Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of an actinium-225 radioimmunoconjugate for targeted alpha therapy against SARS-CoV-2†
Roger M.
Pallares‡
 a,
Matthew
Flick
a,
Katherine M.
Shield
a,
Matthew
Flick
a,
Katherine M.
Shield
 ab,
Tyler A.
Bailey
ab,
Nileena
Velappan
c,
Antonietta M.
Lillo
*c and
Rebecca J.
Abergel
ab,
Tyler A.
Bailey
ab,
Nileena
Velappan
c,
Antonietta M.
Lillo
*c and
Rebecca J.
Abergel
 *ab
*ab
aChemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. E-mail: abergel@berkeley.edu
bDepartment of Nuclear Engineering, University of California, Berkeley, CA 94720, USA
cBioscience Division, Los Alamos National Laboratory, Los Alamos, NM 87545, USA
First published on 29th June 2022
Abstract
Targeted alpha therapy offers unique opportunities for the treatment of tumours and infections. Here, we report the development of a new radioimmunoconjugate construct that targets SARS-CoV-2 infected cells, which act as viral reservoirs and promote virus replication and infection spread. The chosen antibody selectively binds to the ACE2-receptor binding domain of the spike protein, and prevents the protein binding to the receptor. Furthermore, the antibody has been radiolabelled with 225Ac, and the therapeutic performance of the resulting radioimmunoconjugate has been demonstrated in vitro against cells mimicking SARS-CoV-2 infection.
Public awareness of Coronavirus Disease 2019 (COVID-19) first came in late 2019, where early reports of a new pulmonary disease emerged in Wuhan, China.1,2 SARS-CoV-2, a single stranded ribonucleic acid virus, is the causative agent for the COVID-19 pandemic, which has led to over 2.5 million deaths globally.1,3 SARS-CoV-2 presents spike glycoproteins on its exterior that bind to specific antigens on the surface of host cells called ACE2 receptors.4 The high transmissibility of SARS-CoV-2 viral particles expedited its spread on the global scale, resulting in what has become one of the greatest public health crises of the last century, while acting as an impetus for unprecedented biomedical efforts to discover new antiviral therapies. Despite millions of vaccines per day being distributed to the world's population, significantly curbing the spread and severity of COVID-19 cases, effective antivirals are still needed to treat patients with severe symptoms that require hospitalization. The pandemic continues to highlight the need for drug candidates that are both adaptable to new pathogens and variants, and which can yield biological effectiveness for high-risk patients in need of timely treatment.
Targeted alpha therapy (TAT) is a promising type of radiotherapy currently explored in oncology.5–7 TAT relies on radioconjugates composed of a targeting vector, such as monoclonal antibodies, covalently bond to bifunctional chelators, which coordinate α-emitting radionuclides. Because α-particles display high linear energy transfer and low penetration depth, TAT is able to locally deliver high cytotoxic doses of radiation to tumour cells, while leaving healthy surrounding tissue unaffected. The early success of TAT against several types of cancers has resulted in TAT being proposed as a treatment for other pathologies, such as bacterial and viral infections.8,9 Because viruses present high radioresistance due to their small size, radiopharmaceuticals usually target infected cells that act as viral reservoirs and facilitate virus replication, rather than targeting the viruses themselves. This antiviral strategy has been effective against HIV-1 in animal models.10,11 With regards to radionuclide selection, 225Ac may be preferred over other available α-emitters because it has a 10-day half-life, which allows for central production and subsequent distribution, and multiple α-emissions in its decay chain, enhancing the delivered dose.12 Recently, we developed a set of monoclonal antibodies with high affinity and selectivity for SARS-CoV-2 through in vitro evolution of human single-chain antibodies.13 Although the antibodies were obtained for sensitive and selective point of care detection of SARS-CoV-2, we hypothesized that they could also be used as targeting moieties for TAT against the viral infection.
Here, we show the development of a radioimmunoconjugate for TAT against SARS-CoV-2. The antibody is selective against the spike protein, and can prevent its binding to ACE2 receptor. Our radiolabelling protocol resulted in radiochemical yields between 76 and 87%, while the labeled conjugates retained the antibody native macrostructure integrity. Finally, the potential therapeutic performance of the radioimmunoconjugate against SARS-CoV-2 reservoirs was tested with cells displaying spike proteins, which mimicked infected cells.
