Synthesis and catalysis of a Z-stereoretentive ruthenium carbene catalyst chelated by 2,4,5,7-tetrachloro-1,8-dimercaptonaphthalene for olefin metathesis†
Abstract
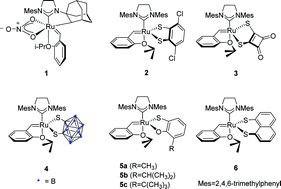
In this work, a 2,4,5,7-tetrachloro-1,8-dimercaptonaphthalene ligand-chelated ruthenium-based carbene olefin metathesis catalyst was synthesized. The synthesized catalyst catalyzed the ring-opening cross-metathesis reactions of norbornene/exo,exo-5-norbornene-2,3-dimethanol with styrene/4-fluorostyrene to obtain high Z-products (97 : 3–99 : 1 Z/E). The cross-metathesis reactions of (Z)-2-butene-1,4-diol with terminal alkenes using the synthesized catalyst gave high Z-stereoretentive products (91 : 9–98 : 2 Z/E). Compared to the previously reported Ru catalysts with naphthalene-1,8-dithiolate and 1,2-dicarbadodecaborane (12)-1,2-dithiolate, the prepared Ru complex exhibited higher catalytic activity under similar catalytic conditions. In addition, the new catalyst tolerates various olefin substrates containing different functional groups.


 Please wait while we load your content...
Please wait while we load your content...