Impact of nanomaterials on the intestinal mucosal barrier and its application in treating intestinal diseases
Wenshuai
Hao
ab,
Ruitao
Cha
 *a,
Mingzheng
Wang
ab,
Pai
Zhang
ab and
Xingyu
Jiang
*a,
Mingzheng
Wang
ab,
Pai
Zhang
ab and
Xingyu
Jiang
 *c
*c
aCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for NanoScience and Technology, Beijing 100190, P. R. China. E-mail: chart@nanoctr.cn
bBeijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences (Beijing), Beijing 100083, P. R. China
cDepartment of Biomedical Engineering, Southern University of Science and Technology, Shenzhen, Guangdong 518055, P. R. China. E-mail: jiang@sustech.edu.cn
First published on 23rd November 2021
Abstract
The intestinal mucosal barrier (IMB) is one of the important barriers to prevent harmful substances and pathogens from entering the body environment and to maintain intestinal homeostasis. The dysfunction of the IMB is associated with intestinal diseases and disorders. Nanomaterials have been widely used in medicine and as drug carriers due to their large specific surface area, strong adsorbability, and good biocompatibility. In this review, we comprehensively discuss the impact of typical nanomaterials on the IMB and summarize the treatment of intestinal diseases by using nanomaterials. The effects of nanomaterials on the IMB are mainly influenced by factors such as the dosage, size, morphology, and surface functional groups of nanomaterials. There is huge potential and a broad prospect for the application of nanomaterials in regulating the IMB for achieving an optimal therapeutic effect for antibiotics, oral vaccines, drug carriers, and so on.
1 Introduction
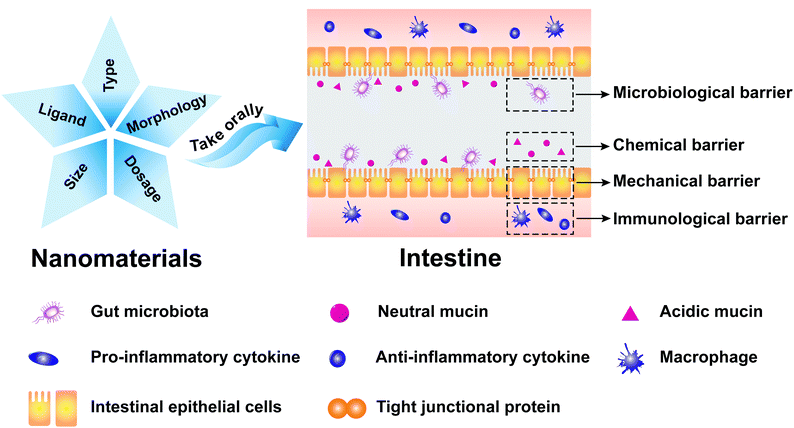
1.1 Definition and function of the intestinal mucosal barrier
The intestinal mucosa is the outermost layer in the intestinal lumen, which is the largest mucosal surface in the human mucosa. The intestinal mucosa includes epithelium, lamina propria, and lamina muscularis mucosae. The intestinal mucosa, commensal bacteria, and other cells related to immune responses collectively form an important component of the intestinal mucosal barrier (IMB).1 The IMB could separate the intestinal lumen from the internal environment of organisms to prevent the invasion of pathogenic antigens and maintain the normal physiological function of the gastrointestinal tract, which can be divided into microbiological barrier, chemical barrier, mechanical barrier, and immunological barrier.2,3The microbiological barrier is dominated by gut microbiota which is resistant to the colonization of foreign strains. The gut microbiota, which constitutes an essential protective barrier to prevent pathogens, can participate in many biological processes, including energy metabolism, immune regulation, and neurotransmitter. The damage to the stability of gut microbiota may lead to the invasion and colonization of intestinal pathogens and trigger inflammation, which is implicated in various human diseases, especially obesity,4,5 inflammatory bowel disease (IBD),6 and cancer.7
The chemical barrier is composed of mucus secreted by the intestinal mucosal epithelial cell, digestive juices (pancreatic juice and bile), and antibacterial substances produced by beneficial intestinal bacteria. It can inactivate intestinal pathogens and provide lubrication to protect the intestinal mucosa from physicochemical damage.
The mechanical barrier is composed of the intestinal epithelial cells and the intercellular tight junction, which could maintain intestinal permeability. The lineages of intestinal epithelial cells mainly include absorptive, goblet, enteroendocrine, and Paneth cells.8,9 Intestinal absorptive cells, which cover the surface of intestinal mucosal, could absorb water, nutrients, and drugs. Goblet cells could secrete gel-forming mucins that are the main component of the intestinal chemical barrier. Enteroendocrine cells could regulate the digestive physiological functions, maintain mucosal homeostasis, and boost innate immunity. Paneth cells could produce large amounts of α-defensins that exhibit strong antibacterial activities to Gram-negative and Gram-positive bacteria. The intercellular tight junction is the main connection form between the intestinal epithelial cells, which could maintain the polarity of epithelial cells, prevent bacteria and endotoxin entering intestines, and control the entry of small molecules and ions into intestines.10,11 Intestinal permeability refers to the physiological function of intestines that selectively allows the passage of microorganisms and their metabolites, nutrients, and drugs.12 The mechanical barrier can prevent macromolecular substances in the intestinal lumen from penetrating intestinal walls. It also can prevent substances in the lamina propria of intestinal walls from penetrating the intestinal lumen.
The immunological barrier is composed of gut-associated lymphoid tissue and intestinal secretory immunoglobulin A (sIgA). Lymphoid tissues, which account for a quarter of intestinal mucosa, can prevent pathogenic antigens from harming the body through cellular immunity and humoral immunity.
1.2 Interactions of four intestinal mucosal barriers
The intestinal mucosa homeostasis is maintained by the dynamic interactions between the gut microbiota, intestinal mucus layer, intestinal epithelial cells, and host immune defense. The change of gut microbiota has the most significant impact on other barriers, affecting mucus secretion, intercellular tight junctions, intestinal epithelial permeability, and intestinal immunity.13Microorganisms and their metabolites play a pivotal role in adjusting mucin production to maintain the integrity of intestinal epithelial tight junctions by changing the synthesis and secretion of mucin, the biochemical components of mucin, and the degradation of mucin. Probiotics such as Lactobacillus and Lactobacillus rhamnosus could resist pathogen infections and modulate the chemical barrier by increasing the expression level of secreted mucins and membrane-bound mucins.14,15
Probiotics such as Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus could up-regulate the expression of the intestinal epithelial tight junction protein and increase the production of short chain fatty acids (SCFAs) through adjusting the gut microbiota, which could maintain the integrity of the intestinal epithelial cell morphology and functions and further improve the intestinal mechanical barrier. However, large amounts of bacterial endotoxins are produced because of intestinal dysbacteriosis, which could cause the increase of intestinal mucosal permeability and the impairment of the intestinal mechanical barrier. Biofilms, which consist of carcinogenic bacteria (e.g., Fusobacterium nucleatum, Escherichia coli, and enterotoxigenic Bacteroides fragilis) in the inner colonic mucus layer, could cause the redistribution of E-cadherin, the increase of intestinal permeability, and the loss of the intestinal mechanical barrier function.16,17
Gut microbiota can maintain the host immune tolerance through inducing proliferation and differentiation of regulatory T cells (Treg).18,19 Treg cells could prevent other immune cells from attacking the own tissues of organisms and other harmless environmental materials (e.g., food and commensal organisms).20 In addition, gut microbiota can prevent the growth of pathogens and improve the capability of host defenses against pathogens through producing antibacterial substances and exerting colonization resistance.21 Low dosage of TiO2 NPs could enhance the immunity of silkworm by reducing the conditional pathogenic bacteria and increasing the abundance of norank_f_Bacteroidales_S24-7_group which was essential for anti-tumor immune functions because of its functions in anti-inflammatory and inhibition of harmful bacteria.22
1.3 Reasons and purposes of review
The IMB could effectively prevent harmful substances and pathogens from entering the body environment and maintain homeostasis. The clinical manifestations of the IMB impairment include the increase of intestinal mucosal permeability and the loss of intestinal digestion and absorption. The dysfunction of IMB could affect the physiological functions of other organs, which may induce multiple organ lesions and multiple organ dysfunction syndromes.Nanomaterials by definition must have at least one dimension that is in nanometer scopes. They possess small particle size, large specific surface area, strong adsorbability, good biocompatibility, and high bioavailability, which have been widely used in medicine and drug carriers.23–25 Nanoparticles are available for the treatment of intestinal diseases because they are easily modified with different ligands. Nano-antibacterial agents (e.g., noble metal nanoparticles and metal oxide nanoparticles) have the strong bacteriostatic ability, which can be used in the regulation of microbiome and as disinfectants in the food industry.26–29 Drug nanocarriers such as functionalized carbon nanomaterials have advantages of good drug targeting efficiency, adjustable drug release rate, and low drug leakage rate, which enable them to be widely used in cancer treatment, vaccine adjuvants, intracellular targeted drug delivery, and oral administration.30,31
Murine, fish, and pigs are the main animals for research of the intestinal mucosal barrier.32,33 Appropriate animal models should be chosen for IMB research. Murine is widely used to simulate the impact of nanomaterials on the IMB of humans, because it is similar to humans in intestine-specific genes, physiological and anatomical functions, and diversity of gut microbiota. Pigs are similar to humans in dietary habits, nutrient absorption, physiological characteristics, and organ size, which make them excellent animal models to study the therapeutic effect of nanomedicine on intestinal diseases (e.g., obesity and inflammatory bowel disease) and the toxicological effect of nanomaterials on the IMB. Fish, crustaceans, silkworms, and enchytraeus crypticus could be used to study the impact of nanomaterials on the IMB of sea creatures and soil organisms.
It is important to understand how nanomaterials affect the IMB, which is conducive to developing and designing novel antibiotic substitutes, drug carriers, medicine, and regulators of microbiome. In recent years, there have been some reviews about the impact of nanomaterials on the gut microbiota and intestinal mucous,34,35 but there have been few reviews regarding the effects of nanomaterials on intestinal epithelial cells and intestinal immunity. Moreover, all of the above-mentioned reviews only focused on metal nanoparticles and carbon nanomaterials.36,37 Thus, we comprehensively summarized the impact of nanomaterials on the microbiological barrier, chemical barrier, mechanical barrier, and immunological barrier in this review (Fig. 1). We discussed the current status and future prospects of nanomaterials in antibiotic substitutes, drug nanocarriers, and regulators of microbiome.
2 Effects of nanomaterials on the intestinal microbiological barrier
The intestinal microbiological barrier with colonization, reproduction, and exclusivity plays an important role in maintaining the host healthy, effectively regulating the intestinal structure and the intestinal immune system, and further affecting other tissues and organs such as nerves, muscles, fat, and the brain. The intestinal beneficial bacteria, which mainly includes mucosal bacteria and intestinal lumen bacteria, can promote intestinal peristalsis, inhibit the growth and colonization of pathogens, and regulate the functions of intestinal epithelial cells and dendritic cells. However, the gut microbiota with carcinogenicity such as Fusobacterium nucleatum, Escherichia coli, and Bacteroides fragilis could induce colorectal cancer.16The imbalance of the intestinal microbiological barrier could lead to the proportion disorder of gut microbiota and the proliferation of pathogens, which in turn induce digestive system-related diseases (e.g., acute diarrhea, acute gastroenteritis, and constipation), intestinal immunity-related diseases (e.g., Crohn's disease and ulcerative colitis),38 and metabolism-related diseases (e.g., obesity and diabetes).
Nanomaterials can affect the intestinal microbiological barrier by changing the diversity of gut microbiota, the ratio of Firmicutes to Bacteroides (F/B), and the abundance of certain specific bacteria. It is helpful for the development of new antibiotic substitutes and regulators of microbiome to study the influence of nanomaterials on the intestinal microbiological barrier. The effects of nanomaterials on the intestinal microbiological barrier are listed in Tables 1 and 2.
| Nanomaterials (characteristics) | Animal models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a mg L−1 represents the concentration of nanomaterials to which animal models were exposed; ↑ represents increase; ↓ represents decrease; F represents female; M represents male. | ||||
| Ag NPs (20 nm; sphere) | C57BL/6NCrl mice (F; 6 weeks old) | 0.76 mg day−1 per mice | Bacteroidete↑; Firmicutes↓ | 46 |
| 45 days | ||||
| Ag NPs (10, 75, and 100 nm; sphere) | SD rats (M and F; 3 weeks old) | 9, 18 and 36 mg kg−1 day−1 | Firmicutes and Lactobacillus↑ | 50 |
| 91 days | ||||
| Ag NPs (55.17 nm; sphere) | C57BL/6 mice (F; 3 months old) | 0.046, 0.46, and 4.6 mg kg−1 day−1 | Disturb α-diversity and β-diversity with dose dependent | 52 |
| 28 days | ||||
| CAgNCs (chitosan-coated) | Wild type zebrafish | Diets containing 2% CAgNCs | Fusobacteria and Bacteroidetes↑ | 53 |
| 60 days | ||||
| Pt NPs (5, 30, and 70 nm; sphere) | C57BL/6 mice (M; 7–8 weeks old) | 0.025 mg kg−1 day−1 | Alter α-diversity and F/B ratio | 54 |
| 8 days | ||||
| Au NCs (200 nm; sphere; glutathione-capped) | BALB/c mice (F; 6 weeks old) | 1.7 mg kg−1 day−1 | Staphylococcus, Ureaplasma, and Methylobacterium↑; Roseburia↓ | 55 |
| 7 days | ||||
| TA-Au NPs (5, 10, 15, 30, and 60 nm; sphere; tannic acid-stabilized) | C57BL/6 mice (M; 7–8 weeks old) | 0.015, 0.018, 0.020, 0.022, and 0.025 mg kg−1 day−1 | α-Diversity and F/B value↓ | 56 |
| 8 days | ||||
| DAPT-Au NPs (5 nm; sphere; 4,6-diamino-2-pyrimidinethiol-coated) | Balb/c mice (M; 6 weeks old) | 17 mg kg−1 day−1 | Treat bacterial infection induced by Escherichia coli in the intestine without harming the gut microbiota | 57 |
| 28 days | ||||
| Cr2O3 NPs (100 nm; sphere) | Broiler chicken | 0.05, 0.10 mg kg−1 day−1 | Bifidobacteria, Staphylococci, and Salmonella↓ in 0.05 mg kg−1 day−1; Enterobacteria↑ in 0.10 mg kg−1 day−1 | 58 |
| 42 days | ||||
| ZnO NPs (72 nm; sphere) | Weaned castrated piglets | 150, 300, 450, and 3000 mg kg−1 day−1 | Escherichia coli↓ in 450 and 3000 mg kg−1 day−1 | 59 |
| 1 day | ||||
| ZnO NPs (23 nm; sphere) | Weaned castrated piglets | 300, 400, 500, and 600 mg kg−1 day−1 | Escherichia coli↓ | 60 |
| 28 days | ||||
| ZnO NPs | Hens (6 weeks old) | 25, 50, and 100 mg kg−1 day−1 | Regulate metabolism of gut microbiota | 61 |
| 63 days | ||||
| Dietary supplemented coated ZnO NPs | Weaned castrated piglets | 100 mg kg−1 day−1 | Alter composition and metabolism of gut microbiota; Richness↑ | 62 |
| 28 days | ||||
| MoO3 NPs (92 nm; sphere) | Zebrafish (1 month old) | 0.2 and 0.4 mg L−1a | Gram-positive microflora↑ | 63 |
| 14 days | ||||
| TiO2 NPs (29 nm; sphere) | SD rats (M; 3 week old) | 2, 10, and 50 mg kg−1 day−1 | L. gasseri, Turicibacter, and L. NK4A136_group↑; Veillonella↓ | 64 |
| 30 days | ||||
| TiO2 NPs (367 nm; sphere) | C57BL/6JAusb mice (M; 5–6 weeks old) | 2, 10, and 50 mg kg−1 day−1 | Alter release of bacterial metabolites | 65 |
| 28 days | ||||
| TiO2 NPs (30 nm; sphere) | Broiler chicken | 1.4 × 10−6, 1.4 × 10−4 mg per egg | Bifidobacterium and Lactobacillus↓ | 66 |
| 21 days | ||||
| TiO2 NPs (33 nm; sphere) | C57BL/6 mice (M; 6 weeks old) | 200 mg kg−1 day−1 | Firmicutes↑; Bacteroidetes, Bifidobacterium, and Lactobacillus ↓ | 67 |
| 56 days | ||||
| TiO2 NPs (29 nm; sphere) | SD rats (M; 3 weeks old) | 2, 10, and 50 mg kg−1 day−1 | Lactobacillus_reuteri↑; Romboutsia↓ | 68 |
| 90 days | ||||
| TiO2 NPs (25, 50, and 80 nm; sphere) | C57BL/6 mice (M; 8 weeks old) | 1 mg kg−1 day−1 | Bifidobacterium↓ | 69 |
| 7 days | ||||
| TiO2 NPs (21 nm; sphere) | C57BL/6J mice (M; 7 weeks old) | 150 mg kg−1 day−1 | Richness and evenness↓ | 70 |
| 30 days | ||||
| TiO2 NPs (10, 50, 100 nm; sphere) | C57BL/6J mice (F; 3 weeks old) | Diets containing 0.1% TiO2 NPs | Bifidobacterium and Lactobacillus↓ | 71 |
| 93 days | ||||
| Nanomaterials (characteristics) | Animal models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a mg L−1 represents the concentration of nanomaterials to which animal models were exposed; ↑ represents increase; ↓ represents decrease; F represents female; M represents male. | ||||
| SWCNTs (diameter: 1.04–1.17 nm; length: 1–5 μm) | ICR mice (M; 7 weeks old) | 0.05, 0.5, and 2.5 mg kg−1 day−1 | Alitipes_uncultured_bacterium and Lachnospiraceae bacterium A4↑ | 90 |
| 7 days | ||||
| MWCNTs (diameter: 20–30 nm; length: 0.5–2.0 μm) | C57BL/6 mice (M; 8–10 weeks old) | 0.025 mg kg−1 day−1 | Helicobacteraceae and Coriobacteriaceae↑ | 92 |
| 15 days | ||||
| C60 | Wistar rats (M; 3–3.5 months old) | 5 mg kg−1 day−1 | Lactobacillus and Turicibacte↑ | 100 |
| 84 days | ||||
| Erivatives of C60 (Fol1: 100 nm; sphere; Fol113: 90 nm; sphere) | C57BL/6 mice (M; 8 weeks old) | 20 mg kg−1 day−1 | Lactobacillus, Bifidenols, Allobaculum, Blautia, Parabacteroides, Akkermansia, and Anaerotruncus↑ | 101 |
| 28 days | ||||
| PS NPs (100 nm; sphere) | Yellow croaker | 5.50 × 10−12, 5.50 × 10−9, and 5.50 × 10−7 mg L−1a | Bacteroidetes, Firmicutes Parabacteroides, and Alistipes↑; Proteobacteria↓ | 102 |
| 14 days | ||||
| PS NPs (100 nm; sphere) | Zebrafish (M and F; 16 weeks old) | 0.5 mg L−1a | Pathogenic bacteria↑ | 103 |
| 21 days | ||||
| PS NPs (50–100 nm) | E. crypticus | Diets containing 0.1% PS NPs | Microbacteriaceae↑; Streptococcaceae, Enterobacteriaceae, Rhodocyclaceae, and Sphinomonadaceae↓ | 104 |
| 14 days | ||||
| PS NPs (50–100 nm) | E. crypticus | Diets containing 0.025, 0.5, and 10% PS NPs | Rhizobiaceae, Xanthobacteraceae, and Isosphaeraceae↓ | 105 |
| 7 days | ||||
| NCC (diameter: 3.24; length: 125 nm; rod) | Wistar rats (M; 4 weeks old) | 250 mg kg−1 day−1 | Bifidobacterium↑; SCFAs↑ | 106 |
| 14 days | ||||
| Se NPs | Broiler chicks (M; 1 day old) | 0.3, 0.9 and 1.5 mg kg−1 day−1 | Lactobacillus and Faecalibacterium↑; butyric acid↑ | 107 |
| 29 days | ||||
| Chitosan NPs (50 nm; sphere) | Piglets (M and F; 3 week old) | 100, 200 and 400 mg kg−1 day−1 | Presumably beneficial gut bacteria↑; conditional pathogenic bacteria↓ | 108 |
| 28 days | ||||
| Amyloid-polyphenol hybrid nanofilaments (long semiflexible rod) | C57BL/6J mice (M; 7 weeks old) | 150 mg kg−1 day−1 | Aestuariispira and Escherichia↓ | 109 |
| 14 days | ||||
| HABN (86–416 nm) | C57BL/6 mice (F; 6 weeks old) | 30 mg kg−1 day−1 | Akkermansia muciniphila and Clostridium XIVα↑ | 110 |
| 6 days | ||||
| mEVs (105 nm; spherical shape with a lipid bilayer) | C57BL/6 mice (F; 3 weeks old) | 0.3, 0.6, and 1.2 mg kg−1 day−1 | Alter composition of gut microbiota; modulate SCFAs | 111 |
| 56 days | ||||
2.1 Noble metal nanoparticles
Noble metals refer to gold, silver, and the platinum group metal.39 Noble metal nanoparticles have been widely applied in food, biomedical engineering, agriculture, and animal husbandry due to their broad-spectrum antibacterial activity.27,40,41 Noble metal nanoparticles can affect the intestinal ecosystem and the intestinal microbiological barrier by changing the diversity of gut microbiota, the F/B value, and the abundance of certain specific bacteria. We mainly discuss gold, silver, and platinum nanoparticles in this review.Silver nanoparticles (Ag NPs) are the most studied noble metal nanoparticles in the intestinal microbiological barrier. They are widely used in antibacterial products, medicine, agricultural materials, industrial products, and household products.42,43 However, the risk of Ag NPs to the ecosystem increases with their total amount entering the environment.