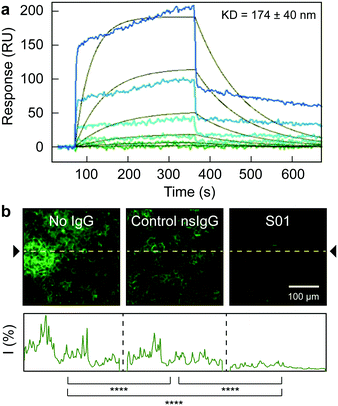
A monoclonal IgG antibody (S01) was selected as model antibody (Table S1, ESI†), since it shows high binding affinity (Kd of 170 ± 40 nM, Fig. 1a) for the ACE2-receptor binding domain of the spike protein (RBD2). The S01 antibody is a member of a library of unique anti-SARS-CoV-2 antibodies produced by our group, via evolution of human single chain antibody libraries, and synergizing phage and yeast display technologies.13 Moreover, the S01 antibody could also block the binding between the RBD2 and the ACE2 receptor, as demonstrated by competition experiment, where the RBD2 labelled with green fluorescent protein (RBD2-sfGFP) was preincubated with the antibody, and later exposed to ACE2-presenting HEK-293T cells. In absence of antibodies or after exposure to a control non-specific IgG (nsIgG), the RBD2-sfGFP could bind to the cells, as observed by confocal microscopy (Fig. 1b). After pre-incubation with S01, however, the RBD2-sfGFP binding to the receptor was mostly prevented. Hence, a radioimmunoconjugate based on S01 antibody, not only would benefit from the radionuclide activity, but also the blocking capabilities of the antibody, as the binding between the spike protein and the ACE2 receptor is an essential step during SARS-CoV-2 infection.
Next, DOTA, a macrocyclic chelator commonly utilized to complex 225Ac in TAT, was coupled to S01 and nsIgG antibodies through lysine-based conjugation, where solvent exposed amine groups of the lysine side chain along the antibodies reacted with p-SCN-Bn-DOTA to form stable thiourea bonds (Fig. 2). The resulting conjugates retained the antibody native macrostructure integrity as confirmed by polyacrylamide gel electrophoresis (Fig. 3a). The S01 and nsIgG conjugates had a chelator to antibody ratio of 1.6 and 2.2, respectively, as determined by matrix assisted laser desorption ionization-time of flight mass spectrometry (Fig. 3b, for full mass spectra refer to Fig. S1, ESI†). The DOTA-antibodies were subsequently radiolabelled with 225Ac at 45 °C for 90 min following a previously established protocol, which maximizes metal binding, while minimizing protein denaturation.14–16 The specific activities for the resulting radioimmunoconjugates (225Ac-S01 and 225Ac-nsIgG) were around 37 kBq μg−1, as quantified by liquid scintillation counter, and the radiolabelling yield ranged between 75.6 and 86.7% (Table 1). Furthermore, the activities remained quantitively bound to the constructs as observed by radio thin layer chromatography (Fig. S2, ESI†).
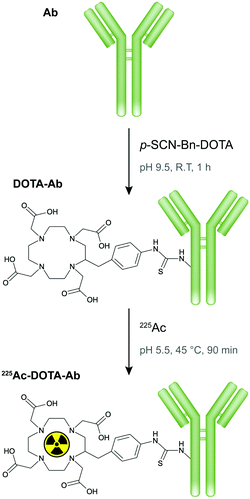 | ||
| Fig. 2 Scheme of antibody conjugation with p-SCN-Bn-DOTA ligand and subsequent radiolabelling with 225Ac. | ||
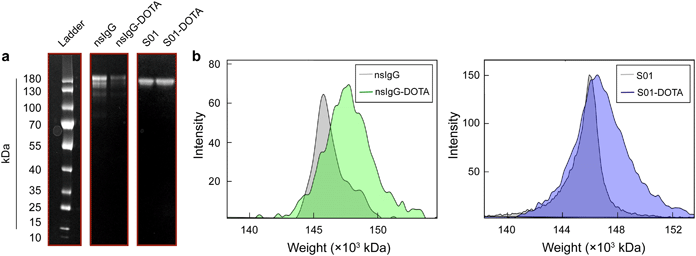 | ||
| Fig. 3 (a) Gel electrophoresis of antibodies used in the experiment before and after conjugation with DOTA. (b) Mass spectra of nsIgG and S01 antibodies before and after DOTA conjugation. | ||
| Chelator/antibody | Recovered activity (MBq) | Radiochemical yield (%) | |
|---|---|---|---|
| Note: 6.03 MBq of 225Ac were used for the radiolabelling of each radioimmunoconjugate. | |||
| 225Ac-S01 | 1.6 | 4.56 ± 0.03 | 75.6 ± 0.5 |
| 225Ac-nsIgG | 2.2 | 5.23 ± 0.04 | 86.7 ± 0.7 |
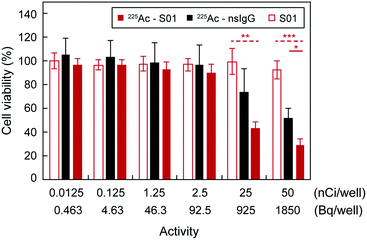
Lastly, we decided to evaluate the therapeutic performance of the radioimmunoconjugates in vitro. It is worth noting that viruses display high radioresistance,17 and there are concerns regarding whether they may be missed by alpha and beta radiation due to their small sizes. Nevertheless, successful radioimmunotherapy has been demonstrated against viral infections, such as HIV, in animal models by targeting viral proteins expressed on the surface of infected cells,10,11 since these cells act as viral reservoirs, where viruses propagate. Similar observations have been reported in cells of COVID-19 patients, where the SARS-CoV-2 infected cells express the spike protein on their surface, which then can interact with ACE2-positive neighbouring cells, causing adverse effects, such as syncytia.18 Furthermore, it has been reported that SARS-CoV-2 spreads through cell-to-cell transmission.19 To mimic infected cells, we used HEK-293T cells expressing the SARS-CoV-2 spike glycoproteins. The cells had been transfected with a SARS-CoV-2 spike glycoprotein expression plasmid to provide consistent expression levels of the protein on the cell membrane. Hence, cell viabilities were determined after a 4-day incubation period with the different radioimmunoconjugate treatments (Fig. 4). The half-maximal inhibitory concentration (IC50) of 225Ac-S01 was 834.9 ± 18.1 Bq per well (22.6 ± 0.5 nCi per well). Furthermore, the antibody did not display observable cytotoxic effects by itself as shown by the consistent cell viability levels after treatment with the unlabelled antibody. Regarding 225Ac-nsIgG, its IC50 was 2-fold higher than that of the targeting radioimmunoconjugate, highlighting the benefits of the targeting vector. These results were consistent with a previous publication, which compared the therapeutic effect of targeting and non-targeting antibody-based radiopharmaceuticals for TAT against tumour cells.14
In summary, we have developed a new radioimmunoconjugate for potential TAT against SARS-CoV-2 infections. The radioimmunoconjugate is based on an antibody that targets the RBD2 of the spike protein. The antibody itself prevents the binding of RBD2 to the cell ACE2 receptor. We radiolabelled the antibody with 225Ac, following a protocol developed in-house, which results in a labelling yield between 75.6 and 86.7%. Furthermore, in vitro studies showed that the radiopharmaceutical induces cytotoxicity to cells mimicking SARS-CoV-2 infection, which are reported to act as virus reservoirs (promoting virus replication and infection propagation). Combined, all of these results indicate that the proposed radioimmunoconjugate construct could be a potential therapeutic agent for TAT against SARS-CoV-2 infections. It is worth noting, however, that as in the case of TAT against cancer (where TAT is reserved for metastatic patients that are not responding to other forms of therapy), TAT would not be expected to be used to treat the overall population, but only extreme cases. Hence, solely patients with severe infections that are not responding to other treatments would be treated, since TAT has inherent risks (e.g. recoil and release of daughters) that would not justify its use in mild cases.
Author contributions
R. M. Pallares: conceptualization, methodology, investigation, formal analysis, visualization, writing – original draft. M. Flick: methodology, investigation, writing – review & editing. K. M. Shield: investigation, writing – review & editing. T. A. Bailey: investigation, writing – review & editing. N. Velappan: investigation, resources, writing – review & editing. A. M. Lillo: funding acquisition, conceptualization, methodology, writing – review & editing. R. J. Abergel: funding acquisition, supervision, conceptualization, methodology, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The conjugation, radiolabelling, and targeted alpha-therapy portions of this work were supported by the University of California Contractor Supporting Research Program at Lawrence Berkeley National Laboratory under US Department of Energy Contract No. DE-AC02-05CH11231 (RJA). Initial antibody development was supported by the US Department of Energy (DOE) Office of Science through the National Virtual Biotechnology Laboratory, a consortium of DOE national laboratories focused on response to COVID-19, with funding provided by the Coronavirus CARES Act. The 225Ac used in this research was supplied by the DOE Isotope Program, managed by the Office of Isotope R&D and Production. The authors acknowledge Dahlia An and Joshua Chen for helpful discussions regarding experimental design, and Drs. Jennifer Wacker and Alyssa Gaiser for their assistance during radiolabelling.Notes and references
- J. F.-W. Chan, S. Yuan, K.-H. Kok, K. K.-W. To, H. Chu, J. Yang, F. Xing, J. Liu, C. C.-Y. Yip, R. W.-S. Poon, H.-W. Tsoi, S. K.-F. Lo, K.-H. Chan, V. K.-M. Poon, W.-M. Chan, J. D. Ip, J.-P. Cai, V. C.-C. Cheng, H. Chen, C. K.-M. Hui and K.-Y. Yuen, Lancet, 2020, 395, 514–523 CrossRef CAS.
