 10 mL of Nafion solution (5 mass% in a mixture of lower aliphatic alcohols and water, 45% water, Sigma-Aldrich) and 20 mL of 0.1 M KOH aqueous solution were mixed to obtain a K+-exchanged Nafion solution. Then, 0.3 mL of the K+-exchanged Nafion solution, 50 mg of the perovskite catalyst, and 10 mg of acetylene black were mixed in a 10 mL volumetric flask, and the flask was filled to 10 mL with a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v) mixture of water and ethanol.
4 (v/v) mixture of water and ethanol.
Electrochemical measurements were conducted using a three-electrode cell with a rotating ring disk electrode (RRDE) system (RRDE-3A, BAS Inc.) at a speed of 1600 rotations per min in 0.1 M KOH aqueous solution. The RRDE consisted of a glassy carbon disk (area ∼ 0.126 cm2) and a platinum ring (area ∼ 0.188 cm2). A Pt wire was used as the counter electrode, and a Hg/HgO (0.1 M KOH aqueous solution) electrode was used as the reference electrode.
Before the electrochemical measurements, oxygen was purged into the electrolyte solution for 20 min to obtain an oxygen-saturated solution. Note that, although oxygen bubbling was stopped after achieving the saturation in the solution, the oxygen flow was maintained above the solution during the measurements. LSV was performed at a scan rate of 10 mV s−1 in the potential range from 0.3 to −0.6 V vs. Hg/HgO (0.1 M aq. KOH). The potential of the ring electrode was set to 0.4 V vs. Hg/HgO to oxidize HO2− formed on the disk electrode.
The production fraction of HO2− and electron transfer number (n) were calculated using the following equations:
 | (6) |
 | (7) |
All the potential values measured vs. Hg/HgO (0.1 M aq. KOH), i.e., E (V vs. Hg/HgO), were converted to the potential values vs. reversible hydrogen electrode, E (V vs. RHE), using the following relationship:
| E (V vs. RHE) = E (V vs. Hg/HgO) + 0.926 | (8) |
3. Results and discussion
3.1 Structure, morphology, and composition
The crystal structures of the prepared La1−xSrxMnO3+δ compounds were analyzed by X-ray diffraction (XRD) (Fig. 1a). The diffraction peaks for all samples with different amounts of Sr matched well with those for the reported perovskite-type structure of LaMnO3 (ICSD#75070).30 The lattice parameters obtained from the XRD patterns are listed in Table S1 (ESI†). The crystal system is rhombohedral and the space group is R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, which has been reported to have a higher ORR catalytic activity than the orthorhombic structure.31 In Fig. 1b–f, the d-spacing obtained from the high-resolution TEM images also confirmed the rhombohedral structure of these samples. In the XRD profile of La0.6Sr0.4MnO3+δ, minor impurity peaks appeared at approximately 12°, which corresponds to SrCO3 (ICSD#15195), possibly formed by a reaction of Sr with citric acid during synthesis. Highly pure samples could not be obtained above x = 0.6, as shown in Fig. S1 (ESI†). SEM images and TEM images in Fig. S2 and S3 (ESI†) suggest that all the samples have similar morphologies. The primary particle diameters of all the samples are mostly in the range of 10–20 nm as shown in Fig. S3 (ESI†), indicating that Sr-substitution does not change the morphology and particle size obviously.
c, which has been reported to have a higher ORR catalytic activity than the orthorhombic structure.31 In Fig. 1b–f, the d-spacing obtained from the high-resolution TEM images also confirmed the rhombohedral structure of these samples. In the XRD profile of La0.6Sr0.4MnO3+δ, minor impurity peaks appeared at approximately 12°, which corresponds to SrCO3 (ICSD#15195), possibly formed by a reaction of Sr with citric acid during synthesis. Highly pure samples could not be obtained above x = 0.6, as shown in Fig. S1 (ESI†). SEM images and TEM images in Fig. S2 and S3 (ESI†) suggest that all the samples have similar morphologies. The primary particle diameters of all the samples are mostly in the range of 10–20 nm as shown in Fig. S3 (ESI†), indicating that Sr-substitution does not change the morphology and particle size obviously.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) and iodometry were performed to determine the numbers of La, Sr, and Mn (Mn3+ and Mn4+) assuming a chemical formula of La1−x−y(VA-site)ySrxMna3+Mnb4+(VB-site)yO3,23 as shown in Table 1. The amount of cation vacancies is decreased, while the fraction of Mn4+ monotonically increased from 0.341 to 0.680 to maintain electrical neutrality as the amount of Sr is increased from 0 to 0.4.
| Sample name | La | Sr | V A-site | Mn3+ | Mn4+ | V B-site |
|---|---|---|---|---|---|---|
| LaMnO3+δ | 0.943 ± 0.002 | 0 | 0.057 ± 0.012 | 0.602 ± 0.012 | 0.341 ± 0.012 | 0.057 ± 0.012 |
| La0.9Sr0.1MnO3+δ | 0.856 ± 0.001 | 0.094 ± 0.001 | 0.050 ± 0.014 | 0.553 ± 0.014 | 0.397 ± 0.014 | 0.050 ± 0.014 |
| La0.8Sr0.2MnO3+δ | 0.749 ± 0.002 | 0.203 ± 0.002 | 0.048 ± 0.011 | 0.457 ± 0.011 | 0.494 ± 0.011 | 0.048 ± 0.011 |
| La0.7Sr0.3MnO3+δ | 0.640 ± 0.002 | 0.315 ± 0.002 | 0.045 ± 0.015 | 0.369 ± 0.015 | 0.586 ± 0.015 | 0.045 ± 0.015 |
| La0.6Sr0.4MnO3+δ | 0.545 ± 0.001 | 0.410 ± 0.001 | 0.044 ± 0.012 | 0.275 ± 0.012 | 0.680 ± 0.012 | 0.044 ± 0.012 |
3.2 Energy spectroscopies
X-Ray photoelectron spectroscopy (XPS) was conducted to analyze the chemical environment of Mn and O. As can be seen from the Mn 2p XPS profiles in Fig. 2a, Mn 2p3/2 and Mn 2p1/2 peaks are centered at 641.5 and 653.3 eV, respectively. The spin–orbit splitting value (ΔE) between Mn 2p3/2 and Mn 2p1/2 is 11.8 eV. Through XPS fitting, both the Mn 2p1/2 and 2p3/2 peaks can be deconvoluted into three peaks, comprising a primary and a satellite peak for Mn3+ and a peak for Mn4+.32,33 The obtained binding energies are listed in Table S2 (ESI†). In all the samples, Mn exists in both +3 and +4 valence states, while the valence number of Mn can be considered +3 in the completely stoichiometric LaMnO3. The atomic ratios of Mn3+ and Mn4+ obtained via XPS fitting are listed in Table 2. The percentage of Mn4+ increased from 32.3% to 67.4%, which is in good agreement with the titration results shown in Table 1. O 1s XPS profiles are shown in Fig. 2b, which can be assigned to two peaks: a low binding energy peak, which represents the lattice oxygen OL, and a high energy peak, which can be attributed to the surface adsorbed oxygen species OS.34,35 The La0.8Sr0.2MnO3+δ sample shows the highest surface adsorbed oxygen species concentration as shown in Table 2. This may partially contribute to ORR catalytic activity.| Sample | Mn3+ (%) | Mn4+ (%) | OS (%) | OL (%) |
|---|---|---|---|---|
| LaMnO3+δ | 67.8 | 32.3 | 37.8 | 62.2 |
| La0.9Sr0.1MnO3+δ | 49.4 | 50.6 | 40.3 | 59.7 |
| La0.8Sr0.2MnO3+δ | 41.1 | 58.9 | 45.5 | 54.5 |
| La0.7Sr0.3MnO3+δ | 34.7 | 65.3 | 37.4 | 62.6 |
| La0.6Sr0.4MnO3+δ | 32.6 | 67.4 | 44.1 | 55.9 |
X-Ray absorption spectroscopy (XAS) is a powerful tool to analyze the oxidation states and bond conditions. Fig. 3a presents the X-ray absorption near-edge spectra (XANES, Mn K-edge) of the La1−xSrxMnO3+δ samples. The spectra of all the samples are located between the spectra of standard Mn2O3 and MnO2, which again indicates that the valence states of Mn in all these samples are between +3 and +4, consistent with the XPS and ICP/iodometry analyses. Fig. 3b shows the extended X-ray absorption fine structure (EXAFS) spectra of the Mn K-edge in the R space. The first peak between 1.0 and 2.0 Å is related to the Mn–O bond.36,37 To evaluate the Mn–O bond length, EXAFS fitting was performed by assuming a rhombohedral LaMnO3 structure with the first nearest shell model using the Artemis program integrated with the IFEFFIT package in the 1.0–2.0 Å range.29 The Mn–O bond lengths, determined from the fitting (cf. Table S3, ESI†), are shown in Fig. 3c. When x was increased from 0 to 0.2, the Mn–O bond length gradually decreased and reached a minimum at x = 0.2. The shortest bond length may be a result of the combination of an increase in the oxidation state of Mn and a decrease in Mn vacancies as shown in Tables 1 and 2. In addition, the shortest Mn–O bond length of the La0.8Sr0.2MnO3+δ sample may result in the strongest interaction between Mn and O, which can explain the highest surface adsorbed oxygen species concentration obtained from O 1s XPS spectral fitting results.
3.3 ORR catalytic properties
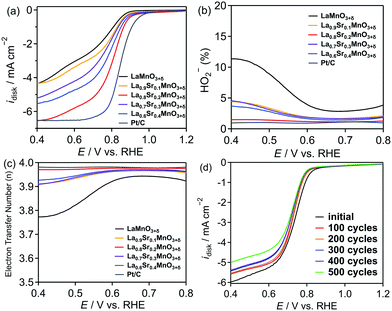
The ORR catalytic activity of La1−xSrxMnO3+δ (x = 0, 0.1, 0.2, 0.3, and 0.4) was evaluated using a rotating ring disk electrode (RRDE) system in a 0.1 M KOH aqueous solution (Fig. 4). A comparison of the linear sweep voltammograms (LSVs) in Fig. 4a suggests that La0.8Sr0.2MnO3+δ exhibits the highest current density among all perovskites in the La1−xSrxMnO3+δ series. The current density is not linearly correlated with the value of x, and it decreases in the following order: x = 0.2 > x = 0.4 > x = 0.3 > x = 0.1 > x = 0. The overpotentials (Eover) determined at −2 mA cm−2 and the onset potentials (Eonset) determined at −0.1 mA cm−2 obtained from the LSVs are listed in Table 3. Eover, Eonset, and the current density are all correlated; a lower Eover corresponds to a higher current density. Therefore, La0.8Sr0.2MnO3+δ exhibited both the lowest Eover and the highest current density, indicating the highest catalytic activity among others with different Sr substitution. In the ORR, the 4-electron pathway is preferred over the 2-electron pathway because of the higher energy conversion efficiency of the former pathway. The production fraction of HO2− (HO2−%) and electron transfer number (n)38 were calculated using eqn (6) and (7) (see the Experimental section for details) using the disk and ring currents (Fig. S4, ESI†), as shown in Fig. 4b and c, respectively. LaMnO3+δ produces >10% HO2− at 0.4 V vs. RHE through the 2-electron pathway, resulting in the lowest value of n (3.78) in the series. With 10% Sr substitution (i.e., La0.9Sr0.1MnO3+δ), the HO2−% decreases significantly to <5%, and n is close to 4. In particular, the HO2−% and n for La0.8Sr0.2MnO3+δ are very close to those of Pt, indicating the excellent 4-electron selectivity of the catalyst. As shown in Fig. 3c and Table 3, the trends for the changes in overpotential and Mn–O bond length are fully consistent. The above trends are in agreement with the report by Suntivich et al.31 who stated that the ORR catalytic activity is related to the B–O covalency in the ABO3 perovskite, where a strong covalency of the B–O bond can increase the driving force of the rate-limiting O2−/OH− exchange reaction occurring on the surface B ions. According to the EXAFS fitting results, the La0.8Sr0.2MnO3+δ sample had the shortest Mn–O bond length with a high degree of covalency, resulting in the highest ORR catalytic activity in the series.| Sample | E over (V) | E onset (V vs. RHE) |
|---|---|---|
| LaMnO3+δ | 0.494 | 0.900 |
| La0.9Sr0.1MnO3+δ | 0.456 | 0.961 |
| La0.8Sr0.2MnO3+δ | 0.398 | 1.135 |
| La0.7Sr0.3MnO3+δ | 0.444 | 1.035 |
| La0.6Sr0.4MnO3+δ | 0.421 | 1.069 |
| Pt/C | 0.355 | 1.085 |
Durability of the catalysts is another important aspect for ORR catalysis. The LSV curves for the best sample, La0.8Sr0.2MnO3+δ, measured during 500 cycles of cyclic voltammetry, are shown in Fig. 4d. For comparison, the LSV curves for La0.8Sr0.2MnO3 over 500 cycles are shown in Fig. S5 (ESI†). After 500 cycles, the current density for La0.8Sr0.2MnO3 was significantly lower than the initial value, indicating poor stability. In contrast, for the cycling using La0.8Sr0.2MnO3+δ, the decrease and increase in the current density and overpotential, respectively, were smaller and gradual. Even after 500 cycles, the overpotential at −2 mA cm−2 increased only by 5.5% compared to the initial value, indicating excellent stability. In addition, the Mn 2p XPS profiles for La0.8Sr0.2MnO3+δ before and after 500 cycles of cyclic voltammetry are shown in Fig. S6 (ESI†), and the atomic ratios of Mn3+ and Mn4+ estimated using the XPS profiles are listed in Table S4 (ESI†). After 500 cycles, the ratio of Mn4+ was decreased, suggesting a gradual reduction of Mn4+ to Mn3+ during 500 cycles. Thus, the slight increase of the overpotential for the ORR of La0.8Sr0.2MnO3+δ of about 5.5% after 500 cycles as shown in Fig. 4d could have been caused by the decrease in the Mn valence number.
4. Conclusions
In this study, we synthesized a series of perovskites with a general formula of La1−xSrxMnO3+δ (x = 0, 0.1, 0.2, 0.3, and 0.4) via Sr substitution of ‘oxygen-excess’ LaMnO3+δ and investigated the effect of Sr substitution on the ORR catalytic activity of the resultant catalysts. Sr substitution leads to an increase in the Mn valence state and a decrease in the amount of cation vacancies, which were verified by iodometry and XPS. The opposite trends in the valence state of Mn and the amount of Mn vacancies may have led to a minimum in the Mn–O bond length. The ORR catalytic activity was found to be correlated with the Mn–O bond length; the shortest Mn–O bond length (and the highest covalency) of La0.8Sr0.2MnO3+δ among others in the series was responsible for the highest ORR catalytic activity of this catalyst. The high catalytic activity, high 4-electron selectivity close to Pt and the excellent stability make this material a promising ORR catalyst that can replace precious metals.Author contributions
Wencong Wang: experiment, analysis, and writing. Wei Liu: XAFS analysis. Masao Kamiko: XPS experimental support. Shunsuke Yagi: supervision, writing, reviewing, and editing.Funding sources
This research was financially supported by a Grant-in-Aid for the University of Tokyo Excellent Young Researcher and Grant-in-Aid for Scientific Research (20H05180, 22H04497) commissioned by the Japan Society for the Promotion of Science. X-Ray absorption spectroscopy (XAS) data were obtained at the BL14B2 beamline (Proposal No. 2021B1902) of SPring-8 with support from the Japan Synchrotron Radiation Research Institute.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that influenced the work reported in this study.Acknowledgements
We thank Prof. Toru H. Okabe and Dr Takanari Ouchi for their support with the ICP-AES measurements. We also thank Prof. Ikuya Yamada and Dr Yuichi Okazaki for giving an opportunity to measure the XAS data.References
- C. Tarhan and M. A. Çil, A study on hydrogen, the clean energy of the future: Hydrogen storage methods, J. Energy Storage, 2021, 40, 102676 CrossRef.
- C. Liao, J. T. Erbaugh, A. C. Kelly and A. Agrawal, Clean energy transitions and human well-being outcomes in Lower and Middle Income Countries: A systematic review, Renew. Sustainable Energy Rev., 2021, 145, 111063 CrossRef.
- L. Ye and X. Li, A dynamic stability design strategy for lithium metal solid state batteries, Nature, 2021, 593, 218–222 CrossRef CAS PubMed.
- M. Singh, D. Zappa and E. Comini, Solid oxide fuel cell: Decade of progress, future perspectives and challenges, Int. J. Hydrogen Energy, 2021, 46, 27643–27674 CrossRef CAS.
- J. Han, S. Yagi, H. Takeuchi, M. Nakayama and T. Ichitsubo, Catalytic mechanism of spinel oxides for oxidative electrolyte decomposition in Mg rechargeable batteries, J. Mater. Chem. A, 2021, 9, 26401–26409 RSC.