After exposure to naked Ag NPs, the F/B value decreased and the community density of gut microbiota dropped dramatically in mice.44,45 The relative abundance of Rikenellaceae was increased while that of Erysipelotrichaceae and Lactobacillaceae was decreased in the feces of naked Ag NP-ingested mice.46,47 The influence of Ag NPs on gut microbiota is sex dependent. Ag NPs could increase the relative abundance of Fusobacteria and Cetobacterium, and decrease the relative abundance of Proteobacteria in male zebrafish, while it did not affect those in female zebrafish.48
The effects of Ag NPs on the intestinal microbiological barrier are affected by their shape, particle size, given dosage, and surface modifier. The relative abundance of Clostridium, Bacteroides uniformis, Christensenellaceae, and Coprococcus eutactus decreased in the silver nanocube (Ag NC) exposed group, whereas that of Oscillospira, Dehalobacterium, Peptococcaceae, Corynebacterium, and Aggregatibacter pneumotropica decreased in the silver nanosphere (Ag NS)-exposed group.49 The proportions of Gram-negative bacteria in gut microbiota increased after Ag NPs were given orally to Sprague-Dawley rats, and the increasing trend is size dependent and dose dependent. Furthermore, exposure to low-dose and small-size Ag NPs decreased the populations of Firmicutes and Lactobacillus in Sprague-Dawley rats.50 The gut microbiota mediated the toxicity of zooplankton and the toxicity level was related to the exposure time at low concentrations of Ag NPs, while at high concentrations of Ag NPs, the toxicity of zooplankton was largely determined by the combined effects of dead gut microbiota and cumulative Ag NPs toxicity (Fig. 2a).51 Surface modifiers could affect the performance of Ag NPs, which will influence the effects of Ag NPs on gut microbiota. After the oral administration of polyvinylpyrrolidone (PVP)-coated Ag NPs, the F/B value increased in mice mainly due to the changes of Lachnospiraceae and S24-7.52 Compared with the control group, chitosan silver nanocomposites (CAgNCs) as regulators of microbiome could increase beneficial bacteria in the intestine of zebrafish, especially the abundance of Fusobacteria and Bacteroidetes, which enhanced the microbiological barrier.53 Liquid glutathione-stabilised Ag NPs (GSH-Ag NPs) increased the abundance of Lactobacillus in the human colon.72
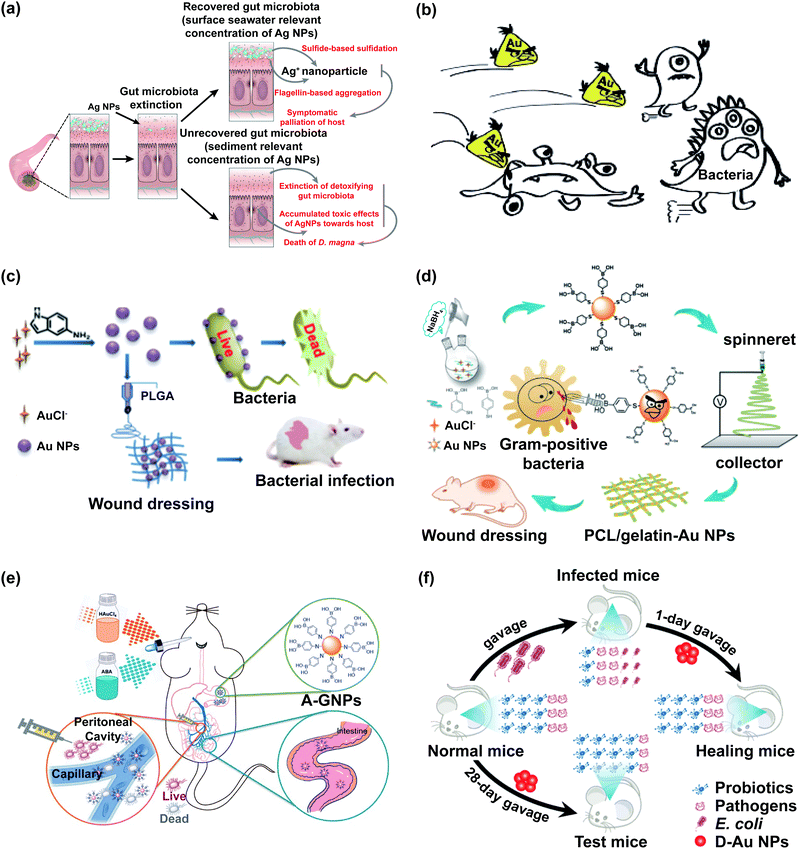 | ||
| Fig. 2 The impact of noble metal nanoparticles on bacteria is related to their concentration and ligands. (a) Schematic representation of the main biological pathways in zooplankton and the gut microbiota affected by Ag NPs; adapted with permission from ref. 51. Copyright 2019, The Royal Society of Chemistry. (b) Au NCs protected with rationally selected ligands could be potent nano-antibiotics against multidrug-resistant Gram-positive bacteria in vivo; adapted with permission from ref. 74. Copyright 2018, Wiley-VCH. (c) The synthesis of indole derivative-capped gold nanoparticles via a straightforward route and their antibacterial mechanism; adapted with permission from ref. 26. Copyright 2018, American Chemical Society. (d) Schematic illustration of the preparation process of ES nanofibrous membranes loaded with mercaptophenylboronic acid-activated Au nanoparticles as antibacterial wound dressings; adapted with permission from ref. 75. Copyright 2020, American Chemical Society. (e) Schematic illustration of the preparation process of ABA-Au NPs (A-GNPs) as an orally administered antibacterial agent; adapted with permission from ref. 76. Copyright 2021, American Chemical Society. (f) Treatment of oral gold nanoparticles on the bacterial infection without effecting probiotics; adapted with permission from ref. 57. Copyright 2019, American Chemical Society. | ||
Platinum nanoparticles (Pt NPs), gold nanoclusters (Au NCs), gold nanoparticles (Au NPs), and bimetallic nanoparticles have a negative impact on the intestinal microbiological barrier by changing the diversity of gut microbiota and the abundance of certain specific bacteria. Pt NPs caused the intestinal dysbacteriosis of mice by adversely altering the α-diversity and F/B value.54 Au NCs could induce the imbalance of gut microbiota. The abundance of Proteobacteria was significantly increased after feeding the mice with Au NCs for 7 days. At the genus level, Au NCs obviously decreased the abundance of Roseburia and notably increased the abundance of Staphylococcus, Ureaplasma, and Methylobacterium.55 The impact of Au NPs on bacteria is related to their ligand (Fig. 2b–f).26,28,57,73–76 Tannic acid (TA)-coated Au NPs (TA-Au NPs) decreased the α-diversity, F/B value, and certain SCFA-producing bacteria, which could exacerbate malign effects on the gut microbiota of mice.56 The aminophenyl boronic acid-activated Au nanoparticles (ABA-Au NPs) were synthesized in vivo, which could be absorbed by the gastrointestinal tract and used to treat peritonitis caused by multidrug-resistant bacteria (Fig. 2e).76 The oral administration of 4,6-diamino-2-pyrimidinethiol coated Au nanoparticles (DAPT-Au NPs) had a better effect in curing the intestinal infection in mice than levofloxacin without harming the gut microbiota, and DAPT-Au NPs had great potential as alternatives to oral antibiotics (Fig. 2f).57
2.2 Non-noble metal oxide nanoparticles
Cr, Zn, and Cu are essential trace elements in the organism, which play an important role in the growth, immunity, and nutrient metabolism of organisms. These non-noble metal oxide nanoparticles have potential applications as new substitutes to overcome antibiotic resistance and antibiotic residues, which can be used in feeds of poultry and livestock to treat diarrhea and promote growth. Cr2O3 NPs and ZnO NPs can be used as dietary sources of Cr, Zn, and Cu in poultry and livestock, which can positively regulate gut microbiota and the contents of SCFAs.Cr2O3 NPs at a dosage of 50 to 100 μg kg−1 in feed could enhance the intestinal microbiological barrier of chicken. Compared with the control group, the number of Staphylococci and Salmonella decreased by 61.3% and 64.0% after feeding chicken with Cr2O3 NPs (50 μg kg−1) for 42 days, while the number of Salmonella decreased by 31.3% in chicken intestines after feeding with Cr2O3 NPs (100 μg kg−1).58
ZnO NPs with high antimicrobial activity can be used as the regulators of intestinal microbiome and antibacterial agents. ZnO NPs could increase the abundance of Lactobacillus in ileum and caecum of weaned piglets and significantly decrease the abundance of Escherichia coli in the caecum of weaned piglets.59,60 ZnO NPs could significantly influence microbiota levels in the ileum of hens, especially the abundance of Lactobacillus.61 Dietary supplemented coated ZnO NPs could change the composition and metabolism of gut microbiota and increase the richness indices (ACE and Chao1) in the intestine of piglets, and 10% dietary supplemented coated ZnO NPs had an even more significant influence on gut microbiota compared with 5% dietary supplemented coated ZnO NPs.62 Besides, ZnO NPs could combine with other drugs to regulate the gut microbiota. Half-fin anchovy hydrolysate (HAHp)/ZnO NP nanocomposites (HAHp(3.0)/ZnO NPs) could have a potential application as the intestinal microbial conditioner, which could increase the abundance of Firmicutes and regulate the gut microbiota in female mice, particularly the increase in abundance of Lactobacillus and Bifidobacterium.77 ZnO NPs combined with L. plantarum BLPL03 fermentation liquor (LFL/ZnO NPs) have beneficial effects for regulating the gut microbiota of weaned piglets, which could significantly enhance the abundance of intestinal beneficial bacteria (e.g., Bifidobacterium) and reduce the abundance of potential enteropathogenic bacteria (e.g., Enterobacteriaceae and Clostridium perfringens).78
Nanoparticles containing molybdenum or titanium could penetrate the environment and then transfer into organisms with their amount increasing applications in industry,79 food, and biomedicine,80,81 which leads to the decrease of gut microbiota diversity and the damage to their intestinal microbiological barrier.82 MoO3 NPs increased the proportion of Gram-positive bacteria in the intestine of zebrafish where Gram-negative bacteria was the dominant bacteria, demonstrating that the composition of gut microbiota was disturbed and the protective mechanisms against colonization by foreign microbiota were suppressed.63 Additionally, nano-MoS2, as the most common two-dimensional molybdenum materials, also changed the metabolism of the intestines and gut microbiota in mice. Nano-MoS2 could cause the alteration of metabolic profiles in the small intestine and large intestine of mice by the change of microbial communities.83
The effects of TiO2 NPs on the intestinal microbiological barrier are influenced by factors as the dosage and crystalline phases of nanoparticles and the physiological state of animal models.84 The high dosage of TiO2 NPs could significantly change the composition of gut microbiota and the release of intestinal metabolites, which might have an adverse effect on the intestinal microbiological barrier (Fig. 3a and b).64,70 TiO2 NPs decreased the relative abundance of Bifidobacterium and Lactobacillus in the intestines of broiler chicken.66,85 Treatment with TiO2 NPs obviously reduced the richness and evenness of Bifidobacterium, Lactobacillus, and Romboutsia,68–70 which might lead to metabolic disruption, weight gain,86 and low-grade inflammation in the intestinal mucosa of mice.67,71 However, a low dosage of TiO2 NPs has a positive effect on the intestinal microbiological barrier. The gut microbiota of lepidopteran insects can accelerate growth and development, and improve insect resistance and disease resistance (Fig. 3c). After feeding silkworms with mulberry leaves soaked in low concentrations of TiO2 NPs, the evenness of gut microbiota improved and the abundance of Staphylococcus, Lachnospiraceae_NK4A136_group, Pseudomonas, and Sphingomonas increased.22
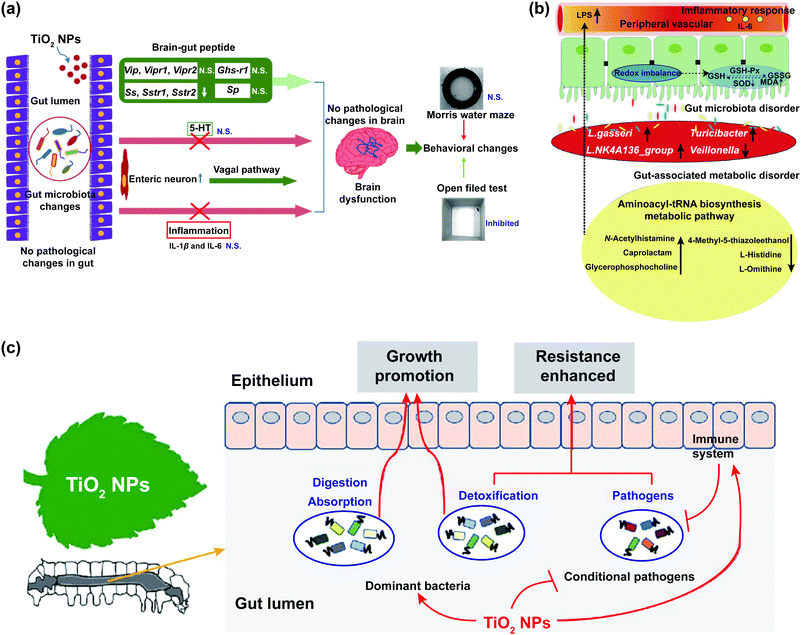 | ||
| Fig. 3 The impact of TiO2 NPs on the intestinal microbiological barrier. (a) Potential mechanisms for neurotoxicity of TiO2 NPs; “N. S.” represents “no significant difference”; adapted with permission from ref. 70. Copyright 2020, Springer Nature. (b) Interaction of gut microbiota, gut-associated metabolism, and toxicity induced by oral exposure to TiO2 NPs; adapted with permission from ref. 64. Copyright 2019, The Royal Society of Chemistry. (c) Summary of TiO2 NPs on the biological functions of silkworms (Bombyx mori); adapted with permission from ref. 22. Copyright 2020, Elsevier. | ||
The crystalline phases of TiO2 NPs mainly include anatase and rutile. Compared with anatase TiO2 NPs, the abundance of Proteobacteria, Firmicutes, and Actinobacteria was significantly increased after the gavage of rutile TiO2 NPs for 28 days.87 TiO2 NPs decreased the abundance of Dehalobacteriaceae and Ellin6075 and increased the abundance of Clostridiales, which might increase the potential risk of gestational diabetes of pregnant rats.88
2.3 Carbon nanomaterials
Carbon nanomaterials, mainly including graphene, C60, and carbon nanotubes (CNTs), are widely applied in industry, agriculture, biomedicine, and daily life due to their favorable chemical versatility, high corrosion resistance, strong adsorption, good biocompatibility, and high productivity.36,89 Carbon nanomaterials might enter the gastrointestinal tract of humans and animals through the skin system, respiratory system, digestive system, and medical injection, causing the change of the intestinal mucosal barrier.90CNTs, including single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), could penetrate the cell membrane, change the gene expression profiles of cells, and affect the gut microbiota. However, they could selectively lyse the walls and membranes of gut microbiota, which is related to the size, shape, and functional groups of CNTs, and the morphology of bacteria. SWCNTs had a more pronounced effect of wall/membrane piercing on spherical bacteria than MWCNTs. As a new antibacterial material, CNTs have selective sterilization effects without causing damage to probiotic bacteria (Fig. 4a).91 The oral administration of SWCNTs could induce great changes of the gut microbiota in mice, which were mainly reflected in the significant shifts of the predominant microbe phyla from Firmicutes to Bacteroidetes and the increased abundance of pro-inflammatory bacteria Alitipes_uncultured_bacterium and Lachnospiraceae bacterium A4.90 MWCNTs aggravated doxorubicin (DOX)-induced intestinal dysbacteriosis, which was manifested in the decreased abundance of Helicobacteraceae and Coriobacteriaceae.92 Tomato exposed to CNTs had minimal to no effect on the human gut microbiota.93
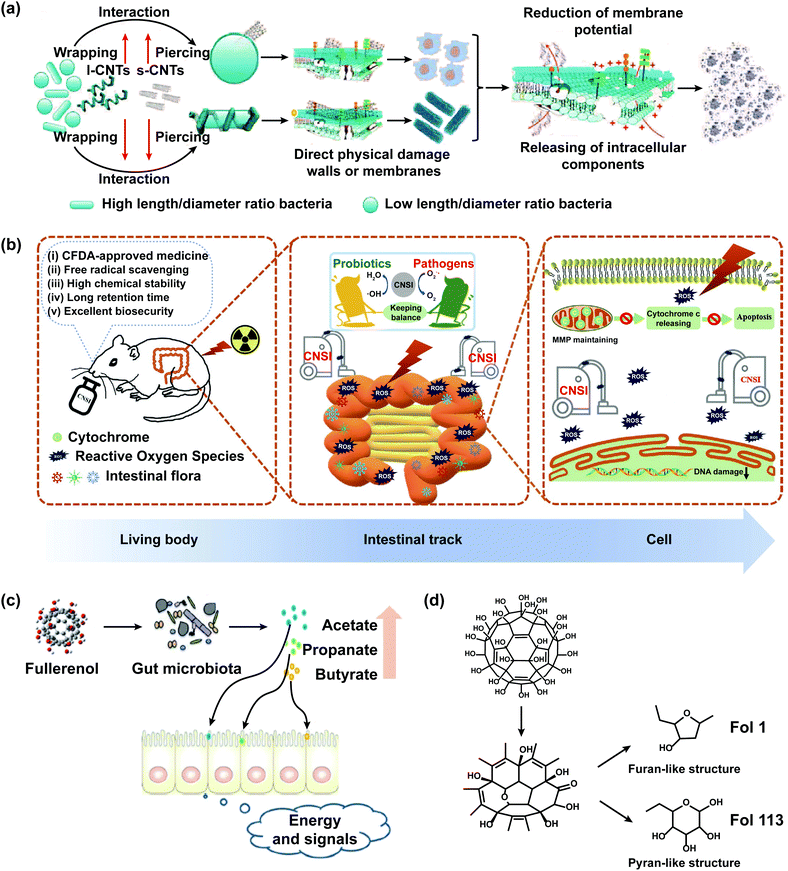 | ||
| Fig. 4 The impact of carbon nanomaterials on the intestinal microbiological barrier. (a) Scheme of the possible mechanisms of antibacterial activity of CNTs against gut microbiota. The present study shows that the antibacterial activity of CNTs is associated with their diameter-dependent piercing and length-dependent wrapping on the lysis of microbial walls and membranes, the induced release of intracellular components DNA and RNA and the loss of bacterial membrane potential, demonstrating the complete destruction of bacteria. CNTs with small diameters showed more effect wall/membrane piercing on spherical bacteria than long CNTs. Long CNTs are prone to wrap around and interact with the bacterial cell surface. The mechanism of antibacterial activity includes the diameter-dependent piercing and length-dependent wrapping of CNTs on the lysis of the microbial wall and membrane; adapted with permission from ref. 91. Copyright 2013, Wiley-VCH. (b) CFDA approved carbon nanoparticles (CNSI) exhibit good performance in intestinal radioprotection, including effective free radical scavenging, high chemical stability, long retention time, and excellent biosafety in the intestines, which strongly protects the crypt cells and maintains the balance of gut microbiota so as to alleviate the symptoms of radiation enteritis; adapted with permission from ref. 99. Copyright 2020, Wiley-VCH. (c) Fullerenol NPs ameliorate hyperlipidemia by modulating the gut microbiota structure and increasing SCFA production; adapted with permission from ref. 101. Copyright 2018, Wiley-VCH. (d) Fullerenol NPs contain furan- and pyran-like structures that could be used by gut microbiota; adapted with permission from ref. 101. Copyright 2018, Wiley-VCH. | ||
Graphene-family materials, including monolayer graphene (GR), graphene oxide (GO), and reduced graphene oxide (rGO), negatively impact the intestinal microbiological barrier by changing the diversity and richness of gut microbiota, thereby causing various immunoreactions of the body.94,95 After exposure to graphene oxide nanosheets, the relative abundance of Fusobacteria, Cetobacterium, and Lactobacillus increased, and the relative abundance of Firmicutes and Pseudomonas decreased.96 Chronic GO exposure increased the pathogenic bacterial community in zebrafish and disturbed the diversity and richness of gut microbiota, which could cause intestinal damage and activate the inflammatory reaction.97 GO could increase the relative abundance of SCFA-producing bacteria in the intestines of high-fat diet (HFD)-induced hyperlipidemic mice, such as Clostridium clusters IV and Allobaculum.98
Carbon nanoparticle suspension injection (CNSI), a conjugated and carbonylated graphene analog containing 12 benzene rings, could act as a new nano-radioprotective agent to cure acute radiation enteritis which was the bowel complication caused by the radiotherapy of pelvic malignant tumors and retroperitoneal malignant tumors (Fig. 4b).99 CNSI has a delocalized π-conjugated structure, numerous carboxyl groups, and a nanoscale size, which exhibited strong free radical scavenging activity, good solubility in water, excellent chemical resistance, and long detention time in the intestines. The probiotics of mice treated with CNSI and X-ray were higher and the pathogenic bacteria was lower than that treated with X-ray, showing that the oral administration of CNSI could maintain the balance of gut microbiota in mice.
C60 and its derivatives could improve the structure of gut microbiota and increase the abundance of SCFA-producing bacteria. Oral C60 significantly increased the abundance of Lactobacillus and Turicibacter, which could influence the lipid homeostasis of rats.100 The orally administered derivatives of C60 (Fol1 C60(OH)7(O)8 and Fol113 C60(OH)11(O)6) could remarkably increase the abundance of Lactobacillus, Bifidenols, Allobaculum, Blautia, Parabacteroides, Akkermansia, and Anaerotruncus, particularly Fol1 C60(OH)7(O)8 (Fig. 4c and d).101
2.4 Nanoplastics
Nanoplastics are obtained by degrading plastics that are widely used in industries, households, and fast-moving consumer goods such as paints, adhesives, electronic products, and cosmetics.112 As the amount of nanoplastics released to the environment increased, it could cause negative ecological effects and disturb the IMB of organisms.113,114 Many reports have demonstrated that exposure to nanoplastics could cross the intestinal mucosa of organisms and result in the damage of IMB. Continuous efforts need to be devoted toward researching the potential toxicity and long-term effects of nanoplastics on the IMB of organisms.Nanoplastics could affect the marine environment and endanger the intestinal microbiological barrier of sea creatures.115 Polystyrene nanoparticles (PS NPs) in oceans had a negative impact on the gut microbiota of whiteleg shrimps.115 Nanoplastics could decrease the viability of gut microbiota and induce intestinal dysbacteriosis, which could damage the digestive organs of the body.116,117 PS NPs could change the proportion of dominant bacteria in juvenile fish (Bacteroidetes, Proteobacteria, and Firmicutes) and increase the abundance of conditional pathogenic bacteria (Parabacteroides and Alistipes), which resulted in a significant increase of total mortality (Fig. 5).102,103
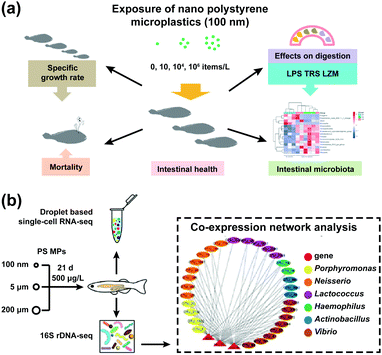 | ||
| Fig. 5 The interaction between PS NPs and the gut microbiota of sea creatures. (a) The juvenile large yellow croaker; adapted with permission from ref. 102. Copyright 2020, Elsevier. (b) Zebrafish; adapted with permission from ref. 103. Copyright 2020, American Chemical Society. | ||
Furthermore, nanoplastics could affect the soil environment and endanger the intestinal microbiological barrier of soil organisms. PS NPs increased the ratio of Planococcaceae to Chitinophagaceae and the ratio of Bacillaceae to Chitinophagaceae, and significantly disturbed the abundance of gut microbiota (such as Streptococcaceae, Enterobacteriaceae, Rhodocyclaceae, Sphingomonadaceae, Rhizobiaceae, Xanthobacteraceae, and Isosphaeraceae) in enchytraeus crypticus (E. crypticus).104,105 Carboxylated PS NPs might migrate from soil to leaves, and then to snails that feed on these leaves, decreasing the viability of gut microbiota in snails and reducing their growth rate.116
2.5 Nanocellulose
Nanocellulose is a general term for pure cellulose with one or more dimensions in the nanometer range, which can be divided into nanocrystalline cellulose (NCC),118,119 bacterial cellulose (BC),120,121 and nanofibrillated cellulose (NFC)122 according to the source and morphology (Fig. 6a).123–126 Nanocellulose has potential applications in food additives, antibacterial agents, regulators of the microbiome, wound dressing, pharmaceutical excipients, and tissue engineering scaffolds due to its large aspect ratio, excellent physicochemical properties, and favourable biocompatibility.122,127–130 | ||
| Fig. 6 The morphology of nanocellulose and their application in antibacterial materials and bacterial growth. (a) From top to bottom: SEM of NCC; adapted with permission from ref. 119. Copyright 2012, Elsevier; SEM of BC; adapted with permission from ref. 121. Copyright 2018, American Chemical Society; TEM of NFC; adapted with permission from ref. 122. Copyright 2019, The Royal Society of Chemistry. (b) Scheme of the experimental process and wound healing of the rat model; adapted with permission from ref. 131. Copyright 2017, The Royal Society of Chemistry.(c) Study design and effects of ingested NFC-50 on live bacterial growth: some description of methods; adapted with permission from ref. 132. Copyright 2020, Elsevier. | ||
2,3-Dialdehyde nanofibrillated cellulose, which was prepared by sodium periodate oxidation, could significantly inhibit the growth of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) (Fig. 6b).131 NFC has a positive impact on the intestinal microbiological barrier, which could alter the diversity of gut microbiota and improve the prebiotic effect (Fig. 6c).132 NFC with exercise increased the abundance of Ruminococcaceae and Eubacteriaceae, improving exercise performance and exerting antiobesity effects.133 The yields of SCFAs and the abundance of Bifidobacterium were significantly increased in the intestines of rats by nanocrystalline cellulose (125 nm) gavage in comparison with those by microcrystalline cellulose.106
2.6 Others
Selenium (Se) is one of the essential trace elements for humans and animals. Se NPs have fine granularity and high bioavailability, and are easily absorbed by the intestines, which could control the abundance of Enterococcus cecorum in poultry intestines without significantly disturbing the total microbial community.134 Se NPs (0.9 mg kg−1 day−1) increased the abundance of probiotics (e.g., Lactobacillus and Faecalibacterium) and the yield of SCFAs, particularly butyric acid, which could remarkably regulate the gut microbiota of chicks and improve their gut health.107 Se NPs synthesized by Lactobacillus casei ATCC 393 (L. casei 393-SeNPs) could maintain the balance of gut microbiota in mice and protect against the intestinal barrier dysfunction caused by Enterotoxigenic Escherichia coli K88.135Chitosan is a new feed additive with antibacterial, antitumor, and growth-promoting activities, which could positively regulate the intestinal microbiological barrier of organisms by modulating the balance of gut microbiota. Chitosan NPs could regulate the colonic microbiota composition of weaned pigs, which increased the abundance of probiotics (e.g., Prevotellaceae and Ruminococcaceae) and inhibited the growth of potential pathogens (e.g., Clostridiaceae).108 (-)-Epigallocatechin 3-O-(3-O-methyl) gallate loaded chitosan-caseinophosphopeptide nanoparticles (EGCG3′′Me-loaded CS-CPP) could promote the growth of probiotics in the intestines of mice (e.g., Bifidobacterium and Lactobacillus) and suppress the proliferation of pathogens (e.g., Bacteroides prevotella and Clostridium histolyticum), which could prevent obesity-related metabolic disorders.136
There are problems in the intestines of patients with IBD (such as ulcerative colitis and Crohn's disease), including disturbance of the balance between symbiotic microorganisms and potential pathogens, reduction of gut microbiota diversity, and disorder of the intestinal microbiological barrier, so that the development of anti-inflammatory drugs which can regulate the gut microbiota is an inevitable trend.137 Amyloid-polyphenol hybrid nanofilaments could improve the IMB of mice with colitis, which could reduce the abundance of gut microbiota related to colitis (e.g., Aestuariispira and Escherichia) and increase the contents of SCFAs.109 Hyaluronic acid bilirubin nanomedicine (HABN) could increase the overall richness and diversity of gut microbiota, especially the abundance of Akkermansia muciniphila and Clostridium XIVα which played a crucial role in maintaining the gut homeostasis.110 Milk-derived extracellular vesicles (mEVs) could act as a novel drug carrier, which could change the composition of gut microbiota and regulate the yield of SCFAs.111
3 Effects of nanomaterials on the intestinal chemical barrier
The intestinal chemical barrier covers the epithelial cells, which lubricates the intestinal epithelium and protects the intestinal epithelium from penetration by nanoparticles.138 The intestinal mucus, chiefly composed of Mucin-2 (MUC2) produced by goblet cells, could protect the body against the toxicity of reactive oxygen species (ROS), keep gastrointestinal epithelial tissues moist, and defend the body from excessive mechanical stress caused by the corrosion of stomach acid and the hydrolysis of digestive protease.139 The digestive juice secreted by the intestines could dilute enterotoxin and clean the intestinal cavity, making it difficult for pathogenic bacteria to adhere to the intestinal epithelium. The antibacterial substances secreted by intestinal beneficial bacteria could inhibit the growth of pathogenic bacteria.The disruption of the intestinal chemical barrier causes mucosal inflammation in the intestines. The effects of nanomaterials on intestinal mucus are affected by the size and surface charge of nanomaterials, and the viscoelasticity of mucus. The diffusion of nanomaterials in the intestinal chemical barrier was hindered by the size of mesh spacing between mucin fibers and the adhesion of mucin granules on mucin fibers. Compared with long nanotubular peptosomes (800–1200 nm), short nanotubular peptosomes (20–30 nm) showed excellent permeability in the mucus, which was due to the fact that nanotubular peptosomes with a smaller size than the mesh spacing could effectively penetrate the mucus layer, prolonging the residence time of nanotubular peptosomes in the intestines.140 Polyphosphate nanoparticles with hydrophilia and negative charges had high mucosal permeability compared to dephosphorylated polyphosphate nanoparticles, because nanoparticles with negative charge could quickly penetrate the mucus layer without chemical actions, while nanoparticles with positive charges could be trapped in the mucus layer by electrostatic interactions.141 Mucous viscoelasticity could affect the penetration of nanoparticles in mucus, which could be changed by surfactants. Sodium dodecyl sulfate increased the viscoelasticity of mucus, while poloxamer showed the opposite tendency. Tween 80 maintained the viscoelasticity of mucus.142
It is important to understand how nanomaterials affect the intestinal chemical barrier, especially on the mucous layer, which is conducive to the development of drug carriers that break through the intestinal mucus and bioadhesive nanomaterials that can be localized to the intestines. The effects of nanomaterials on the intestinal chemical barrier are listed in Table 3.
| Nanomaterials (characteristics) | Animal models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a mg L−1 represents the concentration of nanomaterials to which animal models were exposed; ↑ represents increase; ↓ represents decrease; F represents female; M represents male. | ||||
| Ag NPs (60 nm; sphere) | SD rats (M and F; 4 weeks old) | 30, 300, and 1000 mg kg−1 day−1 | Neutral and acidic mucins↓ | 143 |
| 28 days | ||||
| Ag NPs (<100 nm; sphere) | Brine shrimps | 880.28 mg L−1a | Mucous thickness↑ | 144 |
| 1 day | ||||
| TiO2 NPs (367 nm; sphere) | C57BL/6JAusb mice (M; 5–6 weeks old) | 2, 10, and 50 mg kg−1 day−1 | Gene expression of the colonic MUC2↓ | 65 |
| 28 days | ||||
| PS NPs (100 nm; sphere) | Zebrafish (M and F; 16 weeks old) | 0.5 mg L−1a | Mucus secretion↑ | 103 |
| 21 days | ||||
3.1 Noble metal nanoparticles
Ag NPs could stimulate goblet cells to release mucus granules, and the release amount depended on the dosage of Ag NPs. After treating rats with Ag NPs, the contents of neutral and acidic mucins were decreased in ileal, colonic, and rectal mucosa. In particular, the proportion of sulfated mucins was decreased while that of sialyated mucins was increased in the colon.143 Sulfated mucins and sialyated mucins could prevent bacterial enzymes from breaking down the mucus layer, which significantly affects the stability of the mucus layer. The mucus secretion increased and the mucus layer thickened in the intestines of brine shrimps which were incubated with Ag NPs (880.28 mg L−1) for 24 h.144 The effects of Ag NPs on the intestinal chemical barrier were affected by their particle size. The smaller the Ag NPs, the more easily they could get inside the intestinal mucus and into the intestinal epithelial cells.145 In addition, the effect of Ag NPs on the intestinal chemical barrier is sex dependent. After treating with Ag NPs, the expression of Mucin-3 (MUC3) was more significantly down-regulated in female rats than that in male rats.503.2 Non-noble metal oxide nanoparticles
TiO2 NPs could accumulate in mucus-secreting cells, which could promote mucus secretion, decrease the expression of MUC2, and cause damage to the intestinal chemical barrier.65,85,146,147 The expression levels of MUC2 first increased and then decreased in the intestines of juvenile mice that were administrated with a high dosage of TiO2 NPs by gavage, which could be because TiO2 NPs treatment will damage the intestinal chemical barrier of mice and increase MUC2 transcription to strengthen the host defense response.86The co-culture model of Caco-2/HT29-MTX could be used to study the interaction between TiO2 NPs, gut microbiota, and intestinal mucus, which could stimulate the secretion of mucus by intestinal epithelial cells.146 Caco-2 cells (human clonal colon adenocarcinomas) could form monolayers of differentiated intestinal epithelial cells joined by intercellular tight junctions, which are structurally and functionally similar to the small intestines. HT29-MTX cells (anti-methotrexate human colon cancer cell line) have the function of mucus secretion, which could mimic goblet cells.148
3.3 Carbon nanomaterials
Mesoporous carbon nanoparticles functionalized by polymers could be used as carriers for oral drugs to improve the absorption and utilization of drugs in the intestines. Mesoporous carbon nanoparticles grafted with chitosan and N-(2-hydroxypropyl) methacrylamide (HCMCN) could improve the intestinal ingestion and mucus permeation ability of nanocarriers, because chitosan could transiently open the tight junctions between epithelial cells and HCMCN could be used as a “mucus-insert” material.149 Probucol-loaded HCMCN had a 176% increase in oral bioavailability than Probucol commercial tablets that were insoluble and impermeable (Fig. 7a).150 | ||
| Fig. 7 The impact of nanomaterials on the intestinal chemical barrier and their application as drug carriers. (a) Schematic illustration of synthetic route of HCMCN and the process of intestinal uptake of HCMCN. The pHPMA polymer could be dissociable from the surface of HCMCN in the mucus, thus promoting their mucus permeation, and causing exposure of chitosan after degradation for the following effective transepithelial transport; adapted with permission from ref. 150. Copyright 2020, Elsevier. (b) The interactions between the PET NPs and the intestinal barrier; adapted with permission from ref. 152. Copyright 2018, American Chemical Society. (c) Schematic design of multifunctional MSN, MSN-CPP-PSH, for oral delivery of insoluble drugs to overcome the intestinal barriers; adapted with permission from ref. 157. Copyright 2019, Elsevier. (d) Interactions between the PC6/CS NPs and the intestinal barrier; adapted with permission from ref. 158. Copyright 2020, American Chemical Society. | ||
3.4 Nanoplastics
The effects of PS NPs on intestinal mucus are affected by their particle size (50, 100, 200, 500, 750 nm, 1, 5, and 200 μm) and surface functional groups (amino, carboxylic, and sulfate groups). The optimum particle size and desirable surface charge for the mucus penetration of nanoparticles were found to be 50 nm and neutral.151 100 nm PS NPs induced higher mucus secretion than 5 μm and 200 μm PS MPs, because 100 nm PS NPs altered the small molecular transport pathway in secretory cells and up-regulated the associated genes of mucus secretion.103 Polyethylene terephthalate nanoparticles (PET NPs) possessed a propensity to cross the intestinal chemical barrier, which could cause adverse health effects on organisms (Fig. 7b).152 The intestinal chemical barrier could effectively inhibit the absorption of nanoplastics. When the intestines were exposed to a large dosage of carboxylate-modified PS NPs, most NPs were directly discarded from the intestines or trapped in the mucus.1533.5 Others
Solid lipid nanoparticles (SLNs) fabricated by tristearin and polyethylene glycol (PEG) acylated emulsifiers, whose mucous permeability depended on the size and surface charge of SLNs, could be used for loading curcumin.154 Negatively charged nanoparticles penetrated the mucus layer and were internalized by the intestinal epithelial cells, whereas positively charged nanoparticles were trapped in the mucus layer.155 The permeability of trimethyl chitosan nanoparticles in the mucus layer increased with the decreasing quaternization degree of nanoparticles.156Thiolation polymers could improve the intestinal mucus permeability of drug carriers because their thiol groups could form the disulfide bond with cysteine in mucous glycoprotein. Mesoporous silica nanoparticles functionalized with cell-penetrating peptides and thiolation polymers (MSN-CPP-PSH) possessed high intestinal mucosal permeability and long intestinal residence time, which could load lopinavir to make its relative bioavailability 6.76 times higher than free lopinavir (Fig. 7c).157 Poly(acrylic acid)-cysteine-6-mercaptonicotinic acid coated on chitosan nanoparticles (PC6/CS NPs), which contained reactive thiol groups, could be used as a potential drug carrier for oral protein due to their high-efficiency mucus penetration (Fig. 7d).158
4 Effects of nanomaterials on the intestinal mechanical barrier
The intestinal mechanical barrier not only resists the invasion of pathogens and harmful substances from the external environment into intestines, but also maintains the selective permeability of intestinal epithelium. The integrity and regeneration of intestinal epithelial cells play an essential role in intestinal secretion and absorption.The dysfunction of the intestinal mechanical barrier could lead to a variety of intestinal diseases, such as IBD and chronic diarrhea. Nanomaterials can affect the intestinal mechanical barrier by adjusting the morphology of intestinal epithelial cells, changing the structure of intestinal microvilli, and regulating the expression of tight junctional protein that includes occludin, claudin, zonula occludens 1 (ZO-1), and zonula occludens 2 (ZO-2).
The penetration of nanomaterials in the intestinal mechanical barrier could be influenced by the particle size and surface charge of nanomaterials. SiO2 NPs with negative charges caused tight junction relaxation, inducing an increase in intestinal permeability.159 Small size SiO2 NPs (30 nm) caused a significant decrease in the value of transepithelial electrical resistance (TEER), whereas large size SiO2 NPs (200 nm) would not affect the value of TEER.160 Moreover, the hardness of nanomaterials could influence their penetration into the intestinal mechanical barrier. Semi-elastic nanoparticles as drug carriers had strong uptake and absorption of intestinal epithelial cells. Semi-elastic core shell poly(lactic-co-glycolic acid)-lipid nanoparticles could act as the carrier of peptide drugs to improve the oral bioavailability of peptide drugs.161
It is helpful for the design and development of drug carriers to study the influence of nanomaterials on the intestinal mechanical barrier, which could improve the transepithelial diffusion of drugs. The effects of nanomaterials on the intestinal mechanical barrier are listed in Tables 4 and 5.
| Nanomaterials (characteristics) | Animal models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a mg L−1 represents the concentration of nanomaterials to which animal models were exposed; ↑ represents increase; ↓ represents decrease; F represents female; M represents male. | ||||
| Ag NPs (60 nm; sphere) | SD rats (M and F; 4 weeks old) | 30, 300, and 1000 mg kg−1 day−1 | Shed cell at the tip of the villi | 143 |
| 28 days | ||||
| Ag NPs (10 and 110 nm; sphere) | SD rats (M and F; 7 weeks old) | 9, 18, and 36 mg kg−1 day−1 | Alter intestinal permeability and sex dependent | 162 |
| 91 days | ||||
| Ag NPs (25 nm; sphere) | Zebrafish (M and F) | 0.008, 0.045, and 0.070 mg L−1a | Necrose the intestinal villi | 163 |
| 30 days | ||||
| Ag NPs (<100 nm; sphere) | Brine shrimps | 880.28 mg L−1a | Necrose the intestinal epithelial cells | 144 |
| 1 day | ||||
| CAgNCs (chitosan-coated) | Wild type zebrafish | Diets containing 2% CAgNCs | Villi height of mid intestine↑ | 53 |
| 60 days | ||||
| Pt NPs (5, 30, and 70 nm; sphere) | C57BL/6 mice (M; 7–8 weeks old) | 0.025 mg kg−1 day−1 | Heat-shock protein 25 and tight junction proteins↑ | 54 |
| 8 days | ||||
| DAPT-Au NPs (5 nm; sphere; 4,6-diamino-2-pyrimidinethiol-coated) | Balb/c mice (M; 6 weeks old) | 17 mg kg−1 day−1 | Without damage to the morphology of cells and the structure of mitochondria | 57 |
| 28 days | ||||
| Dietary supplemented coated ZnO NPs | Weaned castrated piglets | 100 mg kg−1 day−1 | ZO-1 and occludin↑ | 62 |
| 28 days | ||||
| ZnO NPs (30 nm; sphere) | Weaned piglets | 400, and 800 mg kg−1 day−1 | Villous height, width, crypt depth, and surface area↑ | 164 |
| 14 days | ||||
| ZnO NPs (72 nm; sphere) | Weaned piglets | 150, 300, and 450 mg kg−1 day−1 | Ratio of villus height to crypt depth in duodenum and jejunum↑ | 165 |
| 21 days | ||||
| TiO2 NPs (12 nm; sphere) | CD1 mice | 12.5 mg kg−1 | Alter paracellular permeability of ileum and colon epithelia | 172 |
| 6 hours | ||||
| TiO2 NPs (20 nm; rutile: 16 nm) | C57BL/6 mice (M; 8 weeks old) | 100 mg kg−1 day−1 | Intestinal villi↑; damage arrangement of villus epithelial cells | 87 |
| 28 days | ||||
| TiO2 NPs (241 nm) | Zebrafish (4 months old) | 2 and 20 mg L−1a | Alter the intestinal epithelial barrier permeability, sex dependent and concentration dependent | 84 |
| 93 days | ||||
| NiO NPs (30–50 nm; sphere) | Earthworm | 5, 50, 200, 500, and 1000 mg kg−1 day−1 | Damage the epithelial layer, microvilli, and mitochondria | 173 |
| 28 days | ||||
| Graphene | ICR mice (M; 4 weeks old) | 0.010, 0.100 mg kg−1 day−1 | Damage the membrane integrality of intestinal epithelial cells | 94 |
| 28 days | ||||
| OH-GQDs (diameter: 3 nm; thickness: 1.5–1.6 nm) | C57BL/6J mice (M; 6–8 weeks old) | 0.05, 0.5, and 5 mg kg−1 day−1 | Enhance the intestinal permeability; improve the shortened villi and crypt loss | 174 |
| 7 days | ||||
| CNSI (10-50 nm; sphere; modified by polyvinylpyrrolidone) | BALB/c mice (M; 6–8 weeks old) | 0.005 and 0.2 mg day−1 per mice | Improve the mitochondrial dysfunction | 99 |
| 8 days | ||||
| Se NPs | C57BL/6 mice (8 weeks old) | 100 mg kg−1 day−1 | ROS, ATP, and MMP↓ | 175 |
| 28 days | ||||
| HABN (86 nm) | C57BL/6 mice (F; 6 weeks old) | 30 mg kg−1 day−1 | Restore epithelium barriers in a murine model of acute colitis | 110 |
| 6 days | ||||
| Nanomaterials (characteristics) | Cell models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a μg mL−1 and μg cm−2 represent the concentration of nanomaterials to which cell models were exposed; ↑ represents increase; ↓ represents decrease. | ||||
| Mn3O4 NPs | Caco2 cell and A549 cell | 100 μg mL−1a | Permeability of Caco-2 cells↑ | 166 |
| 9 days | ||||
| CuO NPs (15–20 nm) | Caco-2/Raji B co-culture and Caco-2/HT29-MTX co-culture | 7.93 and 15.85 μg cm−2a | ZO-1↓ | 167 |
| 1 day | ||||
| CuO NPs (48 nm; sphere; polyvinyl pyrrolidone-coated) | IEC-6 cells | 0.1, 1, 5, 10, 25, and 100 μg mL−1a | ROS concentration↑; damage the mitochondrial membrane | 168 |
| 1 day | ||||
| TiO2 NPs (21 nm) | Caco-2 cells | 42 and 84 μg mL−1a | Damage the tight junction and permeability | 169 |
| 4 hours, 1 day, and 2 days | ||||
| TiO2 NPs (30 nm; sphere) | Caco-2/HT29-MTX co-culture | 0.14 μg mL−1a | Damage intestinal microvilli | 170 |
| 4 hours | ||||
| TiO2 NPs (30 nm) | Caco-2/HT29-MTX co-culture | 1.4 × 10−5, 1.4 × 10−3, and 1.4 × 10−1 μg mL−1a | Absorptive microvilli↓ | 171 |
| 4 hours and 5 days | ||||
| AF-SWCNTs | MDCK cells | 20 μg mL−1a | Damage the tight junction | 176 |
| 1 day | ||||
| PS NPs (100 nm; sphere) | Caco-2 cell | 20 μg mL−1a | ZO-1 and occludin↓ | 177 |
| 4 days | ||||
| PS NPs (50 nm; sphere) | Caco-2 cells | 100 μg mL−1a | ROS-related genes (HO1, SOD2, and GSTP1)↑ | 178 |
| 1 day | ||||
4.1 Noble metal nanoparticles
Naked Ag NPs have an adverse effect on the intestinal mechanical barrier, which are dosage dependent. Naked Ag NPs were found in the lamina propria of intestines, the tip of ileal villus, and the protruding surface of colonic plica in rats, which changed the expression of genes related to cell junctions and increased the permeability of intestines.143,162 Naked Ag NPs induced the disruption of the intestinal mechanical barrier in zebrafish and brine shrimps, which was manifested in the misshapen hyperplasia and blebbing of intestinal epithelial cells, the exfoliation of intestinal musculature, and the large necrosis of intestinal microvillus.144,163 CAgNCs, as the feed supplement, could significantly increase the height of intestinal villus, indicating that the intestinal mechanical barrier of zebrafish was enhanced.53Pt NPs improved the intestinal mechanical barrier through up-regulating the expression of heat shock protein 25 and tight junction proteins (Fig. 8a).54 DAPT-Au NPs were harmless to the intestinal epithelial cells of mice, which embodied in the morphology plumpness of cells and the normal structure of mitochondria (Fig. 8b).57
 | ||
| Fig. 8 The impact of nanomaterials on the intestinal mechanical barrier. (a) Immunolocalization of tight junction proteins by immunofluorescence microscopy after orally treating with Pt NPs in DSS-administered mice (200×); adapted with permission from ref. 54. Copyright 2019, the authors, published by Dove Medical Press. (b) Morphology of the small intestines and MODE-K cells after treating with naked Au NPs (N-Au) and DAPT-Au NPs (D-Au); adapted with permission from ref. 57. Copyright 2019, American Chemical Society. (c) Schematic representation of the proposed mechanism by which biogenic Se NPs synthesized by Lactobacillus casei ATCC 393 protect the intestinal epithelial barrier function against oxidative damage; adapted with permission from ref. 175. Copyright 2019, the authors, published by Dove Medical Press. (d) Schematic illustration of a co-culture system combining intestinal epithelial Caco-2 cells and macrophage RAW264.7 cells as an in vitro model of inflamed intestinal epithelium; adapted with permission from ref. 182. Copyright 2020, Elsevier. | ||