- A. Remuzzi and G. Remuzzi, Lancet, 2020, 395, 1225–1228 CrossRef CAS.
- Y. M. Bar-On, A. Flamholz, R. Phillips and R. Milo, eLife, 2020, 9, e57309 CrossRef.
- M. A. Tortorici and D. Veesler, in Advances in Virus Research, ed. F. A. Rey, Academic Press, 2019, vol. 105, pp. 93–116 Search PubMed.
- C. Parker, V. Lewington, N. Shore, C. Kratochwil, M. Levy, O. Lindén, W. Noordzij, J. H. Park and F. Saad, JAMA Oncol., 2018, 4, 1765–1772 CrossRef PubMed.
- J. Kozempel, O. Mokhodoeva and M. Vlk, Molecules, 2018, 23 Search PubMed.
- R. M. Pallares, P. Agbo, X. Liu, D. D. An, S. S. Gauny, S. E. Zeltmann, A. M. Minor and R. J. Abergel, ACS Appl. Mater. Interfaces, 2020, 12, 40078–40084 CrossRef CAS PubMed.
- E. Dadachova, J. Med. Imaging Radiat. Sci., 2019, 50, S49–S52 CrossRef PubMed.
- R. M. Pallares and R. J. Abergel, ACS Pharmacol. Transl. Sci., 2021, 4, 1–7 CrossRef CAS PubMed.
- E. Dadachova, M. C. Patel, S. Toussi, C. Apostolidis, A. Morgenstern, M. W. Brechbiel, M. K. Gorny, S. Zolla-Pazner, A. Casadevall and H. Goldstein, PLoS Med., 2006, 3, e427 CrossRef PubMed.
- E. Dadachova, S. G. Kitchen, G. Bristol, G. C. Baldwin, E. Revskaya, C. Empig, G. B. Thornton, M. K. Gorny, S. Zolla-Pazner and A. Casadevall, PLoS One, 2012, 7, e31866 CrossRef CAS PubMed.
- R. Andrew Kyle Henderson, R. Caterina Fortunata, S. Paul and R. Valery, Curr. Radiopharm., 2018, 11, 156–172 CrossRef PubMed.
- A. M. Lillo, G. S. Waldo, N. Velappan and H. T. B. Nguyen, US Department of Energy, 2021 DOI:10.2172/1760553.
- A. L. Lakes, D. D. An, S. S. Gauny, C. Ansoborlo, B. H. Liang, J. A. Rees, K. D. McKnight, H. Karsunky and R. J. Abergel, Mol. Pharmaceutics, 2020, 17, 4270–4279 CrossRef CAS.
- M. R. McDevitt, D. Ma, L. T. Lai, J. Simon, P. Borchardt, R. K. Frank, K. Wu, V. Pellegrini, M. J. Curcio, M. Miederer, N. H. Bander and D. A. Scheinberg, Science, 2001, 294, 1537 CrossRef CAS PubMed.
- M. Miederer, M. R. McDevitt, G. Sgouros, K. Kramer, N.-K. V. Cheung and D. A. Scheinberg, J. Nucl. Med., 2004, 45, 129 CAS.
- K. S. B. Rose, J. Environ. Radioact., 1992, 15, 113–133 CrossRef CAS.
- J. Buchrieser, J. Dufloo, M. Hubert, B. Monel, D. Planas, M. M. Rajah, C. Planchais, F. Porrot, F. Guivel-Benhassine, S. Van der Werf, N. Casartelli, H. Mouquet, T. Bruel and O. Schwartz, EMBO J., 2020, 39, e106267 CrossRef CAS PubMed.
- C. Zeng, J. P. Evans, T. King, Y.-M. Zheng, E. M. Oltz, S. P. J. Whelan, L. J. Saif, M. E. Peeples and S.-L. Liu, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2111400119 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj02617a |
| ‡ Current address: Institute for Experimental Molecular Imaging, RWTH Aachen University Hospital, Aachen 52074, Germany. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |