Open Access Article
Open Access ArticleCatalytic confinement effects in nanochannels: from biological synthesis to chemical engineering
Yigang
Shen†
 a,
Xin
Wang†
a,
Jinmei
Lei
a,
Shuli
Wang
a,
Yaqi
Hou
a,
Xin
Wang†
a,
Jinmei
Lei
a,
Shuli
Wang
a,
Yaqi
Hou
 a and
Xu
Hou
a and
Xu
Hou
 *abc
*abc
aState Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
bResearch Institute for Biomimetics and Soft Matter, Fujian Provincial Key Laboratory for Soft Functional Materials Research, College of Physical Science and Technology, Xiamen University, Xiamen, Fujian 361005, China. E-mail: houx@xmu.edu.cn
cInnovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen 361102, Fujian, China
First published on 21st February 2022
Abstract
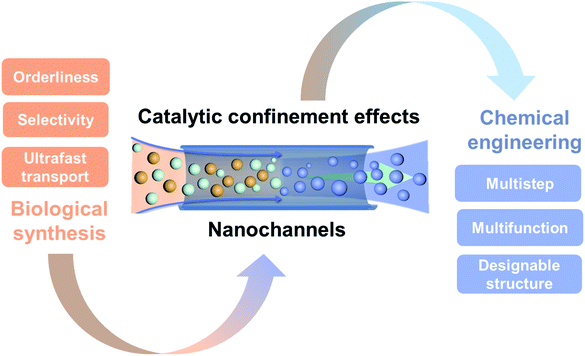
Catalytic reactions within nanochannels are of significant importance in disclosing the mechanisms of catalytic confinement effects and developing novel reaction systems for scientific and industrial demands. Interestingly, catalytic confinement effects exist in both biological and artificial nanochannels, which enhance the reaction performance of various chemical reactions. In this minireview, we investigate the recent advances on catalytic confinement effects in terms of the reactants, reaction processes, catalysts, and products in nanochannels. A systematic discussion of catalytic confinement effects associated with biological synthesis in bio-nanochannels and catalytic reactions in artificial nanochannels in chemical engineering is presented. Furthermore, we summarize the properties of reactions both in nature and chemical engineering and provide a brief overlook of this research field.
1. Introduction
Organisms are engaged in a variety of physiological activities with manifold enzymes all the time. Enzymatic biosynthesis is one of the most fundamental and essential processes in living organisms, for their growth, activity, and survival.1–3 Enzymatic biosynthesis reactions have outstanding properties, such as high selectivity, ultrafast reaction rate, and low energy consumption. In the process of biosynthesis, the confinement and arrangement of molecules in nanochannels or similar structures can fundamentally change their chemical and physical properties using enzymes, reducing the reaction activation energy and showing remarkable efficiency.4,5 For example, a channel-liked or ring-shaped protein, which is formed from DNA polymerases by their linking together, plays important roles in the DNA reproduction process. This biological nanochannel structure facilitates the replication of two strands of DNA in a quick and low energy consumption manner.6 This channel-liked protein surrounds the DNA template and slides along with it, which hugely accelerates the replication speed (750 nucleotides per second).7 Confinement effects not only affect the biosynthesis reaction but also protect protein denaturation and affect the folding rate.8 A theory was presented that the proteins can be prevented from reversible unfolding by the nano confinement space, which can also increase the rates of RNA folding.9 The confinement effects, especially these effects that can improve the synthesis reaction and any other chemical reaction based on the confined space and the catalysts in the confined space, are termed as catalytic confinement effects (CCE) in this review.Living organisms have evolved special nanostructures and functions, which can utilize CCE to govern sophisticated and highly efficient biochemical reactions. Inspired by CCE in biological nanochannels, various artificial nanochannel systems have been developed to improve the performance of chemical reactions in chemical engineering processes.10 Catalytic reactions represent a cornerstone in most chemical engineering processes, covering the field of energy, the environment, and health care. However, with the global backdrop of sustainable development, climate change, and the ecosystem crisis, novel catalytic reactions with high efficiency and resilience are urgently required.11 In conventional bulk catalysis, due to large numbers of surface atoms of the catalysts being exposed to the reaction environment, the active sites become unstable and their catalytic activity diminishes.12 Problems with poisoning, sintering, and the coalescence of catalysts further reduce their activity. As mentioned, the enzymatic biosynthesis in biological systems shows superior reaction performance, which is becoming the solution for chemical engineering processes.13 How to mimic the biological reactions in artificial systems entails understanding the fundamental mechanisms of CCE both in biological and artificial chemical reactions. Historically, Derouane et al. first introduced the confinement effects of zeolite catalysts by proposing a van der Waals model to describe the molecule interactions in the confined space.14 Since then, zeolites as the most desirable catalysts have been well studied in terms of their chemical, environmental, and biological applications.15 However, confinement effects in different nano spaces attract interest from the catalysis, nanotechnology, advanced materials synthesis, and chemical engineering fields. Here, we focus on the confinement effects involved in the chemical reaction processes. Commonly, CCE include the molecular enrichment effect,16 the electronic effect,17 and the physical constraint effect,18 which directly affect the chemical reaction. The nano spaces offering a restricted structure play key roles in CCE. There are different nanoscale structures, such as nanocages, nanocavities, nanopockets, nanopores, and nanochannels, which have been developed as nano reactors for catalysis reactions in artificial systems nanoconfinements.5 Since these closed structures, such as cages and cavities, have been discussed by Dai et al.,19 Yang et al.,10 and Grommet et al.,5 this minireview will focus on the chemical reactions confined within nanochannels or nanotubes (1D structures of which the depth is much larger than the diameter, with the diameter being less than 100 nm). Based on the structural features in dimensions, usually these structures are classified as 0D (porous polymer particles), 1D (nanochannels), 2D (stacking layered 2D materials) and 3D (nanochannels in interconnected porous materials).20 Compared to other structures, 1D nanochannels have a simple and uniform structure, and easily designable features, such as selectivity, regulatability, biomimetic properties, and surface modifying properties,21–26 which have mostly been explored via both experiments27,28 and simulations.29,30 Based on this theoretical research, a number of applications have been developed in the field of chemical engineering within nanochannels, such as materials synthesis,31,32 energy conversion,33 environment protection,34etc. The research field of 1D nanochannels opens up intriguing possibilities to disclose the principle of CCE both in terms of biological reaction systems and chemical engineering systems (Fig. 1). Besides this, most reviews have only discussed the confinement effects and applications in artificial nanochannels and have lacked the discussion that covers the confinement effects both in biological nanochannels and artificial nanochannels. Here, we highlight CCE within nanochannels from the perspective of biological synthesis and chemical engineering. We first introduce the principles and theories of CCE within nanochannels. Then, we investigate the biological reactions involving CCE in bio-nanochannels, especially enzymatic biosynthesis in living organisms. Subsequently, we summarize the recent advances of CCE within nanochannels in chemical engineering processes. Finally, an outlook towards the future development of this research field is presented.