4.2 Non-noble metal oxide nanoparticles
ZnO NPs have beneficial effects on the intestinal mechanical barrier. ZnO NPs could reduce intestinal permeability in the intestines of weaned piglets by increasing the expression levels of occludin and ZO-1 mRNA.62 Moreover, it could protect the intestinal mechanical barrier of weaned piglets by increasing the villus width, villus length, and crypt depth in the intestines.164,165 However, certain animals would present focal epithelial cell exfoliation and villous epithelial cell exfoliation in the small intestines. A short course of ZnO NPs (350 mg kg−1 day−1) caused the damage of gastrointestinal mucosa.Mn3O4 NPs, CuO NPs, and TiO2 NPs cause the disruption of the intestinal mechanical barrier, which are dosage dependent and time dependent. Mn3O4 NPs increased the permeability of Caco-2 cells and decreased the metabolic activity in cells.166 CuO NPs damaged transepithelial electrical resistance (TEER) and decreased the expression level of ZO-1 in Caco-2/Raji B co-culture and Caco-2/HT29-MTX co-culture.167 After treating IEC-6 cells with CuO NPs, the concentration of H2O2 increased while the concentration of glutathione decreased, which led to the increase of ROS concentration and the damage of mitochondrial membrane.168
TiO2 NPs negatively affect the physiological function of the intestinal epithelium layer, which induces chronic damage to the intestines.169 TiO2 NPs significantly decreased the absorption and transportation of nutrients (such as glucose, zinc, iron, and fatty acids), which was due to the fact that the presence of TiO2 NPs caused the microvilli of intestinal absorptive cells and altered the expression of nutrient transporter protein.170,171 TiO2 NPs also increased the paracellular permeability of intestinal epithelial cells and the generation of intracellular ROS, and decreased the activity of intestinal alkaline phosphatase.171,172 Additionally, the effects of TiO2 NPs on the intestinal microbiological barrier are influenced by factors as the gender of animal models and the crystalline phases of nanoparticles. TiO2 NPs had a more significant effect on the intestinal epithelial permeability of male zebrafish than that of female zebrafish, because the expression level of tight junction protein 2 was higher in male zebrafish than that in female zebrafish after exposure to TiO2 NPs.84 Compared with anatase TiO2 NPs, rutile TiO2 NPs led to longer intestinal villi and irregular arrangement of villus epithelial cells in mice.87
NiO NPs have been widely adopted for their high surface energy, large surface area, and excellent catalytic activity, but the waste disposal of NiO NPs makes the environment polluted. The effects of NiO NPs on the mechanical barrier are concentration dependent. The low concentration of NiO NPs (5, 50, and 200 mg kg−1) had no obvious effect on the survival rates of earthworms. However, the high concentration of NiO NPs (500 and 1000 mg kg−1) caused abnormalities of the epithelial layer, microvilli, and mitochondria in the intestines of earthworms.173
4.3 Carbon nanomaterials
GFMs could change the morphology of intestines and the activity of antioxidant enzymes by inducing more intestinal vacuolations and producing more goblet cells.96 Graphene could induce intestinal oxidative stress and damage the membrane integrity of intestinal epithelial cells.94 CNTs could penetrate the membrane of intestinal epithelial cells and change their gene expression.93 Acid functionalized single-walled carbon nanotubes (AF-SWCNTs) up-regulated the expression of tight junction proteins, and decreased TEER, which resulted in the increase of paracellular permeability and the damage of tight junctions.176 The high dosage of hydroxylated graphene quantum dots (OH-GQDs) caused obvious intestine damage, which was embodied in the increase of intestinal permeability, the shortening of intestinal villus, and the loss of crypt.174CNSI could effectively reduce the extent of damage in the intestinal mechanical barrier, because it could improve mitochondrial dysfunction, repair DNA double-strand break, alleviate the apoptosis of intestinal epithelial cells, and inhibit the apoptosis of crypt stem cells.99
4.4 Nanoplastics
Nanoplastics could cause individual Caco-2 cell necrosis and then propagate across the cell monolayer, which was called as the bystander killing effect.179 PS NPs were directly toxic to Caco-2 cells, which was mainly embodied in increasing the concentration of ROS and expression of oxidant stress-related genes, inducing the genotoxicity and DNA oxidative damage.177,178,180 The effects of nanoplastics on the intestinal mechanical barrier are affected by their particle size and surface charge. Nanoplastics with a small size (20 nm) had greater cytotoxicity than that with a large size (40 nm), and nanoparticles with a carboxylated surface had greater cytotoxicity than that with an amino surface.1794.5 Others
Se NPs, as the modulator of the intestinal mechanical barrier, could prevent oxidative stress-related intestinal disease by targeting mitochondria. L. casei 393-SeNPs could relieve intestinal mechanical barrier dysfunction induced by the oxidative damage of intestines in humans and pigs through alleviating the increase of ROS and decrease of adenosine triphosphate (ATP) and mitochondrial membrane potential (MMP) (Fig. 8c).135,175Hyaluronic acid-DOX nanoparticles could effectively protect the integrity of intestinal mucous epithelium in colitis mice treated with dextran sulfate sodium (DSS).181 As a drug carrier to treat the disruption of the intestinal mechanical barrier, ophiopogon japonicus (OJPs)/chitosan (CS)/whey protein (WP) co-assembled nanoparticles could effectively protect the integrity and permeability of the intestinal mechanical barrier (Fig. 8d).182
Carboxymethyl chitosan-coated poly(ortho ester urethane) nanoparticles could enhance the oral delivery of DOX (CMC/POEU/DOX NPs), which could inhibit the spread of H22 tumors without having systemic toxicity reactions. The external coating layer of CMC/POEU/DOX NPs was carboxymethyl chitosan which could improve intestinal absorption through opening the tight junctions and enhancing the paracellular permeability.183 Lipid polymeric nanoparticles (H/VC-LPNs), which were synthesized by poly(N-(2-hydroxypropyl)methacrylamide) and vitamin B12-modified chitosan, enhanced the intracellular uptake and absorption of curcumin.184 PEG derivative nanoparticles coated with poly-L-arginine, which possessed excellent intestinal mucosal permeability and high paracellular permeability, could be loaded with recombination urease subunit B to improve the delivery efficiency of oral vaccine and enhance the levels of mucosal antibody in mice.185
5 Effects of nanomaterials on the intestinal immunological barrier
The intestine is the largest immune organ in the body. The intestinal immunological barrier could prevent bacteria, viruses, and endotoxin. Gut-associated immune cells such as lymphocytes and macrophages could resist bacteria invasion in the intestinal barrier function. SIgA located in the lamina propria of intestinal mucosa is secreted into the intestinal lumen after being processed by intestinal epithelial cells to prevent antigen attachment to the intestinal mucosa and eliminate the foreign antigen. Serum antibodies (IgA and IgG) are important components of innate immunity and adaptive immunity, modulating immune responses.Immunomodulatory cytokines mainly refer to small molecular polypeptides and glycoproteins synthesized and secreted by immune cells, which could regulate the cell growth and immune response, participate in inflammation, and accelerate wound healing. Cytokines can be divided into pro-inflammatory cytokines (e.g., TNF-α, IL-1, IL-6, and IL-8) and anti-inflammatory cytokines (e.g., IL-10, IL-12, and TGF-β), and their combined effects determine the development of inflammation.
The disruption of the intestinal immunological barrier would increase the production of pro-inflammatory cytokines and alter the gene expression of immune cells, causing IBD. Nanomaterials could affect the intestinal immunological barrier through adjusting intestinal immunomodulatory cytokines and immune globulin. It is important to understand how nanomaterials affect the intestinal immunological barrier, which is conducive to the development of novel medicine, drug carriers, and additives used in food and feed. The effects of nanomaterials on the intestinal immunological barrier are listed in Tables 6 and 7.
| Nanomaterials (characteristics) | Animal models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a mg L−1 represents the concentration of nanomaterials to which animal models were exposed; ↑ represents increase; ↓ represents decrease; F represents female; M represents male. | ||||
| Ag NPs (10, 75 and 110 nm; sphere) | SD rats (M and F; 3 weeks old) | 9, 18 and 36 mg kg−1 day−1 | Immunomodulatory genes (TLR2, TLR4, GPR43, and FOXP3)↓ | 50 |
| 91 days | ||||
| Ag NPs (2–35 nm; sphere) | Broiler chickens (7 days old) | 10 and 20 mg kg−1 day−1 | IgG↓ | 187 |
| 36 days | ||||
| Ag NPs | Broiler chickens (480 days old) | 1000 mg L−1 in drinking water | Innate immune system related receptors (TLR4 and TLR2-1)↑ | 186 |
| 42 days | ||||
| CAgNCs (chitosan-coated) | Wild type zebrafish | Diets containing 2% CAgNCs | TNF-α, IL-10, IL-12, Debfl1, and Lyz↑; immune transcriptional factors (IRF-1 and C-Rel)↑ | 53 |
| 60 days | ||||
| ZnO NPs (72 nm; sphere) | Weaned castrated piglets | 150, 300, and 450 mg kg−1 day−1 | IgA, IL-6, and TNF-α↑; IgM↓ | 59 |
| 21 days | ||||
| ZnO NPs | Ducklings (1 day old) | 120 mg kg−1 day−1 | IgA↑ | 188 |
| 35 days | ||||
| ZnO NPs (30 nm; sphere) | Weaned piglets | 400 and 800 mg kg−1 day−1 | IL-1β, IL-10, and TNF-α↑ | 164 |
| 14 days | ||||
| TiO2 NPs (10, 50, and 100 nm; sphere) | C57BL/6J mice (F; 3 weeks old) | Diets containing 0.1% TiO2 NPs | Proportion of CD4+ T cells, Tregs, and macrophages in DSS-treated mice↓ | 71 |
| 93 days | ||||
| TiO2 NPs (12 nm; sphere) | CD1 mice | 12.5 mg kg−1 | Induce the chronic damage in the intestines | 172 |
| 6 hours | ||||
| SWCNTs (diameter: 1.04–1.17 nm; length: 1–5 μm) | ICR mice (M; 7 weeks old) | 0.05, 0.5, and 2.5 mg kg−1 day−1 | IL-1β, IL-6, and TNF-α↑ | 90 |
| 7 days | ||||
| PS NPs (100 nm; sphere) | Zebrafish (M and F; 16 weeks old) | 0.5 mg L−1a | IL-8, IFN-I, and TNF-α↑; T cells↑; M1 macrophage cells↓ | 103 |
| 21 days | ||||
| Amyloid-polyphenol hybrid nanofilaments (long semiflexible rod) | C57BL/6J mice (M; 7 weeks old) | 150 mg kg−1 day−1 | Inhibit the NF-κB signaling pathway | 109 |
| 14 days | ||||
| Curcumin nanoparticles | BALB/c mice (F; 6–8 weeks old) | Diets containing 0.2% curcumin nanoparticles | TNF-α, IL-1β, IL-6 ↓; Chemokines (CXCL1, and CXCL2)↓ | 190 |
| 18 days | ||||
| BSA-SiO2 NPs (130 nm, 430 nm, and 1–2 μm; narrow and long vermiculate pores, mesopores, and wheatlike macrostructure) | BALB/c mice (F) | 0.15 and 0.30 mg mice−1 day−1 | BSA-specific IgG antibody↑ | 191 |
| 56 days | ||||
| CMC/POEU/DOX NPs (350 nm) | ICR mice (F) | 50, 300, and 2000 mg kg−1 day−1 | Transcytosis and lymphatic uptake of M cells↑ | 183 |
| 14 days | ||||
| HABN (86 nm) | C57BL/6 mice (F; 6 weeks old) | 30 mg kg−1 day−1 | IL-10 and TGF-β↑; IL-1β and TNF-α↓ | 110 |
| 6 days | ||||
| mEVs (105 nm; spherical shape with a lipid bilayer) | C57BL/6 mice (F; 3 weeks old) | 0.3, 0.6, and 1.2 mg kg−1 day−1 | IgA and sIgA↑; GATA4, Reg-γ, and MyD88↑ | 111 |
| 56 days | ||||
| Nanomaterials (characteristics) | Cell models | Dosage and time | Results | Ref. |
|---|---|---|---|---|
| a μg mL−1 and μg cm−2 represent the concentration of nanomaterials to which cell models were exposed; ↑ represents increase. | ||||
| Ag NPs (20 and 200 nm; sphere) | Caco-2/HT29-HTX co-culture | 100 μg mL−1a | IL-8↑ | 145 |
| 20 hours | ||||
| ZnO NPs (100–150 nm) | C2BBe1/HT29-MTX/Raji B triculture | 4.05 μg cm−2a | IL-6, IL-1, and IL-8↑ | 189 |
| 1 day | ||||
| TiO2 NPs (30 nm) | Caco-2/HT29-MTX co-culture | 1.4 × 10−5, 1.4 × 10−3, and 1.4 × 10−1 μg mL−1a | Pro-inflammatory signaling↑ | 171 |
| 4 hours and 5 days | ||||
| TiO2 NPs (100–150 nm) | C2BBe1/HT29-MTX/Raji B triculture | 7.90 μg cm−2a | IL-8↑ | 189 |
| 1 day | ||||
| Fe2O3 NPs (80–120 nm; rod) | C2BBe1/HT29-MTX/Raji B triculture | 81.9 μg cm−2a | IL-8↑ | 189 |
| 1 day | ||||
| Fe2O3 NPs (70 nm; acicular) | C2BBe1/HT29-MTX/Raji B triculture | 171.5 μg cm−2a | IL-6, IL-1β, and IL-8↑ | 189 |
| 1 day | ||||
5.1 Noble metal nanoparticles
Naked Ag NPs can directly stimulate the gut-associated mucosal immune response, which might suppress the gastrointestinal immune function. Ag NPs can induce the innate immune function through their impacts on the mRNA expression of jejunal TLR4 and TLR2-1 in broiler chickens.186 The particle size and dosage of Ag NPs could influence the expression of immune-related genes. The expressions of immunomodulatory genes (such as TLR2, TLR4, FOXP3, and GPR43) were down-regulated under low dosage. Ag NPs of size 10 nm and 75 nm could lead to immune-related genetic changes in Sprague-Dawley rats (Fig. 9a).50 The increase of IL-8 secretion was dose dependent and size dependent. Nanoparticles reached the intestinal epithelial cells faster as their size was smaller. The secretion of IL-8 in Caco-2/TC7 cells treated with 20 nm Ag NPs was 8 times higher than that treated with 200 nm Ag NPs, and the secretion of IL-8 in co-culture of Caco-2/TC7 and HT29-MTX cells treated with 20 nm Ag NPs was 4 times higher than that treated with 200 nm Ag NPs.145 In addition, there is a significant interaction between the concentration of Ag NPs and the age of animal models. The IgG level of chicks was significantly decreased in comparison with the control group after feeding with 20 mg kg−1 Ag NPs for 36 days; however, the IgM level of chicks was obviously higher after feeding with 10 mg kg−1 Ag NPs for 36 days than that for 22 days.187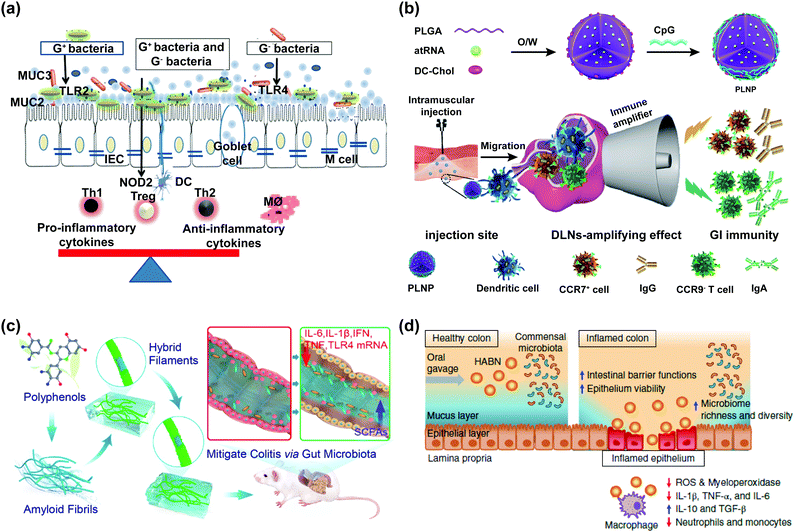 | ||
| Fig. 9 The impact of nanomaterials on the intestinal immunological barrier and their application as drug carriers and medicine to treat IBD. (a) Schematic diagram for the potential interaction of Ag NPs with the intestinal microbiota and gut-associated immune system; adapted with permission from ref. 50. Copyright 2015, Taylor & Francis. (b) Design of PLNP for co-delivery of atRA, CpG, and antigen and the schematic illustration of the DLN-amplifying effect; adapted with permission from ref. 193. Copyright 2019, American Chemical Society. (c) Amyloid-templated polyphenol hybrid nanofilaments and hydrogels mitigate colitis and regulate gut microbial dysbiosis; adapted with permission from ref. 109. Copyright 2020, American Chemical Society. (d) HABN accumulates in the inflamed colon and exerts therapeutic effects against acute colitis; adapted with permission from ref. 110. Copyright 2020, Springer Nature. | ||
CAg NCs supplemented in the zebrafish diet could act as an immunomodulator because chitosan possesses immunostimulants in aquatic animals.53 CAg NCs could positively regulate the expression of immune-related genes (such as TNF-α, IL-10, IL-12, Debfl1, and Lyz) and immune transcription factors, including IRF-1 and C-Rel. After feeding with CAg NPs for 60 days, the mRNA expression levels of TNF-α, IL-10, IL-12, IRF-1, Debfl1, and Lyz increased by more than three times.
5.2 Non-noble metal oxide nanoparticles
ZnO NPs, as a dietary supplement, could significantly increase the concentrations of serum antibodies and improve the intestinal immunological barrier of piglets and ducks.59,188 The mRNA expressions of IL-1β, IL-10, and TNF-α were obviously up-regulated in the intestines of piglets after treating with the dietary supplemented 800 mg kg−1 day−1 ZnO NPs.164The increase of IL-8 secretion was dose dependent when the Caco-2/Raji B co-culture models, which could simulate the function of microfold (M) cells, were treated with CuO NPs.167 M cells could transport nanoparticles and pathogens into potential immune cells by penetrating the intestinal epithelium.
TiO2 NPs intake could induce inflammatory bowel disease, because the interaction between TiO2 NPs and intestinal mucosa could trigger the expression of pro-inflammatory cytokines, stimulate the response of local immune system, and affect the defense of intestinal immunological barrier. Exposure of Caco-2 cells to TiO2 NPs could significantly induce the gene expressions of pro-inflammatory cytokines (IL-8, TNF-α, and NFκB1) in comparison with the control group, which resulted in inflammation in gastrointestinal epithelial cells.169,171 The secretions of pro-inflammatory cytokines (IL-6, IL-1β, and IL-8) were slightly increased in C2BBe1/HT29-MTX/Raji B cell triculture after exposure to TiO2 NPs for 24 h.189 The tendency of cytokines (IL-4, IL-10, IL-17A, and IL-1β) first increased and then decreased in the ileum of mice after treating with high-dose TiO2 NPs.86 Low-dose TiO2 NPs reduced the proportions of CD4+ T cells, Tregs, and macrophages in chronic colitis mice, which resulted in the dysregulation of intestinal immunity and aggravation of colitis.71 In addition, TiO2 NPs could translocate through the epithelial cells of ileum and Peyer's patches, which would induce the deficiency of epithelial cells and chronic damage of the intestines.172
The shape of Fe2O3 NPs affects the interaction between Fe2O3 NPs and the intestinal immunological barrier. After treating with rod-shaped Fe2O3 NPs, the secretions of IL-6, IL-1β, and IL-8 were in C2BBe1 monocultures and C2BBe1/HT29-MTX/Raji B triculture. The acicular Fe2O3 NPs had more notable influence than rod-shaped Fe2O3 NPs.189
5.3 Carbon nanomaterials
CNTs deposited in the lumen of intestines due to the interaction between CNTs and intestinal mucus, thus disrupting the intestinal immunological barrier. The increases in the duodenal and colonic expressions of IL-1β, IL-6, and TNF-α were dose dependent after treating mice with SWCNTs, which suggested that SWCNTs induced intestinal inflammatory responses in mice.90 However, SWCNT-loaded aeromonas hydrophila vaccine (SWCNTs-Aera) could significantly increase the expressions of immune-related genes (IL-8, IFN-I, and TNF-α) in the intestines of grass carp, which suggested that the SWCNT-Aera vaccine induced a better tissue immune response.1925.4 Nanoplastics
Nanoplastics could stimulate the production of immunomodulatory cytokines and influence the relative proportions of phagocytes and lymphocytes, which cause the dysfunction of the intestinal immunological barrier. After treating with PS NPs, the abundance of T cells showed a significant increase while the proportion of M1 macrophage cells decreased in adult zebrafish. M1 macrophage cells can secrete pro-inflammatory cytokines, phagocytize microbiota, and trigger the immune response. PS MPs would also change the IgA production of the intestinal immune network and alter the chemotaxis of B cells.1035.5 Others
Other nanomaterials can also be used as drug carriers, which have attracted extensive attention in the treatment of IBD and cancer. Poly(lactic-co-glycolic acid)/3β-[N-(N,N-dimethylaminoethane)carbamoyl]cholesterol hydrochloride nanoparticles (PLGA/DC-Chol NPs) were used for the delivery of atRNA, CpG oligodeoxynucleotides (CpG), and antigens, which could enhance gastrointestinal immunity via improving the draining lymph node effect. Compared with the group treated with OVA-atRNA-CpG, the concentration of IgA has increased 4.79-fold in the intestines of immunized mice treated with PLGA/DC-Chol NPs. Compared with the group treated with OVA, the proportion of SIINFEKL-pentamer+ T cells has increased 10-fold in the group treated with PLGA/DC-Chol NPs, which could strengthen intestinal immunological barrier defenses (Fig. 9b).193 Ovalbumin nanoparticles combined with cytosine–phosphate–guanine as multifunctional nanovaccine, which could induce the immune response (e.g., DC maturity, T cell activation, and IFN-γ production), possessed the prospect in antigen delivery and cancer immunotherapy.194 Bovine serum albumin (BSA) was loaded into SiO2 NPs with a diameter of about 430 nm and honeycombed mesopores, a diameter of about 130 nm and vermiculate pores, and a diameter of about 400 nm and a wheat-like macrostructure. The particle size and pore structure of SiO2 NPs could influence the titers of BSA-specific IgG antibodies since the large size and the porous structure of the particles could prolong the interaction between the antigen and immune system.191 CMC/POEU/DOX NPs which could promote the intracellular transport and lymph absorption of M cells and suppress the spread of cancer cells.183 Orange juice-derived nanovesicles (ONVs) could treat obesity-associated intestinal complications. ONVs returned the expressions of pro-inflammatory cytokines (TNF-α and IL-1β) and increased the expression of TLR4, accelerating the recovery of the intestinal immune functions of obese patients whose intestines are in a state of low-grade inflammation.195Some nanomaterials can also be used as medicine to directly treat IBD through regulating the intestinal immunological barrier. mEVs could be a potential drug carrier to treat DSS-induced colitis through regulating the immune system. After treating with mEVs (0.6 mg kg−1 day−1), the relative levels of MUC2, GATA4, Reg-γ, and MyD88 were significantly up-regulated, and the concentrations of IgA and sIgA were increased in mice.111 The amyloid–polyphenol hybrid nanofilaments could significantly inhibit the up-regulated expressions of pro-inflammatory cytokines in serum, which was caused by colitis mice treated with dextran sulfate sodium (DSS), including IL-6, IL-1β, IFN-γ, and TNF-α. This was because polyphenol and amyloid fibrils could restrain NF-κB signaling pathways in the epithelial cells and relieve inflammation (Fig. 9c).109 After treating with curcumin nanoparticles, the mRNA expressions of TNF-α, IL-1β, IL-6, CXCL1, and CXCL2 were down-regulated in the colonic epithelial tissues of colitis mice, which demonstrated that curcumin nanoparticles have a potential application in IBD treatment.190
Hyaluronic acid-bilirubin nanomedicine (HABN), which was formed by the nanoaggregation of an amphiphilic conjugate between hyaluronic acid (HA) and bilirubin (BR), had an anti-inflammatory effect on the treatment of acute colitis, because HA could regulate macrophages and CD4+ T (Treg) cells and BR exhibited strong free radical scavenging activity. After treating with HABN, the mRNA expressions of IL-1β, IL-6, and TNF-α were down-regulated while the mRNA expressions of anti-inflammatory IL-10 and TGF-β were up-regulated in colitis mice (Fig. 9d).110,181
6. Conclusion and outlook
The aim of this review is to outline the current state of research about the impact of nanomaterials on the IMB, discuss the treatment of intestinal diseases by using nanomaterials, and propose the future development of nanomaterials. Nanomaterials could affect the IMB by changing the diversity of gut microbiota, adjusting the morphology of intestinal epithelium, influencing the expression of tight junctional protein and mucus protein, and regulating intestinal immune factors and immune globulin. The effects of nanomaterials on the IMB are mainly influenced by the type, dosage, size, morphology, and surface functional groups of nanomaterials. However, despite the considerable developments obtained in this area, there are still some fundamental and essential issues that need more research attention.(1) It is necessary to study the effect of nanomaterials on the small intestinal mucosal barrier. The effect of nanomaterials on the integrity and functionality of the colorectal mucosal barrier has been widely studied, though the effect of nanomaterials on the small intestinal mucosal barrier is still largely unknown. Microbiota in the small intestine is also critically connected with many biological functions.196
(2) It is necessary to focus on strengthening in vitro studies on the regulation of nanomaterials on intestinal anaerobes. There are many in vitro studies on the impact of nanomaterials on aerobes or facultative anaerobes. Anaerobes in the colon, whose number is 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times higher than that of aerobic and facultative anaerobes, are involved in various important biological reactions in the intestines.196,197
000 times higher than that of aerobic and facultative anaerobes, are involved in various important biological reactions in the intestines.196,197
(3) It is necessary to construct and improve the organ-on-a-chip for simulating the complete IMB. At present, the usual methods used to study the effect of nanomaterials on the IMB are in vivo animal experiments and in vitro cell culture. However, animal experiments are ethically challenging and require a long period of time. Cell culture experiments are different from the in vivo environments. The combination of microfluidics198 and organoid chips could play a critical role in constructing the complete IMB to study intestinal diseases and the impact of nanomaterials on the intestines.
(4) It is important to focus on the metabolic process of nanomaterials in the intestines. Nanomaterials have broad prospects in the clinical application of intestinal diseases, such as theranostics of colorectal cancer, treatment of bacterial infection, and delivery of mucosal vaccines.199 However, the application of nanomaterials in biomedicine is still in the early stages, and the safety assessment of nanomaterials in the IMB is not comprehensive enough.
(5) It is important to optimize the structural design and surface modification of nanomaterials as drug carriers. The functionalization of nanomaterials could improve their oral bioavailability by enhancing their mucus permeability, increasing their epithelial absorption, and adjusting the intercellular tight junction. Nanomaterials with negative charge or hydrophilic surface have good mucus permeability because the domain glycosylation of intestinal mucin makes mucus negatively charged.200 At the same time, nanomaterials with negative charges could open the intercellular tight junction and promote them into the blood when they are used to deliver insulin and other protein drugs. However, nanomaterials with positive charge or hydrophobic surface have high epithelial absorption because the outer surface charges of the phospholipid bilayer were negative. The surface charge and hydrophilicity/hydrophobicity of nanomaterials mostly depend on the target organs of drugs.
Continuous efforts need to be devoted toward researching the effects of nanomaterials on the IMB, including the effect on the small intestinal mucosal barrier and the regulation on intestinal anaerobes in vitro. A more challenging direction is to functionalize new drug nanocarriers across the IMB and to study their metabolic processes in the IMB. We believe that nanomaterials will find uses as antibiotics, oral vaccines, drug carriers, and so on.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the National Natural Science Foundation of China (32171397, 21875050, 21535001, 81730051, 21761142006), the National Key R&D Program of China (2018YFA0902600, 2017YFA0205901), and Ningxia Hui Autonomous Region Key Research and Development Program (2020ZDYF0786) for financial support.References
- J. R. Turner, Nat. Rev. Immunol., 2009, 9, 799–809 CrossRef CAS PubMed.
- E. C. Martens, M. Neumann and M. S. Desai, Nat. Rev. Microbiol., 2018, 16, 457–470 CrossRef CAS.
- A. C. Luissint, C. A. Parkos and A. Nusrat, Gastroenterology, 2016, 151, 616–632 CrossRef CAS PubMed.
- C. A. Thaiss, D. Zeevi, M. Levy, G. Zilberman-Schapira, J. Suez, A. C. Tengeler, L. Abramson, M. N. Katz, T. Korem, N. Zmora, Y. Kuperman, I. Biton, S. Gilad, A. Harmelin, H. Shapiro, Z. Halpern, E. Segal and E. Elinav, Cell, 2014, 159, 514–529 CrossRef CAS.
- C. A. Thaiss, S. I. Tav, D. Rothschild, M. T. M. Eijer, M. Levy, C. Moresi, L. Dohnalova, S. Braverman, S. Rozin, S. Malitsky, M. Dori-Bachash, Y. Kuperman, I. Biton, A. Gertler, A. Harmelin, H. Shapiro, Z. Halpern, A. Aharoni, E. Segal and E. Elinav, Nature, 2016, 540, 544–551 CrossRef CAS PubMed.
- R. Caruso, B. C. Lo and G. Nunez, Nat. Rev. Immunol., 2020, 20, 411–426 CrossRef CAS PubMed.
- G. D. Sepich-Poore, L. Zitvogel, R. Straussman, J. Hasty, J. A. Wargo and R. Knight, Science, 2021, 371, eabc4552 CrossRef CAS.
- H. J. Kim, H. Li, J. J. Collins and D. E. Ingber, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E7–E15 CrossRef CAS PubMed.
- L. W. Peterson and D. Artis, Nat. Rev. Immunol., 2014, 14, 141–153 CrossRef CAS.
- T. Suzuki, Cell. Mol. Life Sci., 2013, 70, 631–659 CrossRef CAS.
- J. L. Madara, Annu. Rev. Physiol., 1998, 60, 143–159 CrossRef CAS PubMed.
- I. Bjarnason, A. Macpherson and D. Hollander, Gastroenterology, 1995, 108, 1566–1581 CrossRef CAS.
- P. Paone and P. D. Cani, Gut, 2020, 69, 2232–2243 CrossRef CAS PubMed.
- D. R. Mack, S. Michail, S. Wei, L. McDougall and M. A. Hollingsworth, Am. J. Physiol., 1999, 275, G941–G950 Search PubMed.
- M. Zhang, K. J. Sun, Y. J. Wu, Y. Yang, P. Tso and Z. L. Wu, Front. Immunol., 2017, 8, 942 CrossRef PubMed.
- S. S. Chew, L. T. H. Tan, J. W. F. Law, P. Pusparajah, B. H. Goh, N. S. Ab Mutalib and L. H. Lee, Cancers, 2020, 12, 2272 CrossRef CAS PubMed.
- H. W. Zheng, N. Singh, G. S. Shetye, Y. C. Jin, D. N. Li and Y. Y. Luk, Bioorg. Med. Chem., 2017, 25, 1830–1838 CrossRef CAS PubMed.
- S. Becattini, Y. Taur and E. G. Pamer, Trends Mol. Med., 2016, 22, 458–478 CrossRef CAS PubMed.
- T. Ichinohe, I. K. Pang, Y. Kumamoto, D. R. Peaper, J. H. Ho, T. S. Murray and A. Iwasaki, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5354–5359 CrossRef CAS PubMed.
- S. Sakaguchi, T. Yamaguchi, T. Nomura and M. Ono, Cell, 2008, 133, 775–787 CrossRef CAS PubMed.
- R. A. Britton and V. B. Young, Gastroenterology, 2014, 146, 1547–1553 CrossRef CAS PubMed.
- M. X. Li, F. C. Li, Z. T. Lu, Y. L. Fang, J. W. Qu, T. T. Mao, H. Wang, J. Chen and B. Li, Sci. Total Environ., 2020, 704, 135273 CrossRef CAS PubMed.
- X. J. Cui, L. Bao, X. Y. Wang and C. Y. Chen, Small, 2020, 16, 1907665 CrossRef CAS.
- H. M. Ding and Y. Q. Ma, Nanoscale Horiz., 2018, 3, 6–27 RSC.
- M. Natan and E. Banin, FEMS Microbiol. Rev., 2017, 41, 302–322 CrossRef CAS PubMed.
- X. H. Zhao, Y. X. Jia, J. J. Li, R. H. Dong, J. J. Zhang, C. X. Ma, H. Wang, Y. K. Rui and X. Y. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 29398–29406 CrossRef CAS PubMed.
- Y. Y. Zhao, C. J. Ye, W. W. Liu, R. Chen and X. Y. Jiang, Angew. Chem., Int. Ed., 2014, 53, 8127–8131 CrossRef CAS.
- X. Q. Xing, W. S. Ma, X. Y. Zhao, J. Y. Wang, L. Yao, X. Y. Jiang and Z. H. Wu, Langmuir, 2018, 34, 12583–12589 CrossRef CAS.
- S. T. Wang, Y. F. Gao, Q. Jin and J. Ji, Biomater. Sci., 2020, 8, 6825–6839 RSC.
- J. P. Xu, J. Ji, W. D. Chen and J. C. Shen, Key Eng. Mater., 2005, 288–289, 465–468 CAS.
- V. Brega, F. Scaletti, X. Z. Zhang, L. S. Wang, P. Li, Q. B. Xu, V. M. Rotello and S. W. Thomas, ACS Appl. Mater. Interfaces, 2019, 11, 2814–2820 CrossRef CAS.
- N. Goyal, A. Rana, A. Ahlawat, K. R. V. Bijjem and P. Kumar, Inflammopharmacology, 2014, 22, 219–233 CrossRef PubMed.
- L. M. Gonzalez, A. J. Moeser and A. T. Blikslager, Transl. Res., 2015, 166, 12–27 CrossRef CAS.
- L. M. Ensign, R. Cone and J. Hanes, Adv. Drug Delivery Rev., 2012, 64, 557–570 CrossRef CAS.
- D. K. Dahiya, Renuka and A. K. Puniya, Future Microbiol., 2018, 13, 483–492 CrossRef CAS.
- A. Anand, B. Unnikrishnan, S. C. Wei, C. P. Chou, L. Z. Zhang and C. C. Huang, Nanoscale Horiz., 2019, 4, 117–137 RSC.
- B. Lamas, N. M. Breyner and E. Houdeau, Part. Fibre Toxicol., 2020, 17, 19 CrossRef PubMed.
- R. B. Sartor, Gastroenterology, 2008, 134, 577–594 CrossRef CAS.
- T. Britannica, Noble metal, Encyclopedia Britannica, 2021 Search PubMed.
- X. N. Hu, Y. Y. Zhao, Z. J. Hu, A. Saran, S. Hou, T. Wen, W. Q. Liu, Y. L. Ji, X. Y. Jiang and X. C. Wu, Nano Res., 2013, 6, 822–835 CrossRef CAS.
- S. W. Wang, J. S. Sun, Y. X. Jia, L. Yang, N. X. Wang, Y. L. Xianyu, W. W. Chen, X. H. Li, R. T. Cha and X. Y. Jiang, Biomacromolecules, 2016, 17, 2472–2478 CrossRef CAS PubMed.
- Y. Liu, Y. L. Balachandran, D. Li, Y. M. Shao and X. Y. Jiang, ACS Nano, 2016, 10, 3589–3596 CrossRef CAS.
