Copper(ii) complexes of quinoline-based ligands for efficient photoredox catalysis of atom transfer radical addition (ATRA) reaction†
Abstract
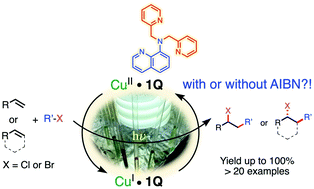
Copper(II) complexes of quinoline-based ligands were synthesized, and their catalytic properties in atom transfer radical addition (ATRA) reactions of alkyl halides to alkenes were studied. Under white light irradiation at room temperature, the complexes effectively catalyze the addition reaction and inhibit radical polymerization of the olefins. The reactions can be conveniently carried out by using the complexes generated in situ from a mixture of common copper(II) halides and the ligand. The photoelectrochemical properties of the copper(II)-quinoline complexes suggest their effective reduction by light-induced homolysis to form the corresponding active copper(I) species without the necessity of an external reducing agent. Excellent yields of regio- and stereoselective addition products (>20 examples) can be obtained by using 1 mol% or less of the catalyst. The role of commonly employed additives AIBN and Na2CO3 is evaluated, suggesting that these alleviate catalyst poisoning by preventing the build-up of HX in the course of the reactions.


 Please wait while we load your content...
Please wait while we load your content...