The versatile roles of neutral donor ligands in tuning catalyst performance for the ring-opening polymerization of cyclic esters
Abstract
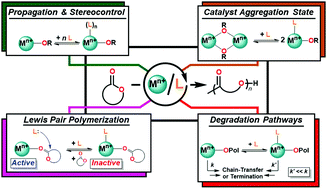
The ring-opening polymerization (ROP) of cyclic esters provides access to biodegradable polymers with materials properties that can be tailored through appropriate catalyst design. Introduction of neutral donor ligands has emerged as an attractive opportunity to tune catalyst performance in a rational, inexpensive, and operationally simple manner; however, broader application of these ligands has been limited by our mechanistic-level understanding of these additives. In this perspective, we highlight the versatile roles of neutral donor ligands in modulating catalyst performance in the ROP of cyclic esters, and identify several unifying concepts and trends that emerge across a diverse range of catalyst systems (group I, II, XII–XIV, and the rare-earths (group III and the lanthanides)), neutral donor ligands (ethers, crown-ethers, amines, guanidines, carbenes, phosphines, and phosphine-oxides), and cyclic esters (ε-caprolactone, lactide, β-lactones). Shared learnings in design strategies, bonding preferences, and mechanisms of action help solidify relationships between catalyst structure and function, and illuminate promising future directions to leverage these effects in the synthesis of sustainable polymers.
- This article is part of the themed collections: 2021 Focus and Perspective articles and NJC Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...