- M. Wang, J. Han, W. Liu, M. Kamiko and S. Yagi, Energy storage mechanism of monocrystalline layered FePS3 and FePSe3 as active materials for Mg batteries and pseudocapacitors, J. Alloys Compd., 2021, 883, 160822 CrossRef CAS.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanostructured carbon-metal oxide composite electrodes for supercapacitors: A review, Nanoscale, 2013, 5, 72–88 RSC.
- M. Secanell, J. Wishart and P. Dobson, Computational design and optimization of fuel cells and fuel cell systems: A review, J. Power Sources, 2011, 196, 3690–3704 CrossRef CAS.
- F. Yang, X. Bao, Y. Zhao, X. Wang, G. Cheng and W. Luo, Enhanced HOR catalytic activity of PGM-free catalysts in alkaline media: The electronic effect induced by different heteroatom doped carbon supports, J. Mater. Chem. A, 2019, 7, 10936–10941 RSC.
- Y. J. Wang, W. Long, L. Wang, R. Yuan, A. Ignaszak, B. Fang and D. P. Wilkinson, Unlocking the door to highly active ORR catalysts for PEMFC applications: Polyhedron-engineered Pt-based nanocrystals, Energy Environ. Sci., 2018, 11, 258–275 RSC.
- C. Lo Vecchio, A. Serov, H. Romero, A. Lubers, B. Zulevi, A. S. Aricò and V. Baglio, Commercial platinum group metal-free cathodic electrocatalysts for highly performed direct methanol fuel cell applications, J. Power Sources, 2019, 437, 226948 CrossRef CAS.
- Y. Shao, J. P. Dodelet, G. Wu and P. Zelenay, PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges, Adv. Mater., 2019, 31, 1–8 CAS.
- X. Ge, A. Sumboja, D. Wuu, T. An, B. Li, F. W. T. Goh, T. S. A. Hor, Y. Zong and Z. Liu, Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts, ACS Catal., 2015, 5, 4643–4667 CrossRef CAS.
- M. Liu, Z. Zhao, X. Duan and Y. Huang, Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts, Adv. Mater., 2019, 31, 1–8 Search PubMed.
- L. E. Betancourt, A. Rojas-Pérez, I. Orozco, A. I. Frenkel, Y. Li, K. Sasaki, S. D. Senanayake and C. R. Cabrera, Enhancing ORR Performance of Bimetallic PdAg Electrocatalysts by Designing Interactions between Pd and Ag, ACS Appl. Energy Mater., 2020, 3, 2342–2349 CrossRef CAS.
- L. Du, L. Xing, G. Zhang, M. Dubois and S. Sun, Strategies for Engineering High-Performance PGM-Free Catalysts toward Oxygen Reduction and Evolution Reactions, Small Methods, 2020, 4, 1–28 CrossRef.
- E. F. Holby, Improving platinum group metal–free oxygen reduction reaction electrocatalyst activity: suggestions from density functional theory studies, Curr. Opin. Electrochem., 2021, 25, 100631 CrossRef CAS.
- J. Wang, Z. Zhang, J. Ding, C. Zhong, Y. Deng, X. Han and W. Hu, Recent progresses of micro-nanostructured transition metal compound-based electrocatalysts for energy conversion technologies, Sci. China Mater., 2021, 64, 1–26 CrossRef CAS.
- W. J. Niu, J. Z. He, B. N. Gu, M. C. Liu and Y. L. Chueh, Opportunities and Challenges in Precise Synthesis of Transition Metal Single-Atom Supported by 2D Materials as Catalysts toward Oxygen Reduction Reaction, Adv. Funct. Mater., 2021, 31, 1–28 Search PubMed.
- H. Wang, M. Zhou, P. Choudhury and H. Luo, Perovskite oxides as bifunctional oxygen electrocatalysts for oxygen evolution/reduction reactions – A mini review, Appl. Mater. Today, 2019, 16, 56–71 CrossRef.
- P. Ramakrishnan, H. Im, S. H. Baek and J. I. Sohn, Recent Studies on Bifunctional Perovskite Electrocatalysts in Oxygen Evolution, Oxygen Reduction, and Hydrogen Evolution Reactions under Alkaline Electrolyte, Isr. J. Chem., 2019, 59, 708–719 CrossRef CAS.
- T. Hyodo, M. Hayashi, N. Miura and N. Yamazoe, Catalytic Activities of Rare-Earth Manganites for Cathodic Reduction of Oxygen in Alkaline Solution, J. Electrochem. Soc., 1996, 143, L266–L267 CrossRef CAS.
- J. A. M. Van Roosmalen, E. H. P. Cordfunke, R. B. Helmholdt and H. W. Zandbergen, The Defect Chemistry of LaMnO3 ± δ, J. Solid State Chem., 1994, 110, 100–105 CrossRef CAS.
- Y. Xue, H. Miao, S. Sun, Q. Wang, S. Li and Z. Liu, La1-: XAgxMnO3 electrocatalyst with high catalytic activity for oxygen reduction reaction in aluminium air batteries, RSC Adv., 2017, 7, 5214–5221 RSC.
- Y. Xue, S. Sun, Q. Wang, H. Miao, S. Li and Z. Liu, La0.7(Sr0.3-xPdx)MnO3 as a highly efficient electrocatalyst for oxygen reduction reaction in aluminum air battery, Electrochim. Acta, 2017, 230, 418–427 CrossRef CAS.
- F. Lu, J. Sui, J. Su, C. Jin, M. Shen and R. Yang, Hollow spherical La0.8Sr0.2MnO3 perovskite oxide with enhanced catalytic activities for the oxygen reduction reaction, J. Power Sources, 2014, 271, 55–59 CrossRef CAS.
- C. Jin, X. Cao, L. Zhang, C. Zhang and R. Yang, Preparation and electrochemical properties of urchin-like La0.8Sr0.2MnO3 perovskite oxide as a bifunctional catalyst for oxygen reduction and oxygen evolution reaction, J. Power Sources, 2013, 241, 225–230 CrossRef CAS.
- S. K. Chandrabanshi, S. Mukhopadhyay and R. Mukherjee, Oxidation of iodide with a mononuclear manganese(IV) complex ion: Mechanistic investigation of autocatalytic behaviour, Polyhedron, 2020, 187, 114664 CrossRef CAS.
- B. Ravel and M. Newville, ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- S. Angel, F. Schneider, S. Apazeller, W. Kaziur-Cegla, T. C. Schmidt, C. Schulz and H. Wiggers, Spray-flame synthesis of LaMO3(M = Mn, Fe, Co) perovskite nanomaterials: Effect of spray droplet size and esterification on particle size distribution, Proc. Combust. Inst., 2021, 38, 1279–1287 CrossRef CAS.
- J. Suntivich, H. A. Gasteiger, N. Yabuuchi, H. Nakanishi, J. B. Goodenough and Y. Shao-Horn, Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries, Nat. Chem., 2011, 3, 546–550 CrossRef CAS PubMed.
- L. Wang, C. Wang, H. Xie, W. Zhan, Y. Guo and Y. Guo, Catalytic combustion of vinyl chloride over Sr doped LaMnO 3, Catal. Today, 2019, 327, 190–195 CrossRef CAS.
- Y. N. Lee, R. M. Lago, J. L. G. Fierro, V. Cortés, F. Sapiña and E. Martínez, Surface properties and catalytic performance for ethane combustion of La1-xKxMnO3 + δ perovskites, Appl. Catal., A, 2001, 207, 17–24 CrossRef CAS.
- M. O’Connell, A. K. Norman, C. F. Hüttermann and M. A. Morris, Catalytic oxidation over lanthanum-transition metal perovskite materials, Catal. Today, 1999, 47, 123–132 CrossRef.
- M. Imamura, N. Matsubayashi and H. Shimada, Catalytically active oxygen species in La1-xSrxCoO3-δ studied by XPS and XAFS spectroscopy, J. Phys. Chem. B, 2000, 104, 7348–7353 CrossRef CAS.
- K. Selvakumar, S. M. Senthil Kumar, R. Thangamuthu, K. Ganesan, P. Murugan, P. Rajput, S. N. Jha and D. Bhattacharyya, Physiochemical investigation of shape-designed MnO2 nanostructures and their influence on oxygen reduction reaction activity in alkaline solution, J. Phys. Chem. C, 2015, 119, 6604–6618 CrossRef CAS.
- A. Paolone, C. Castellano, R. Cantelli, G. Rousse and C. Masquelier, Evidence of a splitting of the Mn-O distance and of a large lattice disorder in the charge-ordered phase of LiMn2O4 obtained by EXAFS, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 1–5 CrossRef.
- R. Zhou, Y. Zheng, M. Jaroniec and S. Z. Qiao, Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment, ACS Catal., 2016, 6, 4720–4728 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj02619h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |