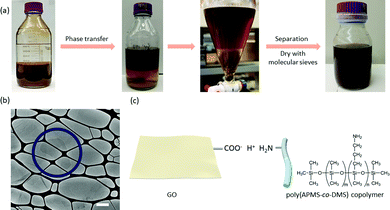
Stable graphene oxide hydrophobic photonic liquids†
Yi-Tao
Xu
a,
Joyce
Li
a and
Mark J.
MacLachlan
 *abcd
*abcd
aDepartment of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia V6T 1Z1, Canada. E-mail: mmaclach@chem.ubc.ca
bStewart Blusson Quantum Matter Institute, University of British Columbia, 2355 East Mall, Vancouver, British Columbia V6T 1Z1, Canada
cWPI Nano Life Science Institute, Kanazawa University, Kanazawa 920-1192, Japan
dBioproducts Institute, University of British Columbia, 2360 East Mall, Vancouver, British Columbia V6T 1Z3, Canada
First published on 11th January 2022
Abstract
Graphene oxide (GO) is an important nanomaterial for producing photonic liquids due to its ability to display full-color reflections in water. However, the poor stability of GO photonic liquids and unsatisfactory dispersibility of GO nanosheets in hydrophobic liquid media have been significant drawbacks to developing photonic materials based on GO. Here, stable GO hydrophobic photonic liquids are demonstrated for the first time. GO nanosheets are directed into different hydrophobic liquid media, including reactive liquid precursors like tetraethoxysilane and ethyl acrylate, in the presence of phase transfer additives. These liquids exhibit tunable reflection wavelength up to ∼1300 nm with improved stability relative to aqueous GO photonic suspensions at elevated temperatures or under ambient conditions. Supported by an entropy-driven depletion mechanism, hydrophobic additives can effectively mediate the self-assembly of GO to produce tunable photonic liquids without the need to adjust GO concentrations. Furthermore, simultaneous infrared and visible light reflection can be achieved, enabling infrared photonic GO liquids to display visible colors. The improved stability and tunable photonic properties of hydrophobic GO liquids will open a way for developing GO-based optical materials and devices.
New conceptsIn 2014, it was discovered that nanostructured graphene oxide (GO) forms photonic suspensions in water, where the ultrathin sheets of GO stack on top of one another with a periodicity that enables the suspensions to reflect visible light. However, the poor stability of GO photonic liquids and inability of GO nanosheets to form photonic liquids in hydrophobic media are two big issues that have hindered the development of GO photonic materials, despite their attractive photonic properties. This study demonstrates for the first time the formation of GO photonic suspensions in various hydrophobic liquid media. These suspensions exhibit highly tunable periodic structures with light reflection extending to the near-infrared region. It was also found that the reflection wavelength can be manipulated not only by adjusting GO concentrations but also by using foreign nanoparticles according to the entropic depletion interaction mechanism. Moreover, the GO photonic suspensions prepared in hydrophobic solvents showed a significantly improved stability compared to aqueous GO suspensions. This study provides important insights for improving the utility of nanostructured GO photonic liquids, developing GO photonic liquids in hydrophobic oganic solvents and liquid precusors. We believe that this study will contribute to the development of GO photonic materials and may broaden their application in photonic sensors, and displays. |
The development of graphene oxide and graphene-based materials has been driven by their many attractive properties, with potential applications including electro-optic devices, energy storage and conversion, reinforced nanocomposites, etc.1–13 GO is a two-dimensional (2D) nanoparticle with abundant oxygen-containing functional groups that increase its dispersibility in water and other hydrophilic media.14,15 In water, GO nanosheets self-assemble into a liquid crystalline phase with nematic or lamellar structures above a critical concentration.3,16 Interestingly, vivid structural color emerges spontaneously when the periodicity of lamellar GO liquid crystals is on the order of the wavelength of visible light.2 Recently, aqueous GO photonic suspensions have demonstrated their possible applications in switchable electro-optical displays and patterning.17–19
However, the development of photonic materials based on GO is fraught with challenges. A common issue is stability; the color of GO photonic suspensions often fades too quickly to be useful.18 Developing durable GO photonic liquids is a prerequisite for applications (e.g., photonic sensors, displays, and making solid-state photonic materials), but this topic remains unexplored.
Another challenge is the fabrication of GO photonic suspensions in hydrophobic liquid media. Exploring this issue is helpful for the development of GO-based composites or organic materials with photonic structures, since many precursors important to material manufacturing are hydrophobic and incompatible with polar solvents used to disperse GO. Hydrophobic GO photonic liquids may also be useful for designing optical sensors or reflective displays. Recently, Song and co-workers investigated other liquid media for GO photonic suspensions and they obtained GO photonic suspensions with tunable structural colors in water-based binary solvents (such as water–dioxane, water–NMP, water–DMF).20 However, the liquid media for GO photonic suspensions were still hydrophilic. Although methods to render GO hydrophobic are known,21,22 these approaches have not led to any reports of GO photonic suspensions in hydrophobic liquid media.
Here, we describe our investigations of GO photonic suspensions with significantly improved stability in hydrophobic liquid media. GO nanosheets were stabilized in hydrophobic liquids by employing the phase transfer additive, aminopropylmethylsiloxane–dimethylsiloxane [poly(APMS-co-DMS)] copolymer. These hydrophobic photonic liquids demonstrate highly tunable periodic structures, allowing for tunable reflection wavelength across the visible and near-infrared (NIR) regions (photonic band gaps up to ∼1300 nm). Directed by an entropic depletion interaction, a hybrid hydrophobic photonic liquid is also developed based on GO and silica nanoparticles. The GO photonic systems can even show second order reflectance, which makes it possible to develop infrared-reflecting GO photonic liquids with visible colors. Additionally, GO hydrophobic photonic liquids are found to be much more stable at room and elevated temperatures compared to aqueous GO photonic suspensions.
GO nanosheets are ∼0.5 to 6 μm in lateral size (Fig. S1, ESI†); they are prepared from the oxidative delamination of graphite in a mixture of KMnO4, NaNO3 and concentrated H2SO4.13,19,23 The successful preparation of GO was confirmed by powder X-ray diffraction (PXRD), infrared (IR) spectroscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. S1 and S2, ESI†). Vivid reflection colors from red to blue emerge by adjusting the GO concentrations in water (Fig. S3, ESI†). To better preserve the structures of the iridescent liquids for SEM analysis, we prepared GO–cellulose nanocrystal (CNC) alcogels to capture GO dispersions, which could be further solidified by supercritical CO2 drying to give GO–CNC aerogels.19,24 The role of CNCs is to induce the gelation of the suspensions for making GO–CNC gels that captured the liquid crystalline structure. The liquid crystalline structure is not significantly disrupted in the presence of a low concentration of CNCs (10 mg mL−1) according to our previous study, though microphase separation may occur to reduce the interlayer separation of GO domains.19 The typical one dimensional (1D) layered structure observed from GO–CNC aerogels indicates the presence of a lamellar structure that is responsible for the structural color (Fig. S4, ESI†).
To direct GO nanosheets into hydrophobic liquid media, we first mixed the dilute GO suspensions (1 mg mL−1) with diethyl ether containing poly(APMS-co-DMS) (5 mg mL−1), a copolymer previously used for the phase transfer of nanoparticles.25,26 The concentration of GO in the water phase became ∼0.17 mg mL−1 after stirring, indicating ∼83% of GO nanosheets went into the diethyl ether phase. The process of phase transfer did not significantly impact the structure of GO (Fig. 1b, Fig. S1c and S2a, ESI†). The GO–ether phase was then separated from the mixture using a separatory funnel and dried with molecular sieves to produce a homogeneous GO suspension in diethyl ether. While the mechanism for the phase transfer process has not yet been fully resolved, it is possible that this process is based on electrostatic interactions via the formation of acid–base pairs between nanoparticles and the phase transfer additives (Fig. 1c).25–27
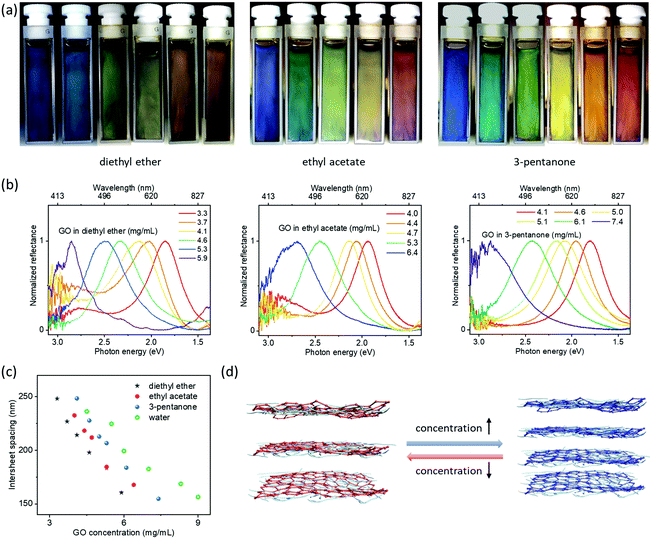
GO suspensions in diethyl ether were concentrated using a rotary evaporator. Interestingly, visible light reflection spanning the entire visible spectrum was observed from the suspensions by tuning the GO concentrations from ∼3.3 to 5.9 mg mL−1 (Fig. 2). The diethyl ether can be easily replaced with other hydrophobic liquid media through rotary evaporation due to its high vapor pressure. Similarly, GO suspensions also exhibited concentration-dependent photonic properties in 3-pentanone and ethyl acetate. As the GO concentration increased, the reflection wavelength was shifted to shorter wavelength; the relationship between concentration and periodicity (or reflection wavelength; see ESI† for the estimation of periodicity based on the reflection wavelength) nearly followed a linear trend (Fig. 2c, d and Fig. S5, ESI†), which is typical of 1D periodic structures in suspensions2,18,28–33 and agrees with the 1D structures observed from the GO–CNC aerogels (Fig. S4, ESI†). Moreover, these hydrophobic photonic liquids displayed angle-dependent structural colors (Fig. S6, ESI†) comparable to other photonic suspensions of 2D nanoparticles in water.29
Surprisingly, a second set of blue-to-red reflection colors was observed after further diluting the suspensions (Fig. 3 and Fig. S7–S9, ESI†). Visible and near-infrared spectra show two distinct reflections at the same time (Fig. 3b, c, Fig. S8 and S9, ESI†): one is in the visible region, while the other is in the near-infrared region with λ extending up to ∼1300 nm. These phenomena indicate the infrared reflective liquids of GO are ordered sufficiently to exhibit second order reflection peaks. For comparison (Fig. S10, ESI†), aqueous GO suspensions only gave reflection wavelengths up to ∼1000 nm with opacity in the visible range, indicating the poor ordering of the dilute aqueous GO suspensions. These observable comparisons demonstrate a degree of ordering within the GO hydrophobic liquids not previously achieved in aqueous suspensions at these large periodicities. Previous works have also demonstrated interesting aqueous suspensions of titanate(IV) with second order reflections.29 However, this requires a very strong magnetic field. Here, GO nanosheets self-organized into periodic structures with interlayer separations up to ∼470 nm without the need to use strong external fields. Near-infrared reflective liquids of dilute GO are also stable for over 1 month. GO hydrophobic liquids with such features may be useful to develop infrared-reflective photonic materials with visible colors.
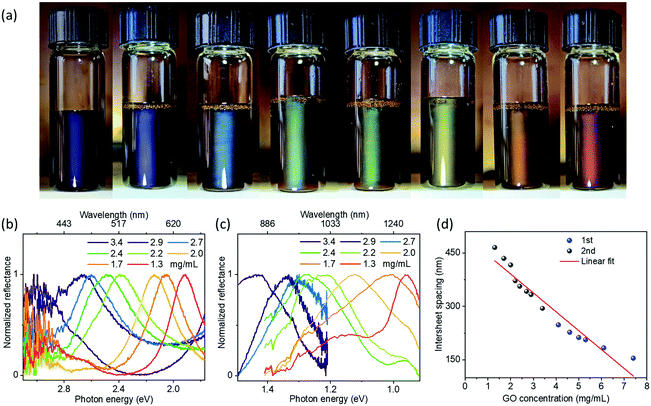 | ||
| Fig. 3 (a) Infrared GO photonic suspensions (∼1.3 to 3.4 mg mL−1 from right to left) in 3-pentanone showing structural colors as a result of second order reflectance. (b) Corresponding visible reflection spectra of samples shown in (a). (c) Corresponding near-infrared reflection spectra of samples shown in (a). (d) Intersheet spacing estimated from the spectra as a function of GO concentration. The intersheet spacing for the blue part of (d) and the grey part of (d) is estimated based on high concentration GO suspensions (1st order reflectance, ∼4.1 to 7.4 mg mL−1) shown in Fig. 2b and the dilute GO suspensions (2nd order reflectance, ∼1.3 to 3.4 mg mL−1) shown in (b), respectively. R2 for the fit is ∼0.93. | ||
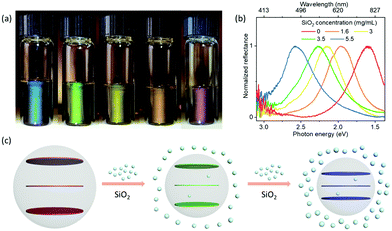
Mediating the self-assembly and interlayer separation of 2D nanoparticles in liquid media has proven challenging.2,18 Luckily, we were able to manipulate the photonic structures of hydrophobic GO liquids in a different manner. We found that using SiO2 nanoparticles as additives could effectively direct the assembly of GO nanosheets in hydrophobic liquid media. The SiO2 particles were functionalized with dichlorodimethylsilane to render them hydrophobic; they are spherical with diameters of ∼25 nm (see Fig. S11 (ESI†) for TEM result, Fig. S12 (ESI†) for size distribution, Fig. S13 (ESI†) for photos of the experimental steps of hydrophobization). Interestingly, the structural color of these liquids composed of sheet/sphere mixtures changed from red to blue when adjusting the SiO2 nanosphere concentration from 0 to 5.5 mg mL−1 at constant GO concentration (3 mg mL−1) (Fig. 4a, b, and Fig. S14, ESI†). This interesting phenomenon is ascribed to the depletion interaction between particles, which is an entropically driven ordering process related to the steric repulsion between particles.19,34–40 As indicated by a proposed model shown in Fig. 4c, in the GO–SiO2 hybrid system, SiO2 nanoparticles serve as depletants to induce depletion attraction between GO nanosheets. We expect most SiO2 nanoparticles to be outside the GO domains since the free volume of a suspension comprised of a binary mixture of nanoparticles can be increased effectively when the two components phase-separate.35 In order to increase free volume for depletant nanoparticles (e.g., SiO2), GO domains have to experience an ordered aggregation to minimize their excluded volume, leading to a decrease in the interlayer separation and blue-shift in the reflection. These results demonstrate that the self-assembled structure of GO can be tuned in non-aqueous, low polarity solvents without the need to adjust GO concentrations. We consider this can be a general phenomenon that is expected to be extended to other relevant hydrophobic hybrid systems of nanoparticles.
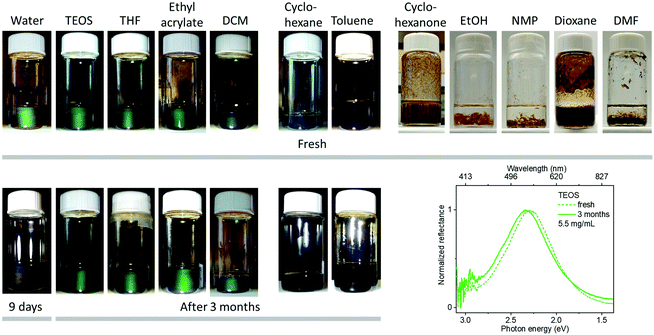
We have tried to disperse GO nanosheets in 17 different liquid media (Fig. 1, 5 and 6). GO nanosheets could be well dispersed in many of them except cyclohexanone, ethanol (EtOH), dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP) and dioxane. In saturated acyclic ketones, esters and ethers (e.g., ethyl acetate, butyl acetate, 3-pentanone, 2-octanone, diethyl ether), GO nanosheets showed not only good dispersion but also the ability to form colorful, photonic liquids. GO photonic liquids were also observed in a halogenated solvent (dichloromethane) and even tetrahydrofuran, a well-known hydrophilic solvent. However, in cyclohexane and toluene, which are hydrocarbon solvents lacking polar functional groups, GO nanosheets only form a stable suspension without any visible reflections, likely due to the strong van der Waals forces between GO themselves or between GO and solvent molecules. Birefringence was observed from these suspensions (Fig. S15, ESI†), suggesting the presence of the GO liquid crystals. We assume that GO nanosheets in these solvents may self-organize into a nematic structure instead of a lamellar structure.
Moreover, GO photonic suspensions were also obtained in tetraethoxysilane (TEOS) and ethyl acrylate, which are important liquid precursors for silica composites and elastomer materials, respectively. The compatibility of GO with reactive liquid precursors will provide new opportunities for the functionalization of GO and developing GO-based organic materials.
The liquid media for forming hydrophobic GO photonic liquids tend to be those that best swell poly(dimethylsiloxane) (PDMS).41 The solubility parameters of the most suitable liquid media are similar (Table S1, ESI†) to those of PDMS, in particular with respect to δHI (Hildebrand solubility parameter) and δd (Hansen dispersion cohesion parameter).41–46 This indicates that the dispersion of GO is mainly determined by the compatibility of the poly(APMS-co-DMS) copolymer with these solvents. We assume it may be possible to extend the range of solvents into which the GO nanosheets will disperse by using other suitable phase transfer additives.
Importantly, the hydrophobic photonic liquids show remarkable stability while maintaining the structural colors even after 3 months at room temperature. For comparison, GO suspensions at room temperature in water typically lose their photonic properties within 9 days. The stability of GO photonic suspensions in liquid media is probably related to the interaction between GO and solvent molecules. It has been proposed by Tour and coworkers that the interaction between GO nanosheets and water may lead to partial C–C bond cleavage and the protonation of water.47 It is conceivable that the released H3O+ ions slowly increase the ionic concentration, finally disrupting the GO lamellae. Such interactions may be related to the large dielectric polarizability of water (dielectric constant, ε ∼ 80, Table S1, ESI†) since the interaction accompanies the ionization of water. The lower dielectric constant of the hydrophobic liquid media (ε of most liquid media in this study is <11) may prevent this from happening, leading to enhanced stability for the hydrophobic GO photonic liquids. The charged GO in low polarity environment is probably stabilized by the protonation of amine groups from the APMS segment of the copolymer. The copolymer thereby adsorbs on the surface of GO and forms a steric barrier to prevent flocculation. These features (e.g., low dielectric constant of liquid media, steric stabilization achieved with the copolymers, decreased activity of water) should be helpful to provide a low ionic strength environment for extending the interlayer separation up to ∼470 nm with extended lifetimes as revealed by optical spectroscopy.
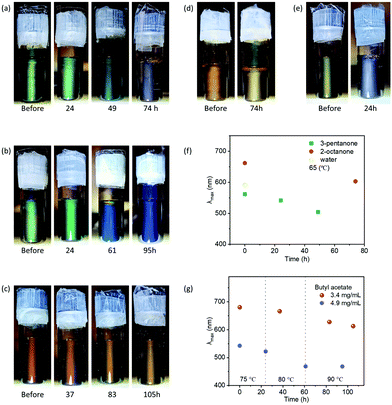
Since many chemical reactions and applications require elevated temperature, we tested the thermal stability of GO hydrophobic photonic liquid using GO suspensions in 3-pentanone (green-Fig. 6a), butyl acetate (green-Fig. 6b, and red-Fig. 6c), 2-octanone (orange-Fig. 6d) and water (yellow-Fig. 6e). In water, GO photonic suspensions lost their structural colors at 65 °C within 24 hours. In 3-pentanone, GO photonic suspensions exhibited significant improved thermal stability at 65 °C for up to 49 hours. GO photonic suspensions are even more thermally stable in 2-octanone and butyl acetate, where structural colors were well maintained at 65 °C for 74 h and higher temperatures of ∼75 to 90 °C for 100 hours, respectively. These results agree with the above discussion that liquid media with low dielectric constants contribute to the improved stability of GO photonic liquids. The interaction between GO and liquid media for degrading GO photonic liquids are probably slowed in solvents with intermediate dielectric constant (i.e., 3-pentanone, ∼17), and inhibited in solvents with low dielectric constant (i.e., butyl acetate, ∼5, 2-ocatnone, ∼10). In addition, we also observed blue-shifting of the reflection, which was inconspicuous at room temperature but accelerated at higher temperature (Fig. 6 and Fig. S16 (ESI†), reflection without normalization is shown in Fig. S16f, ESI†). One possibility is that the elevated temperature accelerates the entropy-driven ordering process, which results in reduced interlayer separation for minimizing the excluded volume and maximizing the translational entropy. These blue-shift phenomena did not affect the stability of the photonic structure of GO hydrophobic liquids. The reflection wavelength of GO hydrophobic liquids was tunable by adjusting the GO concentrations after heating (Fig. S17, ESI†).
The GO photonic liquids may be useful for liquid photonic pattern formation. To demonstrate this, GO nanosheets were dispersed in pure poly(APMS-co-DMS) copolymer liquid, which has a high boiling point of >200 °C for preventing liquid evaporation. The GO photonic liquids were slowly injected into a plastic mold with a “UBC” pattern, which displayed vivid colors when it was illuminated with white light (Fig. S18, ESI†). The good fluidity and photonic properties of hydrophobic GO photonic liquids may offer possibilities for making photonic patterns or displays with different colors in the future.
Conclusions
In conclusion, we have prepared GO photonic suspensions in various hydrophobic liquids including reactive liquid precursors such as tetraethoxysilane and ethyl acrylate. GO nanosheets in these liquid media spontaneously self-assemble into a tunable periodic structure with the ability to reflect light up to ∼1300 nm. These reflective liquids can be manipulated not only by adjusting GO concentrations but also by using foreign nanoparticles according to the entropic depletion interaction mechanism. Furthermore, second-order diffraction was observed in the GO infrared photonic liquids, enabling them to exhibit visible colors. GO hydrophobic photonic liquids are more stable than aqueous GO suspensions, being stable during heating, or under ambient conditions for more than 3 months of storage. The enhanced stability of GO hydrophobic photonic liquids is probably related to the low dielectric constants of the solvents. Future efforts may investigate the use of these hydrophobic photonic liquids to prepare photonic organogels, elastomers, and silicone polymers. The improved stability of these liquids may increase their applicability as photonic sensors and displays.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We thank NSERC for funding (CREATE NanoMat and Discovery Grant). We acknowledge the assistance of the UBC Bioimaging Facility with SEM and TEM. We thank RuiFeng Xu, JinQi Xie and JiaLi Sheng from Shenzhen Institutes of Advanced Technology of the Chinese Academy of Science for providing the graphite. We thank Lindsey Abdale and Professor Lee A. Groat from the Department of Earth, Ocean and Atmospheric Sciences at UBC for providing access to their near-infrared spectrometer.References
- Y. W. Zhu, S. Murali, W. W. Cai, X. S. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- P. Li, M. H. Wong, X. Zhang, H. Q. Yao, R. Ishige, A. Takahara, M. Miyamoto, R. Nishimura and H. J. Sue, ACS Photonics, 2014, 1, 79–86 CrossRef CAS.
- J. E. Kim, T. H. Han, S. H. Lee, J. Y. Kim, C. W. Ahn, J. M. Yun and S. O. Kim, Angew. Chem., Int. Ed., 2011, 50, 3043–3047 CrossRef CAS PubMed.
- Z. Xu and C. Gao, Nat. Commun., 2011, 2, 571 CrossRef PubMed.
- N. Behabtu, J. R. Lomeda, M. J. Green, A. L. Higginbotham, A. Sinitskii, D. V. Kosynkin, D. Tsentalovich, A. N. G. Parra-Vasquez, J. Schmidt, E. Kesselman, Y. Cohen, Y. Talmon, J. M. Tour and M. Pasquali, Nat. Nanotechnol., 2010, 5, 406–411 CrossRef CAS PubMed.
- Y. Wang, J. H. Guo, L. Y. Sun, H. X. Chen and Y. J. Zhao, Chem. Eng. J., 2021, 415, 128978 CrossRef CAS.
- H. Wang, Y. X. Liu, Z. Y. Chen, L. Y. Sun and Y. J. Zhao, Sci. Adv., 2020, 6, eaax8258 CrossRef PubMed.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. X. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS PubMed.
- T. T. Gao, G. C. Xu, Y. Y. Wen, H. H. Cheng, C. Li and L. T. Qu, Nanoscale Horiz., 2020, 5, 1226–1232 RSC.
- R. Luo, Q. Yu, Y. Lu, M. Zhang, T. Peng, H. Yan, X. Liu, J. K. Kim and Y. Luo, Nanoscale Horiz., 2019, 4, 531–539 RSC.
- M. Hegde, L. Yang, F. Vita, R. J. Fox, R. van de Watering, B. Norder, U. Lafont, O. Francescangeli, L. A. Madsen, S. J. Picken, E. T. Samulski and T. J. Dingemans, Nat. Commun., 2020, 11, 830 CrossRef CAS PubMed.
- J. Chang, M. Zhang, Q. Zhao, L. T. Qu and J. Y. Yuan, Nanoscale Horiz., 2021, 6, 341–347 RSC.
- Y. T. Xu, P. X. Wang and M. J. MacLachlan, J. Phys. Chem. C, 2019, 123, 17049–17055 CrossRef CAS.
- J. I. Paredes, S. Villar-Rodil, A. Martinez-Alonso and J. M. D. Tascon, Langmuir, 2008, 24, 10560–10564 CrossRef CAS PubMed.
- S. Park, J. H. An, I. W. Jung, R. D. Piner, S. J. An, X. S. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed.
- Z. Xu and C. Gao, ACS Nano, 2011, 5, 2908–2915 CrossRef CAS PubMed.
- S. H. Hong, T. Z. Shen and J. K. Song, Opt. Express, 2015, 23, 18969–18974 CrossRef CAS PubMed.
- T. Z. Shen, S. H. Hong, B. Lee and J. K. Song, NPG Asia Mater., 2016, 8, e296 CrossRef CAS.
- Y. T. Xu, U. V. Mody and M. J. MacLachlan, Nanoscale, 2021, 13, 7558–7565 RSC.
- T. K. Ekanayaka, S. H. Hong, T. Z. Shen and J. K. Song, Carbon, 2017, 123, 283–289 CrossRef CAS.
- G. Wang, B. Wang, J. Park, J. Yang, X. Shen and J. Yao, Carbon, 2009, 47, 68–72 CrossRef CAS.
- M. Karthika, H. Chi, T. Li, H. Wang and S. Thomas, Composites, Part B, 2019, 173, 106978 CrossRef.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Y. T. Xu, Y. L. Dai, T. D. Nguyen, W. Y. Hamad and M. J. MacLachlan, Nanoscale, 2018, 10, 3805–3812 RSC.
- B. A. Evans, B. L. Fiser, W. J. Prins, D. J. Rapp, A. R. Shields, D. R. Glass and R. Superfine, J. Magn. Magn. Mater., 2012, 324, 501–507 CrossRef CAS PubMed.
- C. Sotebier, A. Michel and J. Fresnais, Appl. Sci., 2012, 2, 485–495 CrossRef CAS.
- J. Yuan, A. Luna, W. Neri, C. Zakri, T. Schilling, A. Colin and P. Poulin, Nat. Commun., 2015, 6, 8700 CrossRef CAS PubMed.
- M. H. Wong, R. Ishige, T. Hoshino, S. Hawkins, P. Li, A. Takahara and H. J. Sue, Chem. Mater., 2014, 26, 1528–1537 CrossRef CAS.
- K. Sano, Y. S. Kim, Y. Ishida, Y. Ebina, T. Sasaki, T. Hikima and T. Aida, Nat. Commun., 2016, 7, 12559 CrossRef CAS PubMed.
- M. X. Zeng, D. King, D. Huang, C. Do, L. Wang, M. F. Chen, S. J. Lei, P. C. Lin, Y. Chen and Z. D. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18322–18327 CrossRef CAS PubMed.
- J. C. P. Gabriel, F. Camerel, B. J. Lemaire, H. Desvaux, P. Davidson and P. Batail, Nature, 2001, 413, 504–508 CrossRef CAS PubMed.
- P. Davidson, C. Penisson, D. Constantin and J. C. P. Gabriel, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6662–6667 CrossRef CAS PubMed.
- W. Q. Yang, S. Yamamoto, K. Sueyoshi, T. Inadomi, R. Kato and N. Miyamoto, Angew. Chem., Int. Ed., 2021, 60, 8466–8471 CrossRef PubMed.
- S. Asakura and F. Oosawa, J. Chem. Phys., 1954, 22, 1255–1256 CrossRef CAS.
- M. Adams, Z. Dogic, S. L. Keller and S. Fraden, Nature, 1998, 393, 349–352 CrossRef CAS.
- G. H. Koenderink, G. A. Vliegenthart, S. G. J. M. Kluijtmans, A. van Blaaderen, A. P. Philipse and H. N. W. Lekkerkerker, Langmuir, 1999, 15, 4693–4696 CrossRef CAS.
- K. Park, H. Koerner and R. A. Vaia, Nano Lett., 2010, 10, 1433–1439 CrossRef CAS PubMed.
- F. M. van der Kooij and H. N. W. Lekkerkerker, Phys. Rev. Lett., 2000, 84, 781–784 CrossRef CAS PubMed.
- R. Kato, A. Kakugo, K. Shikinaka, Y. Ohsedo, A. M. R. Kabir and N. Miyamoto, ACS Omega, 2018, 3, 14869–14874 CrossRef CAS PubMed.
- T. Nakato, A. Takahashi, S. Terada, S. Yamaguchi, E. Mouri, M. Shintate, S. Yamamoto, Y. Yamauchi and N. Miyamoto, Langmuir, 2019, 35, 14543–14552 CrossRef CAS PubMed.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544–6554 CrossRef CAS PubMed.
- J. L. M. Abboud and R. Notario, Pure Appl. Chem., 1999, 71, 645–718 CAS.
- L. Safa, O. Zaki, Y. Leprince and A. Feigenbaum, Packag. Technol. Sci., 2008, 21, 149–157 CrossRef CAS.
- X. Su, B. L. Shi and L. L. Wang, J. Macromol. Sci., Part B: Phys., 2015, 54, 1248–1258 CrossRef CAS.
- C. M. Hansen, Hansen solubility parameters: a user's handbook, CRC Press, Boca Raton, 2007 Search PubMed.
- J. E. Mark, Physical properties of polymers handbook, Springer, NewYork, 2007 Search PubMed.
- A. M. Dimiev, L. B. Alemany and J. M. Tour, ACS Nano, 2013, 7, 576–588 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nh00523e |
| This journal is © The Royal Society of Chemistry 2022 |