2. Mechanisms and theory of CCE within nanochannels
It is one of the most significant aims for chemists to control reaction pathways and reaction products. Inspired by the excellent reaction performance in organisms, researchers have designed and prepared bionic artificial nanochannels to mimic the functions of high reaction rate, high conversion rate, and high selectivity in biological and chemical reactions. 1D nanochannels with hollow interiors provide unique physical confinement and affect transport behavior, and chemical and physical properties of the reactant molecules, resulting in chemical reactions in confined space being conducted very differently from those in the bulk space. From a thermodynamics perspective, confinement effects can stabilize reaction intermediate states and change enthalpies and Gibbs free energies (Fig. 2a).35 In depth, we discuss the CCE within nanochannels from four aspects: the reactants, reaction process, catalysts, and reaction products (Fig. 2b). Concentration and arrangement of reactants, the transition state and transport rate during the reaction process, interaction and activity of catalysts, and the concentration and selectivity of products within nanochannels are discussed.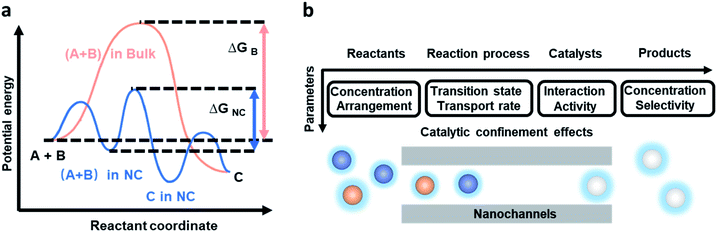 | ||
| Fig. 2 Simplified reaction energy profiles during the overall process of the reaction in the nanochannels and the bulk environment, and the factors affecting the chemical reaction. (a) An example reaction between A and B to form product C in the bulk environment (orange) and confine it in the nanochannels (NC) (blue). The catalytic confinement effects can stabilize the reaction intermediate state and change the enthalpies and Gibbs free energies (ΔG), thereby changing the reaction path. Reproduced from ref. 35 with permission from the Royal Society of Chemistry. (b) CCE within nanochannels in terms of four aspects: reactants, reaction process, catalysts, and reaction products. | ||
In the first aspect, the interaction between restricted reactants and the nanochannels can increase the local concentration of the reactants and also enhance reaction pressure.36 Meanwhile, high concentration causes synergy between crowding effects and confinement effects, which together influence the reaction progress.37 And also, the nanochannels limit the volume or size of the molecules and make the reactants be arranged in a certain order. Liu et al. proposed a concept of an ordered-assembly reaction (OAR) based on frontier molecular orbital (FMO) theory.28 Based on the electronic structure of reactant molecules and the electronic interactions between reactants and nanochannels, if orbital symmetry matching is satisfied, the function of a “acceptor–donor” interaction scenario can be realized. After the matching process, when the size of the nanochannel is close to the distance of the van der Waals equilibrium distance of the molecules, the ion or molecule undergoes ultrafast transport, and is referred to as a quantum-confined superfluid (QSF). Considering both of these effects, reactant molecules arrange in a certain sequence within optimized nanochannels, causing ultrafast directional flow in nanochannels, thereby achieving a high reaction rate.
In the second aspect, there are two parameters that influence the reaction process: transition state and transport rate. The nanochannels enhance the stability of the transition states by adjusting the underlying thermodynamics, which reduces the activation energy barrier and accelerates the reaction kinetics.38 Two scenarios can be distinguished to explain the reasons for how confinement effects reduce the activation energy barrier in nanochannels (these might be combined in practice). One is that the nanochannels can change the activation parameters.35 Fiedler et al. demonstrated that the confined space can reduce both the entropic and enthalpic barriers by rearranging the reactant molecules.39 Secondly, when the nanochannels are integrated with catalysts, the catalysts are able to reduce the activation energy during reactions. Compared to catalysts in bulk environments, problems with the aggregation and sintering of catalysts can be avoided when the reactions are proceeding in nanochannels, which further enhance the catalytic effect.40 Apart from that, size effects make a difference in the reaction process by influencing the transport rate and mode of transport. When the size of the nanochannels is close to the size of several molecules, for example, the spatial constraints are mainly reflected in the interactions between the molecules and the confined inner wall of the nanochannels. The narrow size of a carbon nanotube (CNT) has been shown to act as a selective channel that prevents large molecules (over the diameter of the CNT) from being transported into the channel, which raises the selectivity rate for the para-bromination of N-phenylacetamide from 68 to 97%.41 Interestingly, too narrow nanotubes might hinder the reaction performance. Xiao et al. exhibited a volcano relationship between catalytic reaction and CNT diameter.30 If the size of a nanochannel is too small (<1 nm), the binding between reactants and catalysts weakens, which inhibits the adsorption and activation of reactants. Then, the turnover frequency (TOF) is decreased and the catalytic activation of reaction molecules becomes sluggish. It is noted that the optimum diameter of nanochannels is dependent on the specific reactions and chosen catalysts. Jiang's group proposed the concept that the ideal size of the nanochannel to achieve high flux and 100% selectivity in the reaction process is the van der Waals equilibrium distance for molecules and Debye length for ions. On the contrary, when the size of nanochannels increases (>100 nm), the interactions between the molecules and the confined inner wall gradually weaken.42 In addition to size, wettability, surface charge, recognition, and van der Waals forces, hydrogen bonds of the nanochannels can influence the transport rate and transportation phenomena of molecules,20,43 which indirectly change the reaction process.
In the third part, for chemical reactions with catalysts, the nanochannels can influence the interactions between catalysts and reactants and the activity of the catalysts. The catalytical efficiency can be modified by the electronic interaction between the confined metal or metal oxide (catalyst) and the inner wall of the nanochannels, which could also hinder the migration and sintering of the catalyst.44 Furthermore, the anchoring of catalysts in the nanostructure can improve the selectivity and stability of the reactants.41 Lastly, for the products, the nanochannels can adjust the selectivity of different products, resulting in enhancing the selectivity of one product and impairing the others.45 On the other side, unfavourable interactions of specific products may promote the release of products from nanochannels, decreasing the local concentration of products and increasing the TOF. Zhang et al. demonstrated a benzenehydroxylation reaction in a CNT to separate reaction products from reactants, which showed 4 times higher activity than under the same conditions on the outer walls of the CNT.16
The CCE have such a great impact on the whole process of chemical reactions that many theories related to this have been developed. Shermukhamedov et al. used electron transfer theory and classical molecular dynamics simulations to make qualitative predictions of electron transfer in aqueous solutions inside nanochannels.46 The total reorganization energy λ in the reaction process was estimated using the expression:
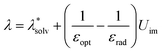 | (1) |
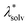 represents the reorganization energy resulting from non-equilibrium solvation; εopt and εrad are the optical dielectric constant and the radial component of static dielectric constant, respectively; and Uim is the image potential related to the ion-wall distance. To simplify the model, εrad is replaced by the static dielectric εeff:
represents the reorganization energy resulting from non-equilibrium solvation; εopt and εrad are the optical dielectric constant and the radial component of static dielectric constant, respectively; and Uim is the image potential related to the ion-wall distance. To simplify the model, εrad is replaced by the static dielectric εeff: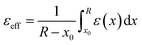 | (2) |
| Econ = Eb(in) − Eb(out) | (3) |
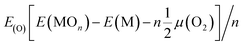 | (4) |
However, sometimes complex parameters might have synergistic influences between each other, which is difficult to deconvolute and disclose the influences from single parameters and build a uniform theory. Until now, there have been no unambiguous mechanisms and theories proposed to directly judge how the confinement effects influence chemical reactions.
3. Biological reactions with CCE in bio-nanochannels
Almost all of the biological reactions in living organisms are performed by enzymes. Enzymes, as fundamental and functional molecules, have evolved to become the sophisticated molecules that govern the activities of cells. However, the underlying working principles of enzymes are still unclear. Generally, enzymes act as templates for the reactions, which bind to their substrate (recognition) and hold them in a specific position (active site) to form products. Enzymes surround the substrate with reactive groups (confinement space) that stabilize the transition state, which makes reactions occur easily. Besides this, substrate preorganization, reaction medium, restricted reactant motion, and protein dynamics play an important role in the catalytic process.35 Many enzymes surround their substrates to form the perfect confinement environment for reactions, which is also an interesting point for disclosing the working principle of enzymes. The numbers of catalytic proteins participating in DNA metabolism (DNA replication, DNA repair, and DNA recombination) form the same channel structure: ring or toroidal shapes, in different species (Fig. 3a).7,51 During the process of DNA replication, DNA polymerases, which are a type of distributive proteins, need to keep in contact with the DNA stand to achieve multiple rounds of catalysis. The confinement space in the center of ring-shaped or channel-liked proteins (DNA clamp) encircles the DNA temple and allows the DNA polymerases to slide along the DNA strand (Fig. 3b).52 A CCE with DNA clamp has been shown to greatly accelerate the replication speed (750 nucleotides per second) in Escherichia coli.7 Another essential protein, DNA endonuclease, shows a similar channel structure that presents outstanding selectivity. The powerful technology CRISPR (clustered regularly interspaced short palindromic repeats)-Cas (CRISPR-associated systems) based on the furcation of DNA endonuclease, has brought about a revolutionary shift in the paradigm of gene-editing methods.53 The type II systems of CRISPR utilize a single RNA-guided endonuclease, Cas 9, to achieve double-stand breaks and change the target DNA. The structure pictures of Cas 9 by electron microscopy show that the two structural lobes form a central channel where DNA is bound and the DNA template can slide along the channel (Fig. 3c).54 The confined space of nanochannels provides outstanding properties of selectivity and effectivity to recognize and cut the target DNA, which is becoming the most robust tool in the field of gene editing. CCE in channel-shaped proteins are essential for DNA replication and editing, and also are one of the reasons why biosynthesis is conducted in a highly efficient and low energy consumption way within organisms.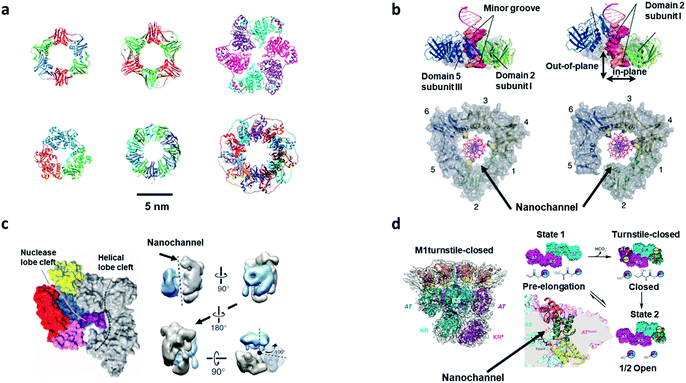 | ||
| Fig. 3 Biological reactions with CCE in bio-nanochannels. (a) Ring proteins in different species. First line: the β-clamp of E. coli (PDB: 2POL), the proliferating cell nuclear antigen of H. sapiens (PDB: 1AXC), helicase of bacteriophage (PDB: 1QAW). Second line: the exonuclease of bacteriophage (PDB: 1AVQ), RNA binding protein of Bacillus stearothermophilus (PDB: 1QAW), and DNA binding protein of Homo sapiens (PDB: 1KN0). Scale bar: 5 nm. Reproduced from ref. 7 with permission from the Royal Society of Chemistry. (b) The nanochannel of ring-shaped (DNA clamp) encircles the DNA temple and allows the DNA polymerases to slide along the DNA strand in an effective and fast way. Reproduced with permission.52 Copyright 2011, American Association for the Advancement of Science. (c) Left: crystal structure of Cas 9 with nanochannels and nucleic acid binding clefts. Right: rotation view of the structures of Cas 9 (black dashed line represents the central channel). Reproduced with permission.54 Copyright 2014, American Association for the Advancement of Science. (d) Structure of DEBS M1 and the turnstile-closed/opened state. Left: the structure of DEBS M1 by cryogenic electron microscopy (4.3 Å-resolution). Right: three states of the DEBS M1. State 1 (pre-elongation) with the accessibility of the AT and KS active site. Then, the proteins undergo a conformational change to a turnstile-closed state with the inaccessibility of the AT and KS. After that, acyl carrier proteins are hindered by the KS active site but have access to the AT active site. Reproduced with permission.57 Copyright 2021, American Association for the Advancement of Science. | ||
On the basis of these single functional enzymes, there are also many multifunctional enzymes that can achieve complex catalytic reactions in nanochannels based on the selectivity and orderliness of CCE.55 Assembly-line polyketide synthases (APKSs), called enzyme factories, are multienzyme systems that generate specific and complex polyketide products by guiding protein-tethered substrates with different active sites in a designed order.56 Cogan et al. applied cryogenic electron microscopy to disclose the structure of 6-deoxyerythronolide B synthase module 1 (DEBS M1), which was used to present a product-bound module that could match the turnstile mechanism in the synthesis process of APS.57 DEBS M1 turns its own components of KS–acyltransferase (AT) and ketosynthase (KS) to change their spatial arrangement and realize two conditions: turnstile-open and turnstile-closed (Fig. 3d), which drives the synthesis process in sequence along the assembly-line (which would allow only one single chain to be tied to the target module) and blocks retrograde chain translocation. Similarly, a turn mechanism also exists in the adenosine triphosphate (ATP) synthesis process that is the most prevalent reaction in living organisms. ATP synthase is a multisubunit complex, which has a water-soluble domain (F1) with three active sites and a membrane domain (F0) that contains nanochannel structures for proton translocation.58 Protons travel through the nanochannel to the c-ring in F0, where they drive the rotation of the rotor (subunits of F1). During the rotation process, conformational changes in the catalytic sites occur, providing the energy for the phosphorylation of adenosine diphosphate (ADP). The dynamic adjustment of the structures in enzymes shows the unique properties of CCE in biological reactions.
From simple transport through the nanochannel to the complex and sophisticated dynamic control of the channel structure in biosynthesis reactions, the fascinating phenomena and mechanisms in cells give us unique inspirations. Jiang's group proposed a new concept of a quantum-confined superfluid (QSF) reaction based on FMO theory, which could help researchers to construct new chemical systems with ultra-high flux and selectivity in a moderate environment.59 These outstanding features (high selectivity, low energy consumption, and ultrafast transport) in biological reactions are what model chemical engineering needs, and the more we explore and demystify the intrinsic mechanism of confinement effects in biological reactions, the better researchers can design and develop efficient systems for chemical engineering.
4. Chemical engineering with CCE in nanochannels
In the trend towards green and sustainable development, transformative and novel chemical engineering systems with high efficiency and low consumption are urgently required.60 In addition, in terms of traditional chemical engineering, there are some problems that hamper the reactions in catalytic processes: transport limitations, maldistribution, and inadequate interactions between reactants and catalysts.13 Although the issues are clear, effective solutions and out-of-the-box thinking are needed. Learning from nature is one of the most promising ways to address such issues.61–65 In terms of the outstanding properties of reactions in biological systems previously mentioned, scientists are inspired by biosynthesis in nature and then design nanochannels to achieve much higher chemical reaction rate and better selectivity.66–68 However, the nanotechnology of fabricating various nanochannels is accelerating the research on the exploration of mass transport, and unique physical and chemical properties in nanochannels.69–72 Therefore, a number of chemical engineering applications with CCE in nanochannels are being reported, which cover the research fields of energy conversion and the environment.33,34,73,74The oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) are two significant processes for exploring new clean and green energy technologies. However, conventional catalytic systems for the ORR and HER always suffer the dilemma that the activity of the catalysts diminishes after repeated cycles and dissociation/aggregation becomes serious.33 One of the most promising methods is carrying out catalytic reactions in nano space. Nanochannels provide an ideal nano space to enhance the interactions among reactants and increase the stability of catalyts.75–77 Yang et al. demonstrated a metal/nitrogen-doped carbon (TM/N/C) catalyst with a mesoporous carbon (MC) architecture. The diameter of each nanochannel in the MC was around 3.5 nm. Due to the highly ordered nanochannel structure, the single-atom catalytic sites can be fully exposed to the reactants, improving the ORR activity and exhibiting an excellent onset potential of 1.03 V vs. the reversible hydrogen electrode (RHE) in an alkaline medium (Fig. 4a).78 Similarly, Guo et al. designed another TM/N/C catalyst with nanochannels (4–10 nm diameter), which showed a lower onset potential of 0.81 V vs. RHE (Fig. 4b).79 They found that the CCE in the nanochannels boosted thermal stability and improved the stability of nitrogen-enriched active sites. In another research study on the HER, Tang et al. developed novel electrocatalysts with hierarchical MoS2/CoS2 nanochannel arrays (Fig. 4c), which exhibited superior catalytic activity (a low overpotential of 90 mV vs. RHE at 10 mA cm−2) and a small Tafel slope of 30 mV dec−1 in acidic media.80 Because of the CCE, the interaction between MoS2 and CoS2 became strong, which reduced the Gibbs free energy, lowered the energy barrier of water dissociation, and increased the electronic states at S–S edges. Except for enhancing the interactions, CCE can also improve the selectivity of molecules to accelerate reactions. A new graphdiyne (GDY) based anodic electrode with a nanochannel (4.2 nm diameter) structure was prepared (Fig. 4d).81 This anode material shows negative selectivity towards methanol and positive selectivity towards proton, exhibiting superior power density (90 mW cm−2) at 80 °C, with the methanol crossover subdued to 33%. Recently, Zou et al. used a carbon nitride nanotube (CNN) membrane as a research carrier for studying the CCE in photocatalysis (Fig. 5a).66 Every single channel of the membrane had an inner diameter of ∼40 nm with a length of 0.2 mm. The reaction in the nanochannels showed a turnover frequency of (9.63 ± 1.87) × 105 s−1 as a result of CCE, comparable to enzyme-catalyzed reaction rates. Such a high rate was due to the internal electric field produced by the polarized surface of the nanochannels.
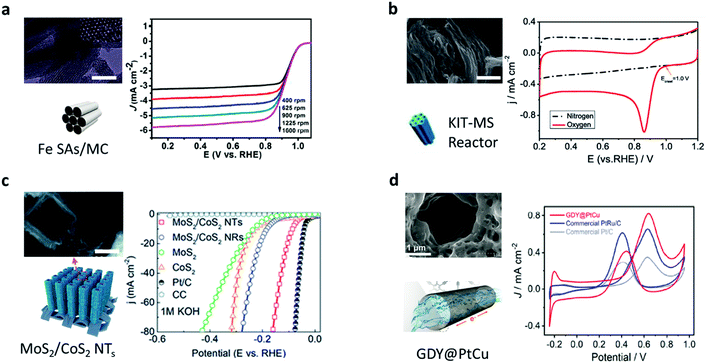 | ||
| Fig. 4 Catalytic reaction systems in artificial nanochannels. (a) Transmission electron microscopy (TEM) and schematic illustration of the Fe SAs/MC (950) catalyst. Linear sweep voltammetry (LSV) curves of the catalyst in 0.1 M KOH at various rotation speeds. Reproduced with permission.78 Copyright 2018, American Chemical Society. (b) Scanning electron microscopy (SEM) images and schematic illustration of Fe/N/C catalyst. Cyclic voltammetry (CV) curves of the catalyst in N2 and O2 saturated 0.1 M KOH solution. Reproduced with permission.79 Copyright 2020, Springer Nature. (c) SEM image and schematic illustration of MoS2/CoS2 nanotube arrays. LSV curves of MoS2/CoS2 nanotube arrays in 1 M KOH compared to other materials. Reproduced from ref. 80 with permission from the Royal Society of Chemistry. (d) SEM image and illustration of the channels in the GDY@PtCu. CV curves of the GDY@PtCu, PtRu/C, and Pt/C. Reproduced with permission.81 Copyright 2021, Elsevier. | ||
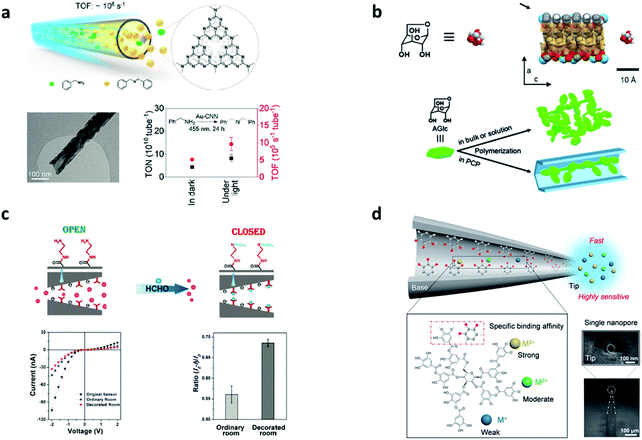 | ||
| Fig. 5 Catalytic reaction systems in artificial nanochannels. (a) Schematic illustration of flow photocatalytic reactions in nanochannels. TEM image of a single Au carbon nitride nanotube. Turnover number (TON) and turnover frequency (TOF) of benzylamine oxidation under different reaction conditions. Reproduced with permission.66 Copyright 2021, American Chemical Society. (b) Schematic illustration of the nanochannel structure and reactants. Polymerization of 6-anhydro-b-D-glucose (AGlc) in the bulk environment compared to nanochannels of porous coordination polymers (PCPs). Reproduced from ref. 32 with permission from the Royal Society of Chemistry. (c) The two states (open and closed) of the gating performance of the nanochannels. Current changes of the EDA-modified nanochannels with and without HCHO. The ionic current ratios of the EDA-modified and original nanochannels after interaction with HCHO in different current directions. Reproduced with permission.83 Copyright 2019, Wiley. (d) Schematic illustration of a tannic acid-modified single nanochannel and binding energies with different ions. SEM images of the single conical nanochannel. Reproduced with permission.21 Copyright 2020, Elsevier. | ||
CCE are highly interesting to be applied for the efficient generation of environmentally-friendly products, sensitive pollutant detection, and effective environmental protection.82 1D nanochannels of [La(1,3,5-benzenetrisbenzoate)(H2O)]n have been used to polymerize glucose monomers with controlled structures (Fig. 5b).32 Based on the CCE in nanochannels, the regulated structure of poly-glucose (PGlc) has superb processability and thermal stability compared to the bulk environment, making it useful for generating new bioplastic. However, the selectivity and limited space of CCE provide a new strategy for dealing with pollution or toxic agents. A nanochannel membrane with ethylenediamine (EDA) functionalized in its inner surface was developed for detecting and removing formaldehyde (HCHO) based on CCE (Fig. 5c).83 The interaction of HCHO molecules with EDA tethered on the inner surface of the nanochannels caused a change in ion current and the CCE increased the detecting sensitivity and selectivity of the HCHO molecules. Compared to the nanochannel membrane, Zhan et al. used ion recognition nanochannels to develop a single conical glass nanochannel with tannic acid that could achieve highly sensitive metal ion detection based on CCE (Fig. 5d).21 Lastly, for reactions in a green reaction medium, Xing et al. developed tube-in-tube structures (outside: 12 nm in diameter, inside: 6 nm in diameter) of amphiphilic confined TiO2@Pt/CNTs and hydrophobic confined C@Pt/CNTs for biphasic reactions. These new amphiphilic confined catalysts enhanced the ion transport rate during the reaction process, which increased the catalytic activity based on CCE.84
However, the catalytic reactions in artificial systems might suffer several disadvantages during practical applications, such as blockage of the nanochannels by the large volume of product, low diffusion rate for molecules in and out of nanochannels, and difficult observation of reaction processes.44 How to figure out these limitations by learning from nature would be a future direction. However, artificial reaction systems with CCE have their own features compared to biological reaction systems, as multistep reactions can be designed in nanochannels,85 multifunction catalysts can be inserted into nanochannels,86 and designable structures and controllable surface properties of nanochannels can be fabricated based on the research purpose. Besides this, these reactions in artificial nanochannels can endure extreme conditions, such as high temperature and pressure.
5. Summary and outlook
In summary, CCE exist in both biological and artificial nanochannels for chemical reactions, which enhance the reaction performance. These positive influences are exhibited in terms of four aspects: reactants, reaction processes, catalysts, and reaction products. In each aspect, the confinement effects have different functions, such as effects on the concentration of reactants or products, the order of reactants entering into the nanochannels, and the control of catalyst activity, transport rate, selectivity, etc. From a biological view, the various reactions that take place in cells always give us unique insights. Like in DNA synthesis processes, different enzymes with channel-liked structures have high selectivity, which improves the synthesis reaction with ultrafast reaction rate and low energy consumption. Inspired by nature, the CCE in nanochannels are widely used in chemical engineering in the fields of energy and the environment, which could improve the activity, selectivity, and stability of reactions. Albeit there are great improvements for the reaction performance within nanochannels compared to the bulk environment, these reactions or catalysts do not even come close to the reaction efficiency and selectivity exhibited by enzymes in living organisms. And one of the reasons for this is the insufficient understanding of CCE in nanochannels, especially from mechanistic and theoretical aspects. It is noted that the properties of fluids or gas within the nanochannels are very different,10,46 whereas most of the published works have focused on electrocatalysts.33,87,88 The mass transport of ions or molecules, and the properties of aqueous solvent within the channels also play significant roles.89 Comprehensive theories and in situ characterization techniques should thus be developed to probe the CCE and mechanism of catalysis.90,91For highly effective chemical engineering, creating new catalytic reaction systems that mimic nature by understanding the mechanisms of nature is another strategy. For instance, the turnstile mechanism in the synthesis process of APS and ATP synthesis might inspire scientists to use dynamic nanochannels to build tunable and assembly-line reaction artificial systems.23 And new reaction systems with CCE, such as bioreactors in nanostructures92 and tandem catalytical reactions in nanochannels,93 might play a significant role in future chemical engineering. However, more attention should be devoted to the rational design of chemical reactions within nanochannels, such as the reactant/channel size ratio and the distribution density of catalysts. Aside from 1D nanochannels, other dimensional structures (0D, 2D, and 3D) have also attracted much attention in exploring the confinement effects during catalytical reactions. As materials with different dimensions have unique geometric and electronic structures, it is an interesting point to distinguish the differences and similarities of CCE between them, which will benefit towards disclosing the fundamental mechanism of such systems and building a unified theory of CCE. Future work should also focus on the durability and recyclability of catalysts within nanochannels and the optimized location of active sites. We believe that with an in-depth mechanism study of CCE both in chemical and biological fields, nanochannels could play an essential part in next-generation catalytic systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (Project No. 2018YFA0209500), the National Natural Science Foundation of China (52025132, 21975209, 21621091, 22021001), the Fundamental Research Funds for the Central Universities of China (20720190037), the 111 Project (B16029).References
- M. E. Winkler, in Brenner's Encyclopedia of Genetics, 2013, pp. 341–345, DOI:10.1016/b978-0-12-374984-0.00156-x.
- Y. Deng, T. Wu, M. Wang, S. Shi, G. Yuan, X. Li, H. Chong, B. Wu and P. Zheng, Nat. Commun., 2019, 10, 2775 CrossRef PubMed.
- S. Zhang, D. J. Heyes, L. Feng, W. Sun, L. O. Johannissen, H. Liu, C. W. Levy, X. Li, J. Yang, X. Yu, M. Lin, S. J. O. Hardman, R. Hoeven, M. Sakuma, S. Hay, D. Leys, Z. Rao, A. Zhou, Q. Cheng and N. S. Scrutton, Nature, 2019, 574, 722–725 CrossRef CAS PubMed.
- B. C. Tripp, K. Smith and J. G. Ferry, J. Biol. Chem., 2001, 276, 48615–48618 CrossRef CAS PubMed.
- A. B. Grommet, M. Feller and R. Klajn, Nat. Nanotechnol., 2020, 15, 256–271 CrossRef CAS PubMed.
- M. Hogg, P. Osterman, G. O. Bylund, R. A. Ganai, E. B. Lundstrom, A. E. Sauer-Eriksson and E. Johansson, Nat. Struct. Mol. Biol., 2014, 21, 49–55 CrossRef CAS PubMed.
- B. J. Pieters, M. B. van Eldijk, R. J. Nolte and J. Mecinovic, Chem. Soc. Rev., 2016, 45, 24–39 RSC.
- B. Panganiban, B. Qiao, T. Jiang, C. DelRe, M. M. Obadia, T. D. Nguyen, A. A. A. Smith, A. Hall, I. Sit, M. G. Crosby, P. B. Dennis, E. Drockenmuller, M. Olvera de la Cruz and T. Xu, Science, 2018, 359, 1239–1243 CrossRef CAS PubMed.
- H. X. Zhou and K. A. Dill, Biochemistry, 2001, 40, 11289–11293 CrossRef CAS PubMed.
- F. Yang, D. Deng, X. Pan, Q. Fu and X. Bao, Natl. Sci. Rev., 2015, 2, 183–201 CrossRef CAS.
- R. Gani, J. Bałdyga, B. Biscans, E. Brunazzi, J.-C. Charpentier, E. Drioli, H. Feise, A. Furlong, K. M. Van Geem, J.-C. de Hemptinne, A. J. B. ten Kate, G. M. Kontogeorgis, F. Manenti, G. B. Marin, S. S. Mansouri, P. M. Piccione, A. Povoa, M. A. Rodrigo, B. Sarup, E. Sorensen, I. A. Udugama and J. M. Woodley, Chem. Eng. Res. Des., 2020, 155, A133–A145 CrossRef.
- F. Lei, W. Liu, Y. Sun, J. Xu, K. Liu, L. Liang, T. Yao, B. Pan, S. Wei and Y. Xie, Nat. Commun., 2016, 7, 12697 CrossRef CAS PubMed.
- M. O. Coppens, Annu. Rev. Chem. Biomol. Eng., 2021, 12, 187–215 CrossRef CAS PubMed.
- G. D. Eric, A. Jean-Marie and A. L. Amand, Chem. Phys. Lett., 1987, 137, 336–340 CrossRef.
- L. H. Chen, M. H. Sun, Z. Wang, W. Yang, Z. Xie and B. L. Su, Chem. Rev., 2020, 120, 11194–11294 CrossRef CAS PubMed.
- H. Zhang, X. Pan, X. Han, X. Liu, X. Wang, W. Shen and X. Bao, Chem. Sci., 2013, 4, 1075–1078 RSC.
- W. Chen, X. Pan and X. Bao, J. Am. Chem. Soc., 2007, 129, 7421–7426 CrossRef CAS PubMed.
- X. Pan, Z. Fan, W. Chen, Y. Ding, H. Luo and X. Bao, Nat. Mater., 2007, 6, 507–511 CrossRef CAS PubMed.
- J. Dai and H. Zhang, Small, 2021, 17, e2005334 CrossRef PubMed.
- Z. Zhu, D. Wang, Y. Tian and L. Jiang, J. Am. Chem. Soc., 2019, 141, 8658–8669 CrossRef CAS PubMed.
- K. Zhan, Z. Li, J. Chen, Y. Hou, J. Zhang, R. Sun, Z. Bu, L. Wang, M. Wang, X. Chen and X. Hou, Nano Today, 2020, 33, 100868 CrossRef CAS.
- Y. Zhu, K. Zhan and X. Hou, ACS Nano, 2018, 12, 908–911 CrossRef CAS PubMed.
- Q. Pi, S. Maharjan, X. Yan, X. Liu, B. Singh, A. M. van Genderen, F. Robledo-Padilla, R. Parra-Saldivar, N. Hu, W. Jia, C. Xu, J. Kang, S. Hassan, H. Cheng, X. Hou, A. Khademhosseini and Y. S. Zhang, Adv. Mater., 2018, 30, e1706913 CrossRef PubMed.
- H. Zhang, Y. Tian, J. Hou, X. Hou, G. Hou, R. Ou, H. Wang and L. Jiang, ACS Nano, 2015, 9, 12264–12273 CrossRef CAS PubMed.
- H. Zhang, X. Hou, Z. Yang, D. Yan, L. Li, Y. Tian, H. Wang and L. Jiang, Small, 2015, 11, 786–791 CrossRef CAS PubMed.
- X. Hou, H. Zhang and L. Jiang, Angew. Chem., Int. Ed. Engl., 2012, 51, 5296–5307 CrossRef CAS PubMed.
- Z. Chen, Z. Guan, M. Li, Q. Yang and C. Li, Angew. Chem., Int. Ed. Engl., 2011, 50, 4913–4917 CrossRef CAS PubMed.
- S. Liu, X. Zhang and L. Jiang, Adv. Mater. Interfaces, 2019, 6, 1900104 CrossRef.
- S. Joseph and N. R. Aluru, Nano Lett., 2008, 8, 452–458 CrossRef CAS PubMed.
- J. Xiao, X. Pan, F. Zhang, H. Li and X. Bao, Chem. Sci., 2017, 8, 278–283 RSC.
- G. Calzaferri, Langmuir, 2012, 28, 6216–6231 CrossRef CAS PubMed.
- Y. Kobayashi, Y. Horie, K. Honjo, T. Uemura and S. Kitagawa, ChemComm, 2016, 52, 5156–5159 RSC.
- T. A. Shifa and A. Vomiero, Adv. Energy Mater., 2019, 9, 1902307 CrossRef CAS.
- X. Fei, P. Wang, D. Zhang, H. Wang and Z. Wu, ChemCatChem, 2021, 13, 2313–2336 CrossRef CAS.
- T. S. Koblenz, J. Wassenaar and J. N. Reek, Chem. Soc. Rev., 2008, 37, 247–262 RSC.
- W. A. Solomonsz, G. A. Rance, B. J. Harris and A. N. Khlobystov, Nanoscale, 2013, 5, 12200–12205 RSC.
- Q. Zhang, L. Zhu, T. Hou, H. Chang, Q. Bai, J. Zhao and D. Liang, Macromolecules, 2019, 52, 4251–4259 CrossRef CAS.
- M. D. Halls and H. B. Schlegel, J. Phys. Chem. B, 2002, 106, 1921–1925 CrossRef CAS.
- D. Fiedler, H. van Halbeek, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2006, 128, 10240–10252 CrossRef CAS PubMed.
- D. Xu, H. Lv and B. Liu, Front. Chem., 2018, 6, 550 CrossRef CAS PubMed.
- S. A. Miners, G. A. Rance and A. N. Khlobystov, ChemComm, 2013, 49, 5586–5588 RSC.
- L. Jing, C. Tang, Q. Tian, T. Liu, S. Ye, P. Su, Y. Zheng and J. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 39763–39771 CrossRef CAS PubMed.
- S. Wang, Y. Liu, P. Ge, Q. Kan, N. Yu, J. Wang, J. Nan, S. Ye, J. Zhang, W. Xu and B. Yang, Lab Chip, 2018, 18, 979–988 RSC.
- S. A. Miners, G. A. Rance and A. N. Khlobystov, Chem. Soc. Rev., 2016, 45, 4727–4746 RSC.
- W. A. Solomonsz, G. A. Rance, M. Suyetin, A. La Torre, E. Bichoutskaia and A. N. Khlobystov, Chemistry, 2012, 18, 13180–13187 CrossRef CAS PubMed.
- S. A. Shermukhamedov, R. R. Nazmutdinov, M. D. Bronshtein and M. Probst, ChemElectroChem, 2021, 8, 563–569 CrossRef CAS.
- A. E. Giannakopoulos, F. Sofos, T. E. Karakasidis and A. Liakopoulos, Int. J. Heat Mass Transfer, 2012, 55, 5087–5092 CrossRef CAS.
- J. Xiao, X. Pan, S. Guo, P. Ren and X. Bao, J. Am. Chem. Soc., 2015, 137, 477–482 CrossRef CAS PubMed.
- A. Avid and I. V. Zenyuk, Curr. Opin. Electrochem., 2021, 25, 100634 CrossRef CAS.
- R. R. Nazmutdinov, M. D. Bronshtein, A. S. Berezin, G. Soldano and W. Schmickler, J. Electroanal. Chem., 2011, 660, 309–313 CrossRef CAS.
- M. M. Hingorani and M. O'Donnell, Nat. Rev. Mol. Cell Biol., 2000, 1, 22–30 CrossRef CAS PubMed.
- B. A. Kelch, D. L. Makino, M. O'Donnell and J. Kuriyan, Science, 2011, 334, 1675–1680 CrossRef CAS PubMed.
- H. Ledford, Nat. Catal., 2015, 522, 20–24 CAS.
- M. Jinek, F. Jiang, D. W. Taylor, S. H. Sternberg, E. Kaya, E. Ma, C. Anders, M. Hauer, K. Zhou, S. Lin, M. Kaplan, A. T. Iavarone, E. Charpentier, E. Nogales and J. A. Doudna, Science, 2014, 343, 1247997 CrossRef PubMed.
- J. M. Crawford, P. M. Thomas, J. R. Scheerer, A. L. Vagstad, N. L. Kelleher and C. A. Townsend, Science, 2008, 320, 243–246 CrossRef CAS PubMed.
- C. T. Walsh, Science, 2004, 303, 1805–1810 CrossRef CAS PubMed.
- D. P. Cogan, K. Zhang, X. Li, S. Li, G. D. Pintilie, S. H. Roh, C. S. Craik, W. Chiu and C. Khosla, Science, 2021, 374, 729–734 CrossRef CAS PubMed.
- A. P. Srivastava, M. Luo, W. Zhou, J. Symersky, D. Bai, M. G. Chambers, J. D. Faraldo-Gomez, M. Liao and D. M. Mueller, Science, 2018, 360, eaas9699 CrossRef PubMed.
- Y. Hao, S. Pang, X. Zhang and L. Jiang, Chem. Sci., 2020, 11, 10035–10046 RSC.
- M. Ge, C. Cao, J. Huang, S. Li, Z. Chen, K.-Q. Zhang, S. S. Al-Deyab and Y. Lai, J. Mater. Chem. A, 2016, 4, 6772–6801 RSC.
- M.-O. Coppens, Curr. Opin. Chem. Eng., 2012, 1, 281–289 CrossRef CAS.
- C. Wang, S. Wang, H. Pan, L. Min, H. Zheng, H. Zhu, G. Liu, W. Yang, X. Chen and X. Hou, Sci. Adv., 2020, 6, eabb4700 CrossRef CAS PubMed.
- X. Hou, Y. Hu, A. Grinthal, M. Khan and J. Aizenberg, Nature, 2015, 519, 70–73 CrossRef CAS PubMed.
- Y. Hou and X. Hou, Science, 2021, 373, 628–629 CrossRef CAS PubMed.
- T. Sun, L. Feng, X. Gao and L. Jiang, Acc. Chem. Res., 2005, 38, 644–652 CrossRef CAS PubMed.
- Y. Zou, K. Xiao, Q. Qin, J. W. Shi, T. Heil, Y. Markushyna, L. Jiang, M. Antonietti and A. Savateev, ACS Nano, 2021, 15, 6551–6561 CrossRef CAS PubMed.
- A. H. Proppe, Y. C. Li, A. Aspuru-Guzik, C. P. Berlinguette, C. J. Chang, R. Cogdell, A. G. Doyle, J. Flick, N. M. Gabor, R. van Grondelle, S. Hammes-Schiffer, S. A. Jaffer, S. O. Kelley, M. Leclerc, K. Leo, T. E. Mallouk, P. Narang, G. S. Schlau-Cohen, G. D. Scholes, A. Vojvodic, V. W.-W. Yam, J. Y. Yang and E. H. Sargent, Nat. Rev. Mater., 2020, 5, 828–846 CrossRef CAS.
- Y. Zhao, J. Wang, X. Y. Kong, W. Xin, T. Zhou, Y. Qian, L. Yang, J. Pang, L. Jiang and L. Wen, Natl. Sci. Rev., 2020, 7, 1793 CrossRef PubMed.
- D. G. Haywood, A. Saha-Shah, L. A. Baker and S. C. Jacobson, Anal. Chem., 2015, 87, 172–187 CrossRef CAS PubMed.
- J. Zhang, Z. Li, K. Zhan, R. Sun, Z. Sheng, M. Wang, S. Wang and X. Hou, Electrophoresis, 2019, 40, 2029–2040 CrossRef CAS PubMed.
- M. Wang, Y. Hou, L. Yu and X. Hou, Nano Lett., 2020, 20, 6937–6946 CrossRef CAS PubMed.
- Z. Zhang, L. Wen and L. Jiang, Chem. Soc. Rev., 2018, 47, 322–356 RSC.
- J. Chen, W. Xin, W. Chen, X. Zhao, Y. Qian, X. Y. Kong, L. Jiang and L. Wen, ACS Cent. Sci., 2021, 7, 1486–1492 CrossRef CAS PubMed.
- S. Hou, W. Ji, J. Chen, Y. Teng, L. Wen and L. Jiang, Angew. Chem., Int. Ed. Engl., 2021, 60, 9925–9930 CrossRef CAS PubMed.
- L. Yan, L. Cao, P. Dai, X. Gu, D. Liu, L. Li, Y. Wang and X. Zhao, Adv. Funct. Mater., 2017, 27, 1703455 CrossRef.
- U. Lačnjevac, R. Vasilić, T. Tokarski, G. Cios, P. Żabiński, N. Elezović and N. Krstajić, Nano Energy, 2018, 47, 527–538 CrossRef.
- L. Zhang, S. Li, C. J. Gomez-Garcia, H. Ma, C. Zhang, H. Pang and B. Li, ACS Appl. Mater. Interfaces, 2018, 10, 31498–31504 CrossRef CAS PubMed.
- Z. K. Yang, C.-Z. Yuan and A.-W. Xu, ACS Energy Lett., 2018, 3, 2383–2389 CrossRef CAS.
- C. Guo, Y. Li, Z. Li, Y. Liu, Y. Si and Z. Luo, Nanoscale Res. Lett., 2020, 15, 21 CrossRef CAS PubMed.
- B. Tang, Z. G. Yu, Y. Zhang, C. Tang, H. L. Seng, Z. W. Seh, Y.-W. Zhang, S. J. Pennycook, H. Gong and W. Yang, J. Mater. Chem. A, 2019, 7, 13339–13346 RSC.
- H. Pan, Z. Jiang, Z. Zuo, F. He, F. Wang, L. Li, Q. Chang, B. Guan and Y. Li, Nano Today, 2021, 39, 101213 CrossRef CAS.
- S. Panić, Á. Kukovecz and G. Boskovic, Appl. Catal., B, 2018, 225, 207–217 CrossRef.
- K. Wu, X. Y. Kong, K. Xiao, Y. Wei, C. Zhu, R. Zhou, M. Si, J. Wang, Y. Zhang and L. Wen, Adv. Funct. Mater., 2019, 29, 1807953 CrossRef.
- S. Xing, Z. Gao, S. Zhao, M. Xiong, P. Wang, J. Zhang, S. Wang, G. Wang and Y. Qin, Green Chem., 2021, 23, 8116–8123 RSC.
- Y. Xu, Q. Wu, Y. Shimatani and K. Yamaguchi, Lab Chip, 2015, 15, 3856–3861 RSC.
- X. Ni, Z. Cui, H. Luo, H. Chen, C. Liu, Q. Wu and A. Ju, Chem. Eng. J., 2021, 404, 126249 CrossRef CAS.
- X. Han, Q. Gao, Z. Yan, M. Ji, C. Long and H. Zhu, Nanoscale, 2021, 13, 1515–1528 RSC.
- M. Seo and T. D. Chung, Curr. Opin. Electrochem., 2019, 13, 47–54 CrossRef CAS.
- D. Munoz-Santiburcio and D. Marx, Chem. Rev., 2021, 121, 6293–6320 CrossRef CAS PubMed.
- B. Dong, Y. Pei, F. Zhao, T. W. Goh, Z. Qi, C. Xiao, K. Chen, W. Huang and N. Fang, Nat. Catal., 2018, 1, 135–140 CrossRef.
- F. Zhang, B. Song and L. Jiang, Nano Res., 2021, 14, 4367–4369 CrossRef CAS.
- H.-L. Nie and J. Huang, Matter, 2019, 1, 1430–1432 CrossRef.
- C. Pei and J. Gong, Science, 2021, 371, 1203–1204 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |