Newly synthesized series of oxoindole–oxadiazole conjugates as potential anti-SARS-CoV-2 agents: in silico and in vitro studies†
Abstract
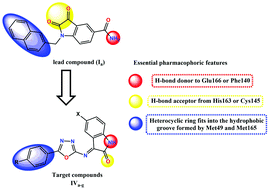
In this study, a series of 1,3,4-oxadiazoles carrying the isatin moiety (IVa–g) as anti-SARS-CoV-2 agents were designed and synthesized. Molecular docking of the compounds (IVa–g) into the SARS-CoV-2 Mpro active site showed promising binding affinities. The docking results were supported using molecular dynamics simulations and MM-GBSA calculations as well. To validate the in silico predictions, all compounds were evaluated for their half-maximal cytotoxicity (CC50) and virus-inhibitory (IC50) concentrations. The CC50 concentrations were remarkably high for most of the tested compounds. However, compounds IVe and IVg showed high activity against SARS-CoV-2 at IC50 values of 13.84 μM and 4.63 μM, with selectivity indices of 4.1 and 5.9, respectively. The most potent antiviral agent IVg demonstrated an IC50 of 16.6 μM against SARS-CoV-2 Mpro, which is considered a moderate activity. However, the represented cellular antiviral activity of IVg could justify further optimization to develop this series of compounds as broad-spectrum anti-SARS-CoV-2 agents.


 Please wait while we load your content...
Please wait while we load your content...