Assessment of hydrogen bond strengths and cooperativity in self- and cross-associating cyclic (HF)m(H2O)n (m + n = 2 to 8) clusters†
Abstract
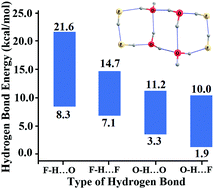
In this work, we investigated the strengths of various self- and cross-associating hydrogen bonds (HBs) in mixed hydrogen fluoride–water cyclic (HF)m(H2O)n (m + n = 2 to 8) clusters, employing a molecular tailoring approach (MTA)-based method. The F–H⋯O HB energies, calculated at the MP2/aug-cc-pVTZ level, were found to be in between 8.3 and 21.6 kcal mol−1. The F–H⋯F HB energies were found to be within 7.1 to 14.7 kcal mol−1, suggesting very strong HBs in these clusters when HF was the HB donor. On the other hand, moderately strong HBs were observed when the O–H of water was the HB donor. For instance, the O–H⋯O HB energies were found to lie between 3.3 and 11.2 kcal mol−1. The O–H⋯F HBs were the weakest (1.9 to 10.0 kcal mol−1) among all the types of HBs. The zero-point energy- and basis set superposition-corrections were negligibly small (less than 1 kcal mol−1). The HB energies in the dimers chopped out of these clusters also followed this rank ordering of the HB strengths, i.e., F–H⋯O (8.5 to 9.9 kcal mol−1) > F–H⋯F (3.6 to 4.7 kcal mol−1) > O–H⋯O (3.4 to 5.2 kcal mol−1) > O–H⋯F (0.7 to 3.6 kcal mol−1). These HB energies in the dimers suggested that the large HB strengths in (HF)m(H2O)n (m + n = 2 to 8) clusters were mainly due to the large cooperativity contribution. For instance, the cooperativity contributions of the other HBs towards the F–H⋯O HBs were found to be −10 to 61%, and those towards the F–H⋯F HBs were 35 to 74%. The O–H⋯O and O–H⋯F HB strengths were enhanced by −6 to 63% and 24 to 92%, respectively. We postulate here that these large cooperativity contributions could be one of the possible reasons for the strong self- and cross-associating HB networks in these cyclic (HF)m(H2O)n (m + n = 2 to 8) clusters.


 Please wait while we load your content...
Please wait while we load your content...