- M. Marchioni, G. Veronesi, I. Worms, W. L. Ling, T. Gallon, D. Leonard, C. Gateau, M. Chevallet, P. H. Jouneau, L. Carlini, C. Battocchio, P. Delangle, I. Michaud-Soret and A. Deniaud, Nanoscale Horiz., 2020, 5, 507–513 RSC.
- R. T. Agans, A. Gordon, S. Hussain and O. Paliy, Toxicol. Sci, 2019, 172, 411–416 CrossRef CAS PubMed.
- H. Q. Chen, R. F. Zhao, B. Wang, C. X. Cai, L. N. Zheng, H. L. Wang, M. Wang, H. Ouyang, X. Y. Zhou, Z. F. Chai, Y. L. Zhao and W. Y. Feng, NanoImpact, 2017, 8, 80–88 CrossRef.
- J. T. Wu, C. Li, J. Zhang, N. W. Menzies, P. M. Bertsch, P. Wang and P. M. Kopittke, Environ. Sci.: Nano, 2020, 7, 1554–1565 RSC.
- C. Catto, E. Garuglieri, L. Borruso, D. Erba, M. C. Casiraghi, F. Cappitelli, F. Villa, S. Zecchin and R. Zanchi, Environ. Pollut., 2019, 245, 754–763 CrossRef CAS.
- Y. B. Ma, L. Y. Song, Y. Lei, P. P. Jia, C. J. Lu, J. F. Wu, C. W. Xi, P. R. Strauss and D. S. Pei, Environ. Sci.: Nano, 2018, 5, 740–751 RSC.
- A. B. Javurek, D. Suresh, W. G. Spollen, M. L. Hart, S. A. Hansen, M. R. Ellersieck, N. J. Bivens, S. A. Givan, A. Upendran, R. Kannan and C. S. Rosenfeld, Sci. Rep., 2017, 7, 2822 CrossRef.
- K. Williams, J. Milner, M. D. Boudreau, K. Gokulan, C. E. Cerniglia and S. Khare, Nanotoxicology, 2015, 9, 279–289 CrossRef CAS.
- Y. D. Li, N. Yan, T. Y. Wong, W. X. Wang and H. B. Liu, Environ. Sci.: Nano, 2019, 6, 3242–3255 RSC.
- S. van den Brule, J. Ambroise, H. Lecloux, C. Levard, R. Soulas, P. J. De Temmerman, M. Palmai-Pallag, E. Marbaix and D. Lison, Part. Fibre Toxicol., 2016, 13, 38 CrossRef PubMed.
- R. M. C. Udayangani, S. H. S. Dananjaya, C. Nikapitiya, G. J. Heo, J. Lee and M. De Zoysa, Fish Shellfish Immunol., 2017, 66, 173–184 CrossRef CAS PubMed.
- S. Q. Zhu, M. Y. Zeng, G. X. Feng and H. H. Wu, Int. J. Nanomed., 2019, 14, 8361–8378 CrossRef CAS.
- L. R. Wang, C. L. Zhang, X. Zhi, W. X. Hou, T. L. Li, S. J. Pan, B. Liu, J. Song, Y. X. Pan, J. Ni and D. X. Cui, J. Biomed. Nanotechnol., 2019, 15, 779–789 CrossRef CAS PubMed.
- S. Q. Zhu, X. M. Jiang, M. D. Boudreau, G. X. Feng, Y. Miao, S. Y. Dong, H. H. Wu, M. Y. Zeng and J. J. Yin, J. Nanobiotechnol., 2018, 16, 86 CrossRef CAS.
- J. J. Li, R. T. Cha, X. H. Zhao, H. B. Guo, H. Z. Luo, M. Z. Wang, F. S. Zhou and X. Y. Jiang, ACS Nano, 2019, 13, 5002–5014 CrossRef CAS PubMed.
- S. V. Lebedev, I. A. Gavrish, I. Z. Gubajdullina and S. V. Shabunin, Selskokhoziaistvennaia Biol., 2019, 54, 820–831 Search PubMed.
- X. Pei, Z. P. Xiao, L. J. Liu, G. Wang, W. J. Tao, M. Q. Wang, J. B. Zou and D. B. Leng, J. Sci. Food Agric., 2019, 99, 1366–1374 CrossRef CAS.
- Y. B. Sun, T. Xia, H. Wu, W. J. Zhang, Y. H. Zhu, J. X. Xue, D. T. He and L. Y. Zhang, Anim. Feed Sci. Technol., 2019, 258, 114312 CrossRef CAS.
- Y. N. Feng, L. J. Min, W. D. Zhang, J. Liu, Z. M. Hou, M. Q. Chu, L. Li, W. Shen, Y. Zhao and H. F. Zhang, Front. Microbiol., 2017, 8, 992 CrossRef PubMed.
- H. N. Liu, M. M. Bai, K. Xu, J. Zhou, X. F. Zhang, R. Yu, R. L. Huang and Y. L. Yin, J. Sci. Food Agric., 2020, 101, 735–745 CrossRef.
- E. Aleshina, E. Miroshnikova and E. Sizova, Int. J. Environ. Sci. Technol., 2020, 17, 721–732 CrossRef CAS.
- Z. J. Chen, S. Han, D. Zhou, S. P. Zhou and G. Jia, Nanoscale, 2019, 11, 22398–22412 RSC.
- G. Pinget, J. Tan, B. Janac, N. O. Kaakoush, A. S. Angelatos, J. O'Sullivan, Y. C. Koay, F. Sierro, J. Davis, S. K. Divakarla, D. Khanal, R. J. Moore, D. Stanley, W. Chrzanowski and L. Macia, Front. Nutr., 2019, 6, 57 CrossRef PubMed.
- N. Kolba, Z. Y. Guo, F. M. Olivas, G. J. Mahler and E. Tako, Food Chem. Toxicol., 2020, 135, 110896 CrossRef CAS PubMed.
- X. Q. Cao, Y. H. Han, M. Gu, H. J. Du, M. Y. Song, X. A. Zhu, G. X. Ma, C. Pan, W. C. Wang, E. M. Zhao, T. Goulette, B. Yuan, G. D. Zhang and H. Xiao, Small, 2020, 16, 2001858 CrossRef CAS.
- Z. J. Chen, D. Zhou, S. Han, S. P. Zhou and G. Jia, Part. Fibre Toxicol., 2019, 16, 48 CrossRef CAS PubMed.
- X. B. Li, Y. S. Zhang, B. Li, J. Cui, N. Gao, H. Sun, Q. T. Meng, S. S. Wu, J. Z. Bo, L. C. Yan, J. Wu and R. Chen, NanoImpact, 2019, 14, 100164 CrossRef.
- S. S. Zhang, X. J. Jiang, S. Q. Cheng, J. C. Fan, X. Qin, T. X. Wang, Y. J. Zhang, J. Zhang, Y. Qiu, J. F. Qiu, Z. Zou and C. Z. Chen, Arch. Toxicol., 2020, 94, 1173–1190 CrossRef CAS PubMed.
- W. Mu, Y. Wang, C. Huang, Y. J. Fu, J. Q. Li, H. Wang, X. D. Jia and Q. Ba, J. Agric. Food Chem., 2019, 67, 9382–9389 CrossRef CAS.
- C. Cueva, I. Gil-Sanchez, A. Tamargo, B. Miralles, J. Crespo, B. Bartolome and M. V. Moreno-Arribas, Food Chem. Toxicol., 2019, 132, 110657 CrossRef CAS PubMed.
- J. J. Zhang, L. Mou and X. Y. Jiang, Chem. Sci., 2020, 11, 923–936 RSC.
- Y. Z. Y. Xie, Y. Liu, J. C. Yang, Y. Liu, F. P. Hu, K. Zhu and X. Y. Jiang, Angew. Chem., Int. Ed., 2018, 57, 3958–3962 CrossRef CAS.
- L. Wang, J. C. Yang, X. L. Yang, Q. H. Hou, S. Q. Liu, W. F. Zheng, Y. Z. Long and X. Y. Jiang, ACS Appl. Mater. Interfaces, 2020, 12, 51148–51159 CrossRef CAS.
- L. Wang, J. C. Yang, S. X. Li, Q. Z. Li, S. Q. Liu, W. F. Zheng and X. Y. Jiang, Nano Lett., 2021, 21, 1124–1131 CrossRef CAS.
- R. Song, J. B. Yao, Q. Q. Shi and R. B. Wei, Mar. Drugs, 2018, 16, 23 CrossRef PubMed.
- L. Zheng, Y. Hu, X. He, Y. Zhao and H. Xu, J. Appl. Microbiol., 2020, 128, 1764–1775 CrossRef CAS.
- T. Zhai, X. H. Lu, F. X. Wang, H. Xia and Y. X. Tong, Nanoscale Horiz., 2016, 1, 109–124 RSC.
- J. Chen, H. M. Meng, Y. Tian, R. Yang, D. Du, Z. H. Li, L. B. Qu and Y. H. Lin, Nanoscale Horiz., 2019, 4, 321–338 RSC.
- X. H. Chang, Y. Zhang, M. Tang and B. Wang, Nanoscale Res. Lett., 2013, 8, 1–10 CrossRef PubMed.
- Y. C. Zhao, J. Y. Xu and X. Y. Jiang, Environ. Sci. Technol., 2021, 55, 4037–4044 CrossRef CAS.
- B. Wu, L. Chen, X. M. Wu, H. Hou, Z. Z. Wang and S. Liu, Environ. Sci.: Nano, 2019, 6, 1594–1606 RSC.
- L. G. Chen, Y. Y. Guo, C. Y. Hu, P. K. S. Lam, J. C. W. Lam and B. S. Zhou, Environ. Pollut., 2018, 234, 307–317 CrossRef CAS.
- R. Limage, E. Tako, N. Kolba, Z. Y. Guo, A. Garcia-Rodriguez, C. N. H. Marques and G. J. Mahler, Small, 2020, 16, 2000601 CrossRef CAS PubMed.
- C. C. Kurtz, S. Mitchell, K. Nielsen, K. D. Crawford and S. R. Mueller-Spitz, J. Appl. Toxicol., 2020, 40, 1384–1395 CrossRef CAS.
- J. Li, S. M. Yang, R. H. Lei, W. H. Gu, Y. X. Qin, S. H. Ma, K. Chen, Y. A. Chang, X. Bai, S. B. Xia, C. M. Wu and G. M. Xing, Nanoscale, 2018, 10, 7736–7745 RSC.
- Z. L. Mao, Y. Q. Li, T. Y. Dong, L. N. Zhang, Y. Q. Zhang, S. S. Li, H. T. Hu, C. F. Sun and Y. K. Xia, Nanoscale Res. Lett., 2019, 14, 26 CrossRef.
- K. M. Pondman, C. Salvador-Morales, B. Paudyal, R. B. Sim and U. Kishore, Nanoscale Horiz., 2017, 2, 174–186 RSC.
- H. Q. Chen, R. F. Zhao, B. Wang, L. N. Zheng, H. Ouyang, H. L. Wang, X. Y. Zhou, D. Zhang, Z. F. Chai, Y. L. Zhao and W. Y. Feng, Adv. Healthcare Mater., 2018, 7, 1701313 CrossRef.
- H. Q. Chen, B. Wang, D. Gao, M. Guan, L. N. Zheng, H. Ouyang, Z. F. Chai, Y. L. Zhao and W. Y. Feng, Small, 2013, 9, 2735–2746 CrossRef CAS PubMed.
- X. X. Liu, Y. Z. Liu, X. W. Chen, C. L. Wang, X. H. Chen, W. Liu, K. Q. Huang, H. L. Chen and J. Yang, Toxicology, 2020, 435, 152410 CrossRef CAS.
- M. H. Lahiani, S. Khare, C. E. Cerniglia, R. Boy, I. N. Ivanov and M. Khodakovskaya, Nanoscale, 2019, 11, 3639–3655 RSC.
- Y. C. Xie, B. Wu, X. X. Zhang, J. B. Yin, L. Mao and M. J. Hu, Chemosphere, 2016, 144, 1306–1312 CrossRef CAS.
- H. Q. Chen, D. Gao, B. Wang, R. F. Zhao, M. Guan, L. N. Zheng, X. Y. Zhou, Z. F. Chai and W. Y. Feng, Nanotechnology, 2014, 25, 165101 CrossRef.
- M. Zheng, J. G. Lu, G. M. Lin, H. L. Su, J. Y. Sun and T. G. Luan, Environ. Pollut., 2019, 254, 112969 CrossRef CAS.
- P. P. Jia, T. Sun, M. Junaid, Y. H. Xiong, Y. Q. Wang, L. Liu, S. Y. Pu and D. S. Pei, Environ. Sci.: Nano, 2019, 6, 2452–2469 RSC.
- J. Li, S. M. Yang, J. Q. Yu, R. L. Cui, R. Liu, R. H. Lei, Y. N. Chang, H. Geng, Y. X. Qin, W. H. Gu, S. B. Xia, K. Chen, J. L. Kong, G. G. Chen, C. M. Wu and G. M. Xing, RSC Adv., 2018, 8, 31366–31371 RSC.
- C. Y. Wang, J. N. Xie, X. H. Dong, L. Q. Mei, M. R. Zhao, Z. W. Leng, H. X. Hu, L. L. Li, Z. J. Gu and Y. L. Zhao, Small, 2020, 16, 1906915 CrossRef CAS.
- S. Durasevic, G. Nikolic, A. Todorovic, D. Drakulic, S. Pejic, V. Martinovic, D. Mitic-Culafic, D. Milic, T. J. Kop, N. Jasnic, J. Dordevic and Z. Todorovic, Food Chem. Toxicol., 2020, 140, 111302 CrossRef PubMed.
- J. Li, R. H. Lei, X. Li, F. X. Xiong, Q. Y. Zhang, Y. Zhou, S. M. Yang, Y. A. Chang, K. Chen, W. H. Gu, C. M. Wu and G. M. Xing, Part. Fibre Toxicol., 2018, 15, 5 CrossRef PubMed.
- H. X. Gu, S. X. Wang, X. H. Wang, X. Yu, M. H. Hu, W. Huang and Y. J. Wang, J. Hazard. Mater., 2020, 397, 122773 CrossRef CAS.
- W. Q. Gu, S. Liu, L. Chen, Y. X. Liu, C. Gu, H. Q. Ren and B. Wu, Environ. Sci. Technol., 2020, 54, 3417–3427 CrossRef CAS.
- J. Ma, G. D. Sheng, Q. L. Chen and P. O'Connor, J. Hazard. Mater., 2020, 387, 122012 CrossRef CAS.
- B. K. Zhu, Y. M. Fang, D. Zhu, P. Christie, X. Ke and Y. G. Zhu, Environ. Pollut., 2018, 239, 408–415 CrossRef CAS.
- J. Nsor-Atindana, Y. X. Zhou, M. N. Saqib, M. S. Chen, H. D. Goff, J. G. Ma and F. Zhong, Food Res. Int., 2020, 131, 108935 CrossRef CAS.
- S. Gangadoo, I. Dinev, J. Chapman, R. J. Hughes, T. T. H. Van, R. J. Moore and D. Stanley, Appl. Microbiol. Biotechnol., 2018, 102, 1455–1466 CrossRef CAS PubMed.
- Y. L. Xu, H. L. Mao, C. M. Yang, H. H. Du, H. F. Wang and J. E. Tu, J. Anim. Physiol. Anim. Nutr., 2020, 104, 597–605 CrossRef CAS.
- B. Hu, S. J. Yu, C. Shi, J. Gu, Y. Shao, Q. Chen, Y. Q. Li and R. Mezzenga, ACS Nano, 2020, 14, 2760–2776 CrossRef CAS.
- Y. Lee, K. Sugihara, M. G. Gillilland, S. Jon, N. Kamada and J. J. Moon, Nat. Mater., 2020, 19, 118–126 CrossRef CAS.
- L. J. Tong, H. N. Hao, X. Y. Zhang, Z. Zhang, Y. Y. Lv, L. W. Zhang and H. X. Yi, Mol. Nutr. Food Res., 2020, 64, 1901251 CrossRef CAS.
- J. Gigault, A. ter Halle, M. Baudrimont, P. Y. Pascal, F. Gauffre, T. L. Phi, H. El Hadri, B. Grassl and S. Reynaud, Environ. Pollut., 2018, 235, 1030–1034 CrossRef CAS.
- C. J. Rhodes, Sci. Progress, 2018, 101, 207–260 CrossRef.
- J. Gigault, H. El Hadri, B. Nguyen, B. Grassl, L. Rowenczyk, N. Tufenkji, S. Y. Feng and M. Wiesner, Nat. Nanotechnol., 2021, 16, 501–507 CrossRef CAS.
- Y. Chae, D. Kim, M. J. Choi, Y. Cho and Y. J. An, Environ. Int., 2019, 130, 104848 CrossRef CAS.
- Y. Chae and Y. J. An, Environ. Sci.: Nano, 2020, 7, 975–983 RSC.
- R. X. Qiao, Y. F. Deng, S. H. Zhang, M. B. Wolosker, Q. D. Zhu, H. Q. Ren and Y. Zhang, Chemosphere, 2019, 236, 124334 CrossRef CAS PubMed.
- H. Tao, N. Lavoine, F. Jiang, J. T. Tang and N. Lin, Nanoscale Horiz., 2020, 5, 607–627 RSC.
- R. T. Cha, Z. B. He and Y. H. Ni, Carbohydr. Polym., 2012, 88, 713–718 CrossRef CAS.
- F. Wahid, Y. X. Duan, X. H. Hu, L. Q. Chu, S. R. Jia, J. D. Cui and C. Zhong, Int. J. Biol. Macromol., 2019, 132, 692–700 CrossRef CAS.
- J. C. Yang, M. D. Du, L. Wang, S. X. Li, G. R. Wang, X. L. Yang, L. J. Zhang, Y. Fang, W. F. Zheng, G. Yang and X. Y. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 33049–33059 CrossRef CAS.
- Y. Liu, K. Y. Long, H. B. Mi, R. T. Cha and X. Y. Jiang, Nanoscale Horiz., 2019, 4, 953–959 RSC.
- C. L. Zhang, Y. P. Zhang, R. T. Cha, K. Y. Long, J. J. Li and X. Y. Jiang, ACS Sustainable Chem. Eng., 2019, 7, 15404–15412 CrossRef CAS.
- S. L. Cheng, Y. P. Zhang, R. T. Cha, J. L. Yang and X. Y. Jiang, Nanoscale, 2016, 8, 973–978 RSC.
- C. L. Zhang, R. T. Cha, R. Y. Li, L. X. Tang, K. Y. Long, Z. L. Zhang, L. Zhang and X. Y. Jiang, ACS Sustain. Chem. Eng., 2020, 8, 7774–7784 CrossRef CAS.
- W. S. Hao, M. Z. Wang, F. S. Zhou, H. Z. Luo, X. Xie, F. L. Luo and R. T. Cha, Carbohydr. Polym., 2020, 243, 116466 CrossRef CAS.
- J. J. Li, R. T. Cha, K. W. Mou, X. H. Zhao, K. Y. Long, H. Z. Luo, F. S. Zhou and X. Y. Jiang, Adv. Healthcare Mater., 2018, 7, 1800334 CrossRef.
- J. J. Li, R. T. Cha, H. Z. Luo, W. S. Hao, Y. Zhang and X. Y. Jiang, Biomaterials, 2019, 223, 119474 CrossRef CAS.
- K. Y. Long, R. T. Cha, Y. P. Zhang, J. J. Li, F. P. Ren and X. Y. Jiang, Cellulose, 2018, 21, 463–471 CrossRef.
- B. Sun, F. Kong, M. Zhang, W. Wang, K. B. Singh, J. Tjong and M. Sain, Paper Biomater., 2019, 4, 1–14 CAS.
- K. W. Mou, J. J. Li, Y. Y. Wang, R. T. Cha and X. Y. Jiang, J. Mater. Chem. B, 2017, 5, 7876–7884 RSC.
- S. Khare, G. M. DeLoid, R. M. Molina, K. Gokulan, S. P. Couvillion, K. J. Bloodsworth, E. K. Eder, A. R. Wong, D. W. Hoyt, L. M. Bramer, T. O. Metz, B. D. Thrall, J. D. Brain and P. Demokritou, NanoImpact, 2020, 18, 100216 CrossRef.
- T. Nagano and H. Yano, Biosci., Biotechnol., Biochem., 2020, 84, 613–620 CrossRef CAS PubMed.
- S. Gangadoo, B. W. Bauer, Y. S. Bajagai, T. T. H. Van, R. J. Moore and D. Stanley, Anim. Nutr., 2019, 5, 424–431 CrossRef PubMed.
- C. L. Xu, Y. Guo, L. Qiao, L. Ma, Y. Y. Cheng and A. Roman, Front. Microbiol., 2018, 9, 1129 CrossRef.
- X. J. Zheng, J. Y. Zhu, X. Zhang, M. Cheng, Z. C. Zhang and J. X. Cao, J. Food Biochem., 2018, 42, e12501 CrossRef.
- C. Zhou, L. Z. Li, T. M. Li, L. H. Sun, J. H. Yin, H. D. Guan, L. C. Wang, H. B. Zhu, P. Xu, X. Fan, B. F. Sheng, W. D. Xiao, Y. Qiu and H. Yang, J. Mol. Med., 2020, 98, 1189–1202 CrossRef CAS PubMed.
- S. K. Lai, Y. Y. Wang and J. Hanes, Adv. Drug Delivery Rev., 2009, 61, 158–171 CrossRef CAS PubMed.
- Y. Liu, X. J. Yu, J. X. Zhao, H. Zhang, Q. X. Zhai and W. Chen, Int. J. Biol. Macromol., 2020, 164, 884–891 CrossRef CAS PubMed.
- C. Bao, B. Liu, B. Li, J. J. Chai, L. W. Zhang, L. L. Jiao, D. Li, Z. Q. Yu, F. Z. Ren, X. H. Shi and Y. Li, Nano Lett., 2020, 20, 1352–1361 CrossRef PubMed.
- Z. B. Akkus, I. Nazir, A. Jalil, M. Tribus and A. Bernkop-Schnurch, Mol. Pharmaceutics, 2019, 16, 2817–2825 CrossRef CAS PubMed.
- X. Zhang, W. Dong, H. B. Cheng, M. X. Zhang, Y. Q. Kou, J. Guan, Q. Y. Liu, M. Y. Gao, X. H. Wang and S. R. Mao, Asian J. Pharm. Sci., 2019, 14, 543–551 CrossRef PubMed.
- G. N. Jeong, U. B. Jo, H. Y. Ryu, Y. S. Kim, K. S. Song and I. J. Yu, Arch. Toxicol., 2010, 84, 63–69 CrossRef CAS.
- S. Kachenton, V. Whangpurikul, N. Kangwanrangsan, T. Tansatit and W. Jiraungkoorskul, Int. J. Nanosci., 2018, 17, 1850007 CrossRef CAS.
- A. Georgantzopoulou, T. Serchi, S. Cambier, C. C. Leclercq, J. Renaut, J. Shao, M. Kruszewski, E. Lentzen, P. Grysan, S. Eswara, J. N. Audinot, S. Contal, J. Ziebel, C. Guignard, L. Hoffmann, A. J. Murk and A. C. Gutleb, Part. Fibre Toxicol., 2016, 13, 9 CrossRef.
- M. Dorier, D. Beal, C. Tisseyre, C. Marie-Desvergne, M. Dubosson, F. Barreau, E. Houdeau, N. Herlin-Boime, T. Rabilloud and M. Carriere, Environ. Sci.: Nano, 2019, 6, 1549–1561 RSC.
- P. Talbot, J. M. Radziwill-Bienkowska, J. B. J. Kamphuis, K. Steenkeste, S. Bettini, V. Robert, M. L. Noordine, C. Mayeur, E. Gaultier, P. Langella, C. Robbe-Masselot, E. Houdeau, M. Thomas and M. Mercier-Bonin, J. Nanobiotechnol., 2018, 16, 53 CrossRef.
- J. Domenech, A. Hernandez, E. Demir, R. Marcos and C. Cortes, Sci. Rep., 2020, 10, 2793 CrossRef CAS PubMed.
- M. Liu, J. Zhang, X. Zhu, W. Shan, L. Li, J. J. Zhong, Z. R. Zhang and Y. Huang, J. Controlled Release, 2016, 222, 67–77 CrossRef CAS PubMed.
- H. Y. Lu, G. Z. Yang, F. Ran, T. B. Gao, C. S. Sun, Q. F. Zhao and S. L. Wang, Carbohydr. Polym., 2020, 229, 115508 CrossRef CAS PubMed.
- S. P. Bandi, Y. S. Kumbhar and V. V. K. Venuganti, J. Nanopart. Res., 2020, 22, 62 CrossRef CAS.
- D. Magri, P. Sanchez-Moreno, G. Caputo, F. Gatto, M. Veronesi, G. Bardi, T. Catelani, D. Guarnieri, A. Athanassiou, P. P. Pompa and D. Fragouli, ACS Nano, 2018, 12, 7690–7700 CrossRef CAS.
- H. Sinnecker, T. Krause, S. Koelling, I. Lautenschlager and A. Frey, Beilstein J. Nanotechnol., 2014, 5, 2092–2101 CrossRef PubMed.
- C. Ban, M. Jo, Y. H. Park, J. H. Kim, J. Y. Han, K. W. Lee, D. H. Kweon and Y. J. Choi, Food Chem., 2020, 302, 125328 CrossRef CAS PubMed.
- D. T. Pham, C. Tetyczka, S. Hartl, M. Absenger-Novak, E. Frohlich, W. Tiyaboonchai and E. Roblegg, J. Drug Delivery Sci. Technol., 2020, 57, 101484 CrossRef CAS.
- H. Guo, T. Gong and Y. Huang, Chin. Pharmacol. J, 2014, 49, 1146–1151 CAS.
- Y. L. Mao, S. Feng, X. J. Zhang, Q. F. Zhao, Y. Fang and S. L. Wang, J. Drug Delivery Sci. Technol., 2019, 53, 101184 CrossRef CAS.
- S. R. Zhou, H. L. Deng, Y. Zhang, P. Y. Wu, B. He, W. B. Dai, H. Zhang, Q. Zhang, R. S. Zhao and X. Q. Wang, Mol. Pharmaceutics, 2020, 17, 239–250 CrossRef CAS PubMed.
- N. G. Lamson, A. Berger, K. C. Fein and K. A. Whitehead, Nat. Biomed. Eng., 2020, 4, 84–96 CrossRef CAS PubMed.
- R. Cornu, C. Chretien, Y. Pellequer, H. Martin and A. Beduneau, Arch. Toxicol., 2020, 94, 1191–1202 CrossRef CAS PubMed.
- S. N. Zhao, J. H. Li, F. Z. Wang, T. Yu, Y. Zhou, L. L. He, Y. Zhang and J. Yang, Chin. Chem. Lett., 2020, 31, 1147–1152 CrossRef CAS.
- S. E. Orr, K. Gokulan, M. Boudreau, C. E. Cerniglia and S. Khare, J. Nanobiotechnol., 2019, 17, 63 CrossRef PubMed.
- R. Pecoraro, F. Marino, A. Salvaggio, F. Capparucci, G. Di Caro, C. Iaria, A. Salvo, A. Rotondo, D. Tibullo, G. Guerriero, E. M. Scalisi, M. Zimbone, G. Impellizzeri and M. V. Brundo, Front. Physiol., 2017, 8, 1011 CrossRef.
- C. Wang, L. G. Zhang, Z. X. Ying, J. T. He, L. Zhou, L. L. Zhang, X. Zhong and T. Wang, Biol. Trace Elem. Res., 2018, 185, 364–374 CrossRef CAS PubMed.
- C. Wang, L. G. Zhang, W. P. Su, Z. X. Ying, J. T. He, L. L. Zhang, X. Zhong and T. Wang, PLoS One, 2017, 12, e0181136 CrossRef.
- T. Titma, R. Shimmo, J. Siigur and A. Kahru, Cytotechnology, 2016, 68, 2363–2377 CrossRef CAS.
- V. C. Ude, D. M. Brown, V. Stone and H. J. Johnston, J. Nanobiotechnol., 2019, 17, 70 CrossRef PubMed.
- T. E. Henson, J. Navratilova, A. H. Tennant, K. D. Bradham, K. R. Rogers and M. F. Hughes, Nanotoxicology, 2019, 13, 795–811 CrossRef CAS.
- P. Pedata, G. Ricci, L. Malorni, A. Venezia, M. Cammarota, M. G. Volpe, N. Iannaccone, V. Guida, C. Schiraldi, M. Romano and G. Iacomino, Food Res. Int., 2019, 119, 634–642 CrossRef CAS.
- J. W. Richter, G. M. Shull, J. H. Fountain, Z. Y. Guo, L. P. Musselman, A. C. Fiumera and G. J. Mahler, Nanotoxicology, 2018, 12, 390–406 CrossRef CAS PubMed.
- Z. Y. Guo, N. J. Martucci, F. Moreno-Olivas, E. Tako and G. J. Mahler, NanoImpact, 2017, 5, 70–82 CrossRef.
- E. Brun, F. Barreau, G. Veronesi, B. Fayard, S. Sorieul, C. Chaneac, C. Carapito, T. Rabilloud, A. Mabondzo, N. Herlin-Boime and M. Carriere, Part. Fibre Toxicol., 2014, 11, 13 CrossRef PubMed.
- M. Adeel, C. X. Ma, S. Ullah, M. Rizwan, Y. Hao, C. Y. Chen, G. Jilani, N. Shakoor, M. S. Li, L. H. Wang, D. C. W. Tsang, J. Rinklebe, Y. K. Rui and B. S. Xing, Environ. Pollut., 2019, 254, 113032 CrossRef CAS.
- L. Yu, X. Tian, D. X. Gao, Y. Lang, X. X. Zhang, C. Yang, M. M. Gu, J. M. Shi, P. K. Zhou and Z. F. Shang, Nanotoxicology, 2019, 13, 1409–1421 CrossRef CAS.
- C. L. Xu, L. Qiao, L. Ma, Y. Guo, X. N. Dou, S. Q. Yan, B. H. Zhang and A. Roman, Int. J. Nanomed., 2019, 14, 4491–4502 CrossRef CAS PubMed.
- A. P. Singh, M. B. Mia and R. K. Saxena, J. Biosciences, 2020, 45, 23 CrossRef CAS.
- S. Liu, X. M. Wu, W. Q. Gu, J. Yu and B. Wu, Chemosphere, 2020, 256, 127204 CrossRef CAS PubMed.
- C. Cortes, J. Domenech, M. Salazar, S. Pastor, R. Marcos and A. Hernandez, Environ. Sci.: Nano, 2020, 7, 272–285 RSC.
- A. Thubagere and B. M. Reinhard, ACS Nano, 2010, 4, 3611–3622 CrossRef CAS PubMed.
- J. Domenech, A. Hernandez, L. Rubio, R. Marcos and C. Cortes, Arch. Toxicol., 2020, 94, 2997–3012 CrossRef CAS.
- A. Singh, Nat. Mater., 2020, 19, 3–4 CrossRef CAS.
- C. Lin, T. C. Kuo, J. C. Lin, Y. C. Ho and F. L. Mi, Int. J. Biol. Macromol., 2020, 160, 558–570 CrossRef CAS.
- M. Sun, D. P. Li, X. Wang, L. He, X. D. Lv, Y. Xu and R. P. Tang, J. Mater. Chem. B, 2019, 7, 3692–3703 RSC.
- Y. Liu, Z. F. Jiang, X. F. Hou, X. M. Xie, J. P. Shi, J. Y. Shen, Y. Z. He, Z. Wang and N. P. Feng, Nanomed. Nanotechnol., 2019, 21, 102075 CrossRef CAS.
- Y. D. Zhang, H. B. Li, Q. Wang, X. Y. Hao, H. M. Li, H. Q. Sun, L. Han, Z. R. Zhang, Q. M. Zou and X. Sun, Adv. Funct. Mater., 2018, 28, 1802675 CrossRef.
- Z. G. Song, J. D. Lv, A. Sheikhahmadi, J. Uerlings and N. Everaert, Biol. Trace Elem. Res., 2017, 180, 306–313 CrossRef CAS PubMed.
- L. Pineda, A. Chwalibog, E. Sawosz, C. Lauridsen, R. Engberg, J. Elnif, A. Hotowy, F. Sawosz, Y. H. Gao, A. Ali and H. S. Moghaddam, Arch. Anim. Nutr., 2012, 66, 416–429 CrossRef CAS PubMed.
- X. P. Wu, Y. F. Zhu, K. Y. Zhang, X. M. Ding, S. P. Bai, J. P. Wang, H. W. Peng and Q. F. Zeng, Poult. Sci., 2019, 98, 3894–3901 CrossRef CAS.
- I. S. Sohal, G. M. DeLoid, K. S. O'Fallon, P. Gaines, P. Demokritou and D. Bello, NanoImpact, 2020, 17, 100209 CrossRef.
- M. Ohno, A. Nishida, Y. Sugitani, K. Nishino, O. Inatomi, M. Sugimoto, M. Kawahara and A. Andoh, PLoS One, 2017, 12, e0185999 CrossRef PubMed.
- T. Y. Wang, H. T. Jiang, Q. F. Zhao, S. L. Wang, M. J. Zou and G. Cheng, Int. J. Pharm., 2012, 436, 351–358 CrossRef CAS.
- L. Liu, Y. X. Gong, B. Zhu, G. L. Liu, G. X. Wang and F. Ling, Fish Shellfish Immunol., 2015, 45, 175–183 CrossRef CAS PubMed.
- Y. Q. Du, Y. F. Xia, Y. J. Zou, Y. N. Hu, J. Q. Fu, J. Wu, X. D. Gao and G. H. Ma, ACS Nano, 2019, 13, 13809–13817 CrossRef CAS.
- X. Dong, J. Liang, A. F. Yang, Z. Y. Qian, D. L. Kong and F. Lv, ACS Appl. Mater. Interfaces, 2019, 11, 4876–4888 CrossRef CAS PubMed.
- E. Berger, P. Colosetti, A. Jalabert, E. Meugnier, O. P. B. Wiklander, J. Jouhet, E. Errazurig-Cerda, S. Chanon, D. Gupta, G. J. P. Rautureau, A. Geloen, S. El-Andaloussi, B. Panthu, J. Rieusset and S. Rome, Mol. Ther.-Meth. Clin. D., 2020, 18, 880–892 CrossRef CAS.
- A. J. Kastl, N. A. Terry, G. D. Wu and L. G. Albenberg, Cell. Mol. Gastroenter., 2020, 9, 33–45 Search PubMed.
- B. S. Ramakrishna, J. Clin. Gastroenterol., 2007, 41, S2–S6 CrossRef.
- R. H. Dong, Y. Liu, L. Mou, J. Q. Deng and X. Y. Jiang, Adv. Mater., 2018, 31, 1805033 CrossRef.
- Y. Liu, Y. Y. Xu, Y. Tian, C. Y. Chen, C. Wang and X. Y. Jiang, Small, 2014, 10, 4505–4520 CrossRef CAS PubMed.
- X. Zhang, H. B. Cheng, W. Dong, M. X. Zhang, Q. Y. Liu, X. H. Wang, J. Guan, H. Y. Wu and S. R. Mao, J. Controlled Release, 2018, 272, 29–38 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |