Open Access Article
Open Access ArticleTransition metal dichalcogenide magnetic atomic chains†
Kai
Zhang
 a,
Xiaojun
Wu
a,
Xiaojun
Wu
 *ab and
Jinlong
Yang
*ab and
Jinlong
Yang
 ab
ab
aHefei National Research Center of Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: xjwu@ustc.edu.cn
bSchool of Chemistry and Materials Sciences, CAS Key Laboratory of Materials for Energy Conversion, Synergetic Innovation of Quantum Information & Quantum Technology, CAS Center for Excellence in Nanoscience, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 20th October 2022
Abstract
Reducing the dimensions of a material to the atomic scale endows them with novel properties that are significantly different from their bulk counterparts. A family of stoichiometric transition metal dichalcogenide (TMD) MX2 (M = Ti to Mn, and X = S to Te) atomic chains is proposed. The results reveal that the MX2 atomic chains, the smallest possible nanostructure of a TMD, are lattice-dynamically stable, as confirmed from their phonon spectra and ab initio molecular dynamics simulations. In contrast to their bulk and two-dimensional (2D) counterparts, the TiX2 atomic chains are nonmagnetic semiconductors, while the VX2, CrX2, and MnX2 chains are unipolar magnetic, bipolar magnetic, and antiferromagnetic semiconductors, respectively. In addition, the VX2, CrX2, and MnX2 chains can be converted via carrier doping from magnetic semiconductors to half metals with reversible spin-polarization orientation at the Fermi level. Of these chains, the MnX2 chains exhibit either ferromagnetic or antiferromagnetic half metallicity depending on the injected carrier type and concentration. The diverse and tunable electronic and magnetic properties in the MX2 chains originate, based on crystal field theory, from the occupation of the metal d orbitals and the exchange interaction between the tetrahedrally coordinated metal atoms in the atomic chain. The calculated interaction between the carbon nanotubes and the MX2 chains implies that armchair (7,7) or armchair (8,8) carbon nanotubes are appropriate sheaths for growing MX2 atomic single-chains in a confined channel. This study reveals the diverse magnetic properties of MX2 atomic single-chains and provides a promising building block for nanoscale electronic and spintronic devices.
1. Introduction
Reducing the dimensionality of bulk materials has emerged as a practical route to engineer the properties of materials and create a wave of exciting research in two-dimensional (2D) materials.1 One example is atomically thin 2D graphene, which exhibits intriguing physical properties.2 Inspired by the discoveries of 2D atom-thin materials, atom-thin one-dimensional (1D) wires (or atomic chains), made from silicon,3 carbon,4 pnictogens,5 chalcogens,6,7 iodine,8 metal chains,9,10 semiconducting compounds,11,12 metal oxides,13 oxyhydroxides,14 and molecular wires,15–17 have been explored. These materials have received enormous attention for both their exotic electronic properties, such as strong quantum confinement,18,19 novel edge states,20,21 polarization,22,23 and their wide array of applications in nanoconnectors,10,24 transistors,25 optoelectronics,26 catalysts,27,28 and spintronics.15,29Transition metal dichalcogenides (TMDs) are a class of layered van der Waals material with versatile electronic and magnetic properties.30 In the past few years, 2D TMDs, which have different properties to those of bulk TMDs, have been widely investigated due to their unique combination of atomic thickness, direct band gap, strong spin–orbital coupling and enhanced catalytic properties.30,31 Meanwhile, efforts also have been devoted to the reduction of the dimensionality of TMD materials to create atomic chains.32,33 Experimentally, various ultrathin wires based on transition metal chalcogenides, such as transition metal monochalcogenides (TMM),34–38 transition metal trichalcogenides (TMT),39–41 CrSbSe3,42 Mo2S5,21 Mo5S4,43 Mo2Se5 ultrathin wires,24 single-unit-cell Cu9S5 wires,44 and segmented linear chains,23 have been synthesized. Fascinating physical and chemical properties, such as an anisotropic optical response,35 oscillating edge states,21 torsional waves,41 nontrivial spin-polarization,23 and high electrocatalytic activity,44 have been observed. Nevertheless, stoichiometric TMD atomic chains have rarely been reported.
Herein, we reported a family of stable 1D stoichiometric TMDs MX2 (M = Ti to Mn, and X = S to Te) atomic chains via the use of first-principles calculations. Stable MX2 atomic chains consist of a metal backbone, which is tetrahedral coordinated with a chalcogen exterior cap. The computed formation energies of these MX2 chains are on the same order as those of their 2D counterparts. With the exception of the TiX2 chain, the 1D MX2 chains are either ferromagnetic (FM) or antiferromagnetic (AFM) semiconductors, depending on the occupation of metal d orbital. Meanwhile, a transition from a magnetic semiconductor to a half-metal with a tunable magnetic ground state and coupling strength can be achieved via electron or hole doping. The VTe2 chain has a large magnetic anisotropic energy (MAE) of 3.78 meV per metal and strong inter-chain FM coupling. Finally, the MX2 chains may possibly be realized experimentally by growing them as armchair (7,7) or armchair (8,8) carbon nanotubes (CNT). The semiconducting character of TMD atomic chains together with their tunable magnetic and electronic properties make them promising building blocks for use in optoelectronics and spintronics.
2. Results and discussion
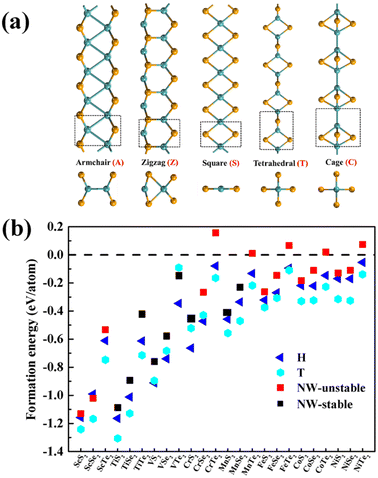
As illustrated in Fig. 1a, different configurations of 1D MX2 (M = transition metal atoms from Sc to Ni; X = S, Se, or Te) atomic chains, including the armchair (A-type), zigzag (Z-type), planar quadrilateral (Q-type), tetrahedral (T-type), and cage-like (C-type)45 atomic arrangements, were investigated to determine the most stable structures. The calculated energy differences between different configurations vary from 0.04 to 2.62 eV per metal atom, as summarized in Table S1 (see ESI†). According to the calculated energies, TiX2, VX2, CrX2, MnS2, MnSe2, FeS2, and FeSe2 chains prefer T-type configurations, while MnTe2, FeTe2, ScX2, CoX2, ad NiX2 chains prefer Z-type configurations. Here, only the most energy favourable configurations were considered to explore their stability. Note that the tetrahedral coordination geometry widely exists in binary compounds (e.g. the zinc blende crystals) and the repeat units have been observed in metal chalcogenide molecules and clusters.46–50The optimized lattice constants range from 5.13 to 6.72 Å and from 3.19 to 4.09 Å for T-type and Z-type MX2 atomic chains, respectively (Table S2†). The variation of the atomic radius is consistent with the calculated lattice constants. The lattice stability of the 1D MX2 atomic chains was further investigated through phonon dispersion spectra calculations and ab initio molecular dynamics simulations (AIMD). From Fig. S1† we can see that TiX2, VX2, CrS2, MnX2, and NiTe2 atomic chains are lattice-dynamically stable as they lack an imaginary vibrational mode. In addition, AIMD simulations were performed at a temperature of 300 K for 5 ps to check the thermodynamical stability of the MX2 chains. A supercell containing five repeat units was used. From Fig. S2† we can see that only the T-type atomic chains, TiX2, VX2, CrX2, and MnX2, can retain their 1D lattice structures at 300 K without significant distortion and that the maximum fluctuation of the total energy is 0.06 eV per atom. This implies that their structures are thermodynamically stable at room temperature. The atomic coordinates of these nine stable chains are summarized in Table S3†.
The thermal stability of the MX2 atomic chains can be understood using the M–X bond strength and formation energy. As summarized in Table 1, Bader charge analysis indicates that about 0.88 to 1.93 electrons are moved from the metal onto the chalcogens.51 These values are larger than those in TMM chains,52 resulting in smaller bond lengths between the metal and chalcogen,53 and thus stronger bonding in the MX2 chains. In addition, the average formation energies per metal atom of the MX2 chains, defined as Ef = (ENW(M2X4) − 2E(M) − 4E(X))/6, were also calculated. ENW(MX2), E(M), and E(X) represent the total energies of the atomic chain and the metal atoms calculated from their most stable bulk or molecular phases. The calculated values of Ef for the MX2 atomic chains are negative, with the exception of the MTe2 atomic chains, implying that the synthesis of MX2 atomic chains from their bulk precursors is exothermic. The calculated Ef increases monotonically with the atomic number of the metals or X, varying from S to Te. The formation energies of nine stable T-type MX2 chains are comparable with those of their 2D MX2 counterparts and previously-synthesized 1D TMM and TMT wires (Fig. 1b and Tables S2 and S4†).53 In particular, nine stable T-type MX2 chains have more negative formation energies than an experimentally synthesized T-phase CrTe2 nanosheet54 and single-chain NWs made from W6Te6, VTe3 and NbTe3 (Fig. 1b),38,39,53 implying that the creation of T-type MX2 atomic chains is experimentally feasible.
| Chains | D | B | C | M | GS | E g |
|---|---|---|---|---|---|---|
| TiS2 | 3.09 | 2.29 | 1.93 | 0.0 | NM-SC | 2.39 (D) |
| TiSe2 | 3.19 | 2.41 | 1.78 | 0.0 | NM-SC | 1.96 (D) |
| TiTe2 | 3.36 | 2.64 | 1.56 | 0.0 | NM-SC | 1.31 (D) |
| VS2 | 2.86 | 2.21 | 1.18 | 0.9 | FM-SC | 1.34 (D) |
| VSe2 | 2.95 | 2.35 | 1.37 | 1.0 | FM-SC | 0.67 (D) |
| VTe2 | 3.09 | 2.56 | 1.08 | 1.2 | FM-SC | 1.26 (D) |
| CrS2 | 2.66 | 2.15 | 1.23 | 2.0 | FM-SC | 1.32 (In) |
| MnS2 | 2.62 | 2.11 | 1.04 | 1.2 | AFM-SC | 1.64 (In) |
| MnSe2 | 2.71 | 2.25 | 0.88 | 1.3 | AFM-SC | 1.07 (D) |
The magnetic configurations of four 1D MX2 chains, including nonmagnetic (NM), FM, and AFM orderings, were considered when determining the magnetic ground state (Fig. S3†). As summarized in Table S5,† the TiX2 chains have NM ground states, while the MnS2 and MnSe2 chains have AFM-1 ground states where the FM-AFM energy differences are equal to 540 and 709 meV per metal atom at the HSE06 level, respectively. In contrast, the VS2, VSe2, VTe2, and CrS2 chains have FM ground states with energy differences ranging from 62 to 426 meV per metal atom. The plotted spin charge density profiles in Fig. S4† reveal that the spin polarization is predominantly distributed on the central metal atom. As shown in Table 1, the local magnetic moments at the metal atom are 0.9 (VS2), 1.0 (VSe2), 1.2 (VTe2), 2.0 (CrS2), 1.2 (MnS2), and 1.3 (MnSe2) μB, respectively.
From Fig. 2 and S5† we can see that all MX2 chains are either direct or indirect band gap semiconductors where the values of the band gap range from 0.67 to 2.39 eV, as summarized in Table 1. TiX2 chains are NM semiconductors with a direct band gap ranging from 1.31 to 2.39 eV. The MnS2 chain is an AFM indirect band gap semiconductor, while MnSe2 is an AFM direct band gap semiconductor. The VX2 and CrX2 chains are unipolar magnetic semiconductors (UMS) and bipolar magnetic semiconductors (BMS).55
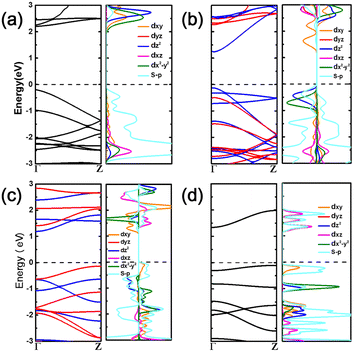 | ||
| Fig. 2 Electronic structures of (a) TiS2, (b) VS2, (c) CrS2, and (d) MnS2 atomic chains, respectively. The spin up and down states are indicated in red and blue colors in the band structure. | ||
The projected density of states (PDOS) and the partial charge density of the valence band maximum (VBM) and conduction band minimum (CBM) were plotted to reveal the contributions of the metal and chalcogen atoms (Fig. 2 and S6†). The orbital energy levels and distributions indicate that the VBM and CBM of the TiX2 chains consist of chalcogen and Ti orbitals, respectively, while the orbitals of both the chalcogens and the Cr atoms contribute to the VBM and CBM of the CrS2 chain. Conversely, the CBM of the VX2 chains are generated, with the exception of the VTe2 chain which is formed from Te orbitals, from the orbitals of the chalcogens. The MnX2 chains consist of orbitals from both the Mn and chalcogen atoms. Meanwhile, the VBM states of the VX2 and MnX2 chains receive contributions from both the metal and chalcogen orbitals.
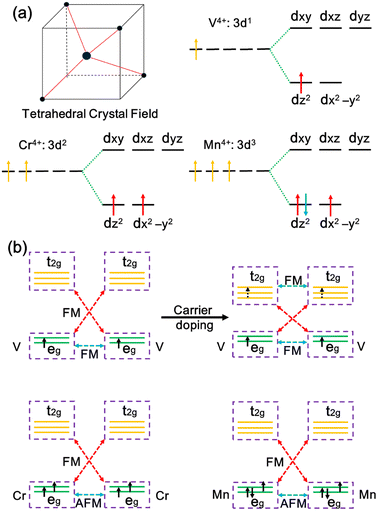
According to crystal field theory, the metal ions in MX2 chains are tetrahedrally coordinated, and the five d orbitals will split into degenerate eg (dx2−y2, dz2) and t2g (dxy, dxz, dyz) orbitals, as illustrated in Fig. 3a. Note that Ti, V, Cr, and Mn atoms in the MX2 chains have a +4 oxidation state with their 3d electron configurations being 3d0, 3d1, 3d2, and 3d3, respectively. The eg orbitals are occupied with one, two, and three electrons, generating one, two, and one unpaired 3d electrons. Thus, 1, 2, and 1 μB magnetic moments are located on the metals in the VX2, CrX2, and MnX2 chains, respectively.
The magnetic ground state in the MX2 chains relies on the occupation of the metal d orbital and can be interpreted by exchange interactions between the metal ions. In the MX2 chains, the metal–ligand–metal bonding angle varies from 74.5 to 85.0°, close to 90°, leading to the coexistence of superexchange and direct exchange interactions between the metal ions.56 As illustrated in Fig. 3b, the lower energy eg orbitals are half-filled while the higher energy t2g orbitals of the Cr ion are empty. As a result, the direct exchange interaction between the half-filled orbitals is AFM, and the virtual hopping between the half-filled eg and empty t2g orbitals via a superexchange interaction is FM according to the Goodenough–Kanamori–Anderson (GKA) rules.57,58 Given that the FM superexchange interaction between the half-filled eg orbitals and empty t2g orbitals is generally stronger than AFM direct exchange in a tetrahedral crystal, FM coupling is induced overall in the CrS2 chain.59 As for the VX2 chains, in addition to the FM coupling driven by superexchange interactions, direct exchange, i.e., real hopping between partially occupied eg orbitals, is also FM.60,61 Therefore, FM coupling is induced in the VX2 chains. However, the direct exchange interactions in the MnX2 chains are enhanced because the eg orbitals are more than half-filled and there is a decreased distance between the metal ions. Thus, the AFM coupling between the eg orbitals dominates over the FM virtual hopping, resulting in an AFM ground state.
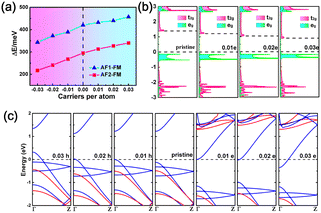
As the exchange interaction between the metal ions is related to the occupation of the metal d orbitals, the magnetic coupling strength and magnetic ground state of the MX2 chains may be tuned by controlling the number of occupied electrons in the metal d orbitals. Next, the electronic structures and magnetic coupling tunability of MX2 chains with carrier doping were investigated. A carrier concentration of up to 9.3 × 1013 cm−2 (0.03 carriers per atom) was used, a level which is experimentally reachable.62 As illustrated in Fig. 4a and S7,† the VX2, CrS2, and MnS2 chains retain their ground state magnetic order following carrier doping. However, an AFM to FM transition is observed in the MnSe2 chain when doping with 0.02 or 0.03 holes per atom. In addition, the energy differences in the VX2 chains increase monotonically with the total electron concentration, as illustrated in Fig. 4a and S7,† which can be explained by the aforementioned exchange interaction mechanism. Taking the VS2 chain as an example, the doped electrons partially occupy the empty t2g orbitals, as depicted in Fig. 2b. Compared with a pristine VS2 chain, the extra FM exchange interactions between the t2g orbitals arising from the doped electrons enlarge the energy differences between the FM and AFM states in the VS2 chain.61 Meanwhile, the gap between the eg and t2g levels narrows when the VS2 chain is doped with electrons, as illustrated in Fig. 4b, which further enhances the virtual hopping between the eg and t2g orbitals.56 On the contrary, after hole injection, fewer electrons occupying the eg orbitals weakens the strength of the FM coupling induced by superexchange and direct exchange, and thus the energy difference between the AFM and FM states is decreased. As for the CrS2 and MnX2 chains, the decrease in occupied electrons shrinks the AFM exchange interaction due to there being no eg orbitals less than half-filled.
Fig. 4c and S8 to S10† show the band structures of the MX2 chains doped with electrons or holes. Transitions from semiconductor to metal are observed in the carrier-doped MX2 chains. The VX2, CrS2, and MnX2 chains are transformed into half metals (HM) following carrier injection. The VX2 chains can be converted from UMS to FM HM via carrier injection. In particular, hole (electron) doping in CrS2 and MnS2 chains would lead to the transition from a BMS and AFM semiconductor to a FM HM and AFM HM with reversible spin-polarization orientation, respectively. However, the MnSe2 chain can be switched from an AFM semiconductor to an AFM HM with electron doping or doping with no more than 0.01 hole per atom. Meanwhile, a FM HM can be induced in a MnSe2 chain by doping with 0.02 or more holes per atom. The results demonstrate the potential that MX2 chains hold for use in electronically controlled spintronics applications.
It has been accepted that no long-range magnetic ordering can exist in infinite strictly 1D isotropic systems above 0 K.63 However, thermal fluctuation is possibly blocked by strong inter-chain coupling and large magnetic anisotropic energy (MAE) and thus, long-range magnetic order could be stabilized.15,17,29,64 For example, Pt substrate supported 1D Co atomic chains present long-range magnetic coupling below 15 K due to a large MAE of 2.0 meV per Co atom.29 Our results indicate the easy axes (EA) of the MX2 chains are parallel to the axial direction, except in the CrS2 and MnS2 chains where they are perpendicular to the axial direction (Table S6†). The calculated MAE values vary from 0.019 to 3.78 meV per metal. Among them, the MAE value of the VTe2 chain reaches 3.78 meV per metal, which is larger than those of Co atomic chains29 and Fe-benzenetetramine coordination polymers.65 Furthermore, the inter-chain interactions of the MX2 chains were also studied. As illustrated in Fig. S11,† MX2 bundles are arranged in AA-, AB-, AC-, and AD-stacking configurations to determine the most energy favorable configuration. The total energies per metal of these bundles are summarized in Table S7.† AD-stacked MX2 bundles are energetically preferred, with inter-chain coupling energies varying from −166 to −318 meV per metal atom and inter-chain distances ranging from 3.59 to 4.12 Å. The inter-chain coupling of VS2, VSe2, and CrS2 chains are AFM with energy differences of −0.3, −20.6, and −14.4 meV per metal, respectively. Meanwhile, the VTe2 chains are FM coupled with an FM-AFM energy difference of 27.3 meV per metal atom. This difference is distinctly larger than those of the MnH3,15 V(Bz), and Mn(Bz) bundles,64 indicating the feasibility of maintaining their FM order at nonzero temperatures.
Motivated by experimentally observed torsional waves in single-chain nanowires,41 here we used the TiS2 atomic chain as a prototype to investigate the atomic and electronic structures of a twisted single chain with a variable rotation angle. Twisted nanowires with 15°, 30°, 45°, 60°, 75°, and 90° rotation angles are displayed in Fig. S12.† First, we calculated the variation of the rotation energy, i.e., ENW(θ) − ENW(θ = 0) as a function of the rotation angle. The results demonstrate that the total energy of the twisted TiS2 nanowire increases monotonically with the rotation angle, and that there is a high energy barrier of 1.4 eV per metal atom between the square and tetrahedral configurations (Fig. S13†). Further, from the calculated band structures of the twisted nanowires (Fig. S14†), we can see that the direct band gap decreases from 1.40 (θ = 0) to 0.06 eV (θ = 60°) and then increases into an indirect band gap of 0.15 eV (θ = 75° and 90°), implying that the electronic structure of these nanowires is dependent on the rotation angle.
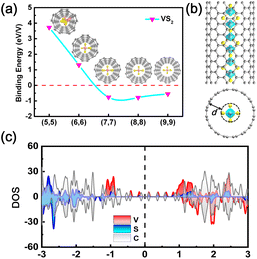
Experimentally, the MX2 chains may be synthesized in ultra-narrow carbon nanotubes (CNTs), analogous with previously reported TMC chains.36,38–41 Here, the suitable diameters of a CNT sheath in which to grow MX2 chains were evaluated by plotting the variation of the binding energy Eb against the diameter of the CNT. The binding energy is defined as Eb = ENW@CNT − ENW − ECNT, where ENW, ECNT, and ENW@CNT are the total energies of the atomic chain, CNT, and their joint system, respectively. The lattice mismatch between the atomic chain and CNT is less than 5.5% (Table S8†). The calculated Eb decreases at first and then increases as the diameter is augmented. This behavior is attributed to the competition between the attraction and repulsion interactions between the chain and CNT (Fig. 5a and S15†). It is clear that insertion of the MX2 chains inside (7,7) or (8,8) CNTs leads to the lowest Eb values, ranging from −0.792 to −0.698 eV per metal, which are on the same order of magnitude as that of NbSe3 inside a CNT.41 Therefore, we believe (7,7) and (8,8) CNTs may be suitable for growing MX2 chains inside them. As presented in Fig. S16,† the equilibrium separation d between the chains and CNTs range from 3.06 to 3.74 Å, and about 0.02 to 0.17 electrons per metal are transferred from the CNT to the chain, as shown in Fig. S17.† A smaller binding energy and larger separation between the chain and CNT than in the case of NbSe3 suggest that the interaction between the MX2 chains and CNT is only a van der Waals interaction.41 Both spin charge density profiles and PDOS analysis indicate that the electronic and magnetic properties of the TiX2 and VX2 chains are preserved inside the (7,7) and (8,8) CNTs, while a transition from a semiconductor to a HM is observed in the CrS2, MnS2, and MnSe2 chains (Fig. 5b and c, S18, and S19†).
Very recently, a new type of platinum-based single-metal-atom chain (SMAC) has been fabricated in a controlled manner via a chemical vapor co-deposition method.66 However, the synthesized Pt-based SMAC differs from our proposed MX2 wires in terms of atomic arrangement, band structure, and magnetic properties. Firstly, although the metal ions in the T-type MX2 chains and Pt-SMAC are both bonded with four chalcogen atoms, the metals in the MX2 and Pt-SMAC structures have tetrahedral and square coordination symmetries, respectively. Secondly, the band structure calculations indicate that the MX2 wires are semiconductors while a metallic behavior is observed in Pt-SMAC. Lastly, Pt-SMAC is intrinsically non-magnetic while the majority of the MX2 wires are magnetic.
3. Conclusions
In conclusion, we have proposed a family of 1D stoichiometric transition metal dichalcogenide (TMD) atomic chains featuring magnetic properties and band gaps. Different from their bulk and 2D counterparts, the TiX2, VX2, CrX2, and MnX2 chains are nonmagnetic semiconductors, unipolar magnetic semiconductors, bipolar magnetic semiconductors, and antiferromagnetic (AFM) semiconductors, respectively. In particular, the VX2, CrX2, and MnX2 chains can be converted by carrier injection from ferromagnetic (FM) or AFM semiconductors to half metals (HMs) with tunable spin-polarization orientation at the Fermi level. The diverse and tunable electronic and magnetic properties in the MX2 chains rely on the occupation of metal d orbitals and the exchange interaction between the tetrahedrally coordinated metal atoms. Of these MX2 chains, VTe2 possesses the largest magnetic anisotropic energy (MAE) of 3.78 meV per metal and the inter-chain coupling is FM, revealing the potential to maintain FM ordering at nonzero temperatures. The calculated interaction between carbon nanotubes (CNTs) and the MX2 chains reveals that armchair (7,7) or armchair (8,8) carbon nanotubes are appropriate sheaths for growing MX2 atomic chains. In addition, both the magnetic and electronic properties of the TiX2 and VX2 chains were maintained inside the CNTs, while a conversion from a semiconductor to a HM is observed in the CrS2, MnS2, and MnSe2 chains. The diverse and tunable electronic and magnetic properties of the MX2 atomic single-chains make them a promising building block in the field of electronics and spintronics.4. Methods
First-principles calculations were performed based on density functional theory as implemented in the Vienna Ab Initio Simulation Package (VASP).67,68 The projector augmented wave (PAW) method was used,69,70 and the generalized gradient approximation (GGA) with Perdew–Burke–Ernzerhof (PBE) parametrization was adopted to deal with exchange–correlation functional.71 The cut-off energy was set to 500 eV. A vacuum space larger than 15 Å was used to eliminate interactions between the adjacent periodic images of the NWs. Brillouin zone integration was performed using a 1 × 1 × 18 Γ-centered grid. The energy and forces convergence criteria were set to 10−6 eV and 10−3 eV Å−1. A DFT-D3 method was adopted to include a van der Waals dispersion correction for the joint systems.72 For accurate calculations of the electronic properties, a well-performed hybrid HSE06 functional was employed.73 Unless stated otherwise, band structures, DOS, charge densities, and magnetic properties were obtained using the HSE06 functional. Spin–orbital coupling calculations were not considered during the structural relaxation but were then added into the MAE calculations by including the interaction between the electron-spin and the orbital angular momentum in the Hamiltonian. To investigate the thermodynamic stability of the designed NWs at the PBE level, the phonon calculations were performed using the PHONOPY code with a finite difference method,74 and Ab Initio Molecular Dynamics (AIMD) simulations were simulated in the NVT ensemble using a Nosé Hoover thermostat at 300 K for 5 ps with a time step of 1 fs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (22073087, 21890751), Innovation Program for Quantum Science and Technology (2021ZD0303302), CAS Project for Young Scientists in Basic Research YSBR-004, the MOST (Grants 2016YFA0200602), the Fundamental Research Funds for the Central University, National Program for Support of Topnotch Young Professional, and the support from Super Computer Centre of the USTCSCC and SCCAS.References
- S. Z. Butler, S. M. Hollen, L. Cao, Y. Cui, J. A. Gupta, H. R. Gutiérrez, T. F. Heinz, S. S. Hong, J. Huang, A. F. Ismach, E. Johnston-Halperin, M. Kuno, V. V. Plashnitsa, R. D. Robinson, R. S. Ruoff, S. Salahuddin, J. Shan, L. Shi, M. G. Spencer, M. Terrones, W. Windl and J. E. Goldberger, ACS Nano, 2013, 7, 2898–2926 CrossRef CAS PubMed.
- K. I. Bolotin, F. Ghahari, M. D. Shulman, H. L. Stormer and P. Kim, Nature, 2009, 462, 196–199 CrossRef CAS.
- E. Do, J. W. Park, O. Stetsovych, P. Jelinek and H. W. Yeom, ACS Nano, 2022, 16, 6598–6604 CrossRef CAS.
- L. Shi, P. Rohringer, K. Suenaga, Y. Niimi, J. Kotakoski, J. C. Meyer, H. Peterlik, M. Wanko, S. Cahangirov, A. Rubio, Z. J. Lapin, L. Novotny, P. Ayala and T. Pichler, Nat. Mater., 2016, 15, 634–639 CrossRef CAS PubMed.
- M. Hart, J. Chen, A. Michaelides, A. Sella, M. S. P. Shaffer and C. G. Salzmann, Inorg. Chem., 2019, 58, 15216–15224 CrossRef CAS.
- P. V. C. Medeiros, S. Marks, J. M. Wynn, A. Vasylenko, Q. M. Ramasse, D. Quigley, J. Sloan and A. J. Morris, ACS Nano, 2017, 11, 6178–6185 CrossRef CAS PubMed.
- T. Fujimori, R. B. dos Santos, T. Hayashi, M. Endo, K. Kaneko and D. Tománek, ACS Nano, 2013, 7, 5607–5613 CrossRef CAS PubMed.
- H.-P. Komsa, R. Senga, K. Suenaga and A. V. Krasheninnikov, Nano Lett., 2017, 17, 3694–3700 CrossRef CAS.
- B. H. Hong, S. C. Bae, C.-W. Lee, S. Jeong and K. S. Kim, Science, 2001, 294, 348–351 CrossRef CAS.
- A. I. Yanson, G. R. Bollinger, H. E. van den Brom, N. Agraït and J. M. van Ruitenbeek, Nature, 1998, 395, 783–785 CrossRef CAS.
- D. Li, S. Hao, G. Xing, Y. Li, X. Li, L. Fan and S. Yang, J. Am. Chem. Soc., 2019, 141, 3480–3488 CrossRef CAS PubMed.
- J.-J. Zhang, J. Guan, S. Dong and B. I. Yakobson, J. Am. Chem. Soc., 2019, 141, 15040–15045 CrossRef CAS PubMed.
- Z. Zhang, T. Murayama, M. Sadakane, H. Ariga, N. Yasuda, N. Sakaguchi, K. Asakura and W. Ueda, Nat. Commun., 2015, 6, 7731 CrossRef PubMed.
- S. Iijima, T. Yumura and Z. Liu, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11759 CrossRef CAS PubMed.
- X. Li, H. Lv, J. Dai, L. Ma, X. C. Zeng, X. Wu and J. Yang, J. Am. Chem. Soc., 2017, 139, 6290–6293 CrossRef CAS.
- H. Sakaguchi, H. Matsumura and H. Gong, Nat. Mater., 2004, 3, 551–557 CrossRef CAS.
- M. Takahashi, P. Turek, Y. Nakazawa, M. Tamura, K. Nozawa, D. Shiomi, M. Ishikawa and M. Kinoshita, Phys. Rev. Lett., 1991, 67, 746–748 CrossRef CAS PubMed.
- F. Münning, O. Breunig, H. F. Legg, S. Roitsch, D. Fan, M. Rößler, A. Rosch and Y. Ando, Nat. Commun., 2021, 12, 1038 CrossRef.
- Y. Tian, M. R. Sakr, J. M. Kinder, D. Liang, M. J. MacDonald, R. L. J. Qiu, H.-J. Gao and X. P. A. Gao, Nano Lett., 2012, 12, 6492–6497 CrossRef CAS PubMed.
- A. Murani, A. Kasumov, S. Sengupta, Y. A. Kasumov, V. T. Volkov, I. I. Khodos, F. Brisset, R. Delagrange, A. Chepelianskii, R. Deblock, H. Bouchiat and S. Guéron, Nat. Commun., 2017, 8, 15941 CrossRef CAS PubMed.
- H. Xu, S. Liu, Z. Ding, S. J. R. Tan, K. M. Yam, Y. Bao, C. T. Nai, M.-F. Ng, J. Lu, C. Zhang and K. P. Loh, Nat. Commun., 2016, 7, 12904 CrossRef CAS.
- J. Wang, S. Gudiksen Mark, X. Duan, Y. Cui and M. Lieber Charles, Science, 2001, 293, 1455–1457 CrossRef CAS.
- T. Pham, S. Oh, S. Stonemeyer, B. Shevitski, J. D. Cain, C. Song, P. Ercius, M. L. Cohen and A. Zettl, Phys. Rev. Lett., 2020, 124, 206403 CrossRef CAS.
- J. Lin, O. Cretu, W. Zhou, K. Suenaga, D. Prasai, K. I. Bolotin, N. T. Cuong, M. Otani, S. Okada, A. R. Lupini, J.-C. Idrobo, D. Caudel, A. Burger, N. J. Ghimire, J. Yan, D. G. Mandrus, S. J. Pennycook and S. T. Pantelides, Nat. Nanotechnol., 2014, 9, 436–442 CrossRef CAS.
- J.-K. Qin, P.-Y. Liao, M. Si, S. Gao, G. Qiu, J. Jian, Q. Wang, S.-Q. Zhang, S. Huang, A. Charnas, Y. Wang, M. J. Kim, W. Wu, X. Xu, H.-Y. Wang, L. Yang, Y. Khin Yap and P. D. Ye, Nat. Electron., 2020, 3, 141–147 CrossRef CAS.
- Y. Li, F. Qian, J. Xiang and C. M. Lieber, Mater. Today, 2006, 9, 18–27 CrossRef CAS.
- X.-t. Li, Y.-y. Wan and X.-j. Wu, Chin. J. Chem. Phys., 2019, 32, 267–272 CrossRef CAS.
- C. Ling, Y. Ouyang, L. Shi, S. Yuan, Q. Chen and J. Wang, ACS Catal., 2017, 7, 5097–5102 CrossRef CAS.
- P. Gambardella, A. Dallmeyer, K. Maiti, M. C. Malagoli, W. Eberhardt, K. Kern and C. Carbone, Nature, 2002, 416, 301–304 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- M. Remskar, A. Mrzel, Z. Skraba, A. Jesih, M. Ceh, J. Demšar, P. Stadelmann, F. Lévy and D. Mihailovic, Science, 2001, 292, 479–481 CrossRef CAS PubMed.
- F. Cheng, H. Xu, W. Xu, P. Zhou, J. Martin and K. P. Loh, Nano Lett., 2017, 17, 1116–1120 CrossRef CAS PubMed.
- J. Kibsgaard, A. Tuxen, M. Levisen, E. Lægsgaard, S. Gemming, G. Seifert, J. V. Lauritsen and F. Besenbacher, Nano Lett., 2008, 8, 3928–3931 CrossRef CAS PubMed.
- H. E. Lim, Y. Nakanishi, Z. Liu, J. Pu, M. Maruyama, T. Endo, C. Ando, H. Shimizu, K. Yanagi, S. Okada, T. Takenobu and Y. Miyata, Nano Lett., 2021, 21, 243–249 CrossRef CAS.
- M. Nagata, S. Shukla, Y. Nakanishi, Z. Liu, Y.-C. Lin, T. Shiga, Y. Nakamura, T. Koyama, H. Kishida, T. Inoue, N. Kanda, S. Ohno, Y. Sakagawa, K. Suenaga and H. Shinohara, Nano Lett., 2019, 19, 4845–4851 CrossRef CAS PubMed.
- L. Venkataraman and C. M. Lieber, Phys. Rev. Lett., 1999, 83, 5334–5337 CrossRef CAS.
- N. Kanda, Y. Nakanishi, D. Liu, Z. Liu, T. Inoue, Y. Miyata, D. Tománek and H. Shinohara, Nanoscale, 2020, 12, 17185–17190 RSC.
- S. Stonemeyer, J. D. Cain, S. Oh, A. Azizi, M. Elasha, M. Thiel, C. Song, P. Ercius, M. L. Cohen and A. Zettl, J. Am. Chem. Soc., 2021, 143, 4563–4568 CrossRef CAS.
- S. Meyer, T. Pham, S. Oh, P. Ercius, C. Kisielowski, M. L. Cohen and A. Zettl, Phys. Rev. B, 2019, 100, 041403 CrossRef CAS.
- T. Pham, S. Oh, P. Stetz, S. Onishi, C. Kisielowski, M. L. Cohen and A. Zettl, Science, 2018, 361, 263 CrossRef CAS PubMed.
- Y. Qu, M. Q. Arguilla, Q. Zhang, X. He and M. Dincă, J. Am. Chem. Soc., 2021, 143, 19551–19558 CrossRef CAS PubMed.
- X. Liu, T. Xu, X. Wu, Z. Zhang, J. Yu, H. Qiu, J.-H. Hong, C.-H. Jin, J.-X. Li, X.-R. Wang, L.-T. Sun and W. Guo, Nat. Commun., 2013, 4, 1776 CrossRef.
- D. Yang, S. Zuo, H. Yang and X. Wang, Adv. Energy Mater., 2021, 11, 2100272 CrossRef CAS.
- R. Allmann, I. Baumann, A. Kutoglu, H. Rösch and E. Hellner, Naturwissenschaften, 1964, 51, 263–264 CrossRef CAS.
- S. C. Lee and R. H. Holm, Angew. Chem., Int. Ed. Engl., 1990, 29, 840–856 CrossRef.
- D. B. Mitzi, Inorg. Chem., 2005, 44, 3755–3761 CrossRef CAS PubMed.
- M. Yuan, M. Dirmyer, J. Badding, A. Sen, M. Dahlberg and P. Schiffer, Inorg. Chem., 2007, 46, 7238–7240 CrossRef CAS.
- D. B. Mitzi, L. L. Kosbar, C. E. Murray, M. Copel and A. Afzali, Nature, 2004, 428, 299–303 CrossRef CAS.
- W. Bronger, Z. Anorg. Allg. Chem., 1968, 359, 225–233 CrossRef CAS.
- M. Yu and D. R. Trinkle, J. Chem. Phys., 2011, 134, 064111 CrossRef.
- W. Zhang, J. Wang, L. Zhao, J. Wang and M. Zhao, Nanoscale, 2020, 12, 12883–12890 RSC.
- C. Shang, L. Fu, S. Zhou and J. Zhao, JACS Au, 2021, 1, 147–155 CrossRef CAS.
- X. Sun, W. Li, X. Wang, Q. Sui, T. Zhang, Z. Wang, L. Liu, D. Li, S. Feng, S. Zhong, H. Wang, V. Bouchiat, M. Nunez Regueiro, N. Rougemaille, J. Coraux, A. Purbawati, A. Hadj-Azzem, Z. Wang, B. Dong, X. Wu, T. Yang, G. Yu, B. Wang, Z. Han, X. Han and Z. Zhang, Nano Res., 2020, 13, 3358–3363 CrossRef CAS.
- X. Li, X. Wu, Z. Li, J. Yang and J. G. Hou, Nanoscale, 2012, 4, 5680–5685 RSC.
- C. Huang, J. Feng, F. Wu, D. Ahmed, B. Huang, H. Xiang, K. Deng and E. Kan, J. Am. Chem. Soc., 2018, 140, 11519–11525 CrossRef CAS.
- J. B. Goodenough, Phys. Rev., 1955, 100, 564–573 CrossRef CAS.
- J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87–98 CrossRef CAS.
- C. Huang, J. Feng, J. Zhou, H. Xiang, K. Deng and E. Kan, J. Am. Chem. Soc., 2019, 141, 12413–12418 CrossRef CAS.
- C. Zener, Phys. Rev., 1951, 82, 403–405 CrossRef CAS.
- Y. Zhao, L. Lin, Q. Zhou, Y. Li, S. Yuan, Q. Chen, S. Dong and J. Wang, Nano Lett., 2018, 18, 2943–2949 CrossRef CAS.
- A. S. Dhoot, C. Israel, X. Moya, N. D. Mathur and R. H. Friend, Phys. Rev. Lett., 2009, 102, 136402 CrossRef.
- N. D. Mermin and H. Wagner, Phys. Rev. Lett., 1966, 17, 1133–1136 CrossRef CAS.
- H. Xiang, J. Yang, J. G. Hou and Q. Zhu, J. Am. Chem. Soc., 2006, 128, 2310–2314 CrossRef CAS PubMed.
- Y. Wan, Y. Sun, X. Wu and J. Yang, J. Phys. Chem. C, 2018, 122, 989–994 CrossRef CAS.
- S. Guo, J. Fu, P. Zhang, C. Zhu, H. Yao, M. Xu, B. An, X. Wang, B. Tang, Y. Deng, T. Salim, H. Du, R. E. Dunin-Borkowski, M. Xu, W. Zhou, B. K. Tay, C. Zhu, Y. He, M. Hofmann, Y.-P. Hsieh, W. Guo, M. Ng, C. Jia, Z. Zhang, Y. He and Z. Liu, Nature Synthesis, 2022, 1, 245–253 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- A. Togo and I. Tanaka, Scr. Mater., 2015, 108, 1–5 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2na00543c |
| This journal is © The Royal Society of Chemistry 2022 |