Open Access Article
Open Access ArticlepH-responsive hollow Fe–gallic acid coordination polymer for multimodal synergistic-therapy and MRI of cancer†
Congcong
Liu
a,
Chengcheng
Li
a,
Sen
Jiang
a,
Cheng
Zhang
b and
Yang
Tian
 *a
*a
aDepartment of Chemistry, Analytical Instrumentation Center, 105 North Road of Western Third Ring, Haidian District, Beijing 100048, China. E-mail: tianyang@cnu.edu.cn
bCollege of Life Science, Capital Normal University, 105 North Road of Western Third Ring, Haidian District, Beijing 100048, China
First published on 8th November 2021
Abstract
Tumor-microenvironment (TME) responsive nanostructures are attractive for drug delivery in clinical cancer treatment. The coordination polymer Fe–gallic acid (Fe–GA) is one of the promising drug carriers due to its pH-response, good biocompatibility, and minimal side effects. However, the hollow nanostructures of Fe–GA have not been reported until now, which seriously limits the quantity of drug delivery. Herein, hollow Fe–GA nanospheres were prepared for the first time with bovine serum albumin (BSA) combination (denoted as Fe–GA/BSA) under mild reaction conditions. Then, the antitumor drug doxorubicin (DOX) was loaded in the hollow Fe–GA/BSA to obtain Fe–GA/BSA@DOX. A series of experiments in vitro and in vivo indicated that the Fe–GA/BSA@DOX could efficiently respond to TME and release DOX and Fe(III) ions. Furthermore, the Fe(III) could consume overexpressed glutathione (GSH) in cancer cells and generate Fe(II) to trigger the Fenton reaction, producing ·OH for chemodynamic treatment (CDT) of cancer. In addition, the Fe–GA/BSA@DOX could effectively convert near-infrared (NIR) light into heat by acting as a photothermal therapy (PTT) agent. Besides that, magnetic resonance imaging (MRI) data also showed that the Fe–GA/BSA had beneficial T1 and T2 imaging effects, demonstrating that the hollow Fe–GA/BSA has potential for multimodal synergistic cancer MRI diagnosis and therapies of drugs, CDT, and PTT.
1. Introduction
Cancer is still a severe threat to human health in the world. Drug treatment is regarded as one of the indispensable means in the clinic to face the cancer menace.1,2 In recent years, drug delivery based on nanomaterials in cancer therapy has been attractive due to the controlled release, targeted delivery, and multiple-drug loading,1,3–5 which can enhance the cancer cure rate and reduce side effects.6 At present, general nanomaterials for drug delivery include inorganic nanoparticle drug binders,7–9 organic polymers,10,11 hydrogel nanoparticles,12–14 and coordination polymers.15–19 Although the use of different nanodrug delivery systems for cancer drug treatment has achieved encouraging results, the potential biosafety problem still hinders the use of most nanodrug carriers.20,21 Especially, biodegradability of nanomaterial carriers is a critical challenge in future clinical applications.22The coordination polymer consisting of Fe(III) and gallic acid (GA) has been widely used in drug delivery,23 in which Fe(III) is coordinated with the –COOH of GA in unsaturated modality.24,25 It can gradually degrade in response to the weakly acidic tumor microenvironment (TME),26 and be effectively excreted from the body without causing long-term retention and toxicity problems.27 Until now, many examples of various drugs have been successfully delivered by the Fe–GA coordination polymer.28 However, most reported drug loadings by the Fe–GA coordination polymer relied on surface adsorption between the drug molecules and Fe–GA, resulting in unsatisfactory drug loading amount.29 Therefore, there is an urgent need to design Fe–GA complexes with a larger inner cavity for drug-loaded cancer treatment.
In the meantime, the development of drug-loaded nanomaterials that can be combined with multiple treatment methods is the current trend in cancer therapy. Among the various treatment systems studied in recent years, photothermal therapy (PTT) employs laser light inducing high temperature at tumor sites to kill cancer cells,30–34 which has higher specificity, minimal invasiveness, and lower side effects. Chemodynamic therapy (CDT) is an emerging treatment strategy that uses chemical reactions such as the Fenton reaction to produce reactive oxygen species (ROS) to kill tumor cells in the tumor in situ with minor side effects.7,35 It uses the iron-catalyzed Fenton reaction to convert hydrogen peroxide (H2O2),28,36 which is less active, into hydroxyl radicals (·OH) with a significantly more vital oxidizing ability.37,38 Therefore, drug-delivery treatment combined with PTT and CDT treatments is promising for enhancing cancer therapy efficiency.39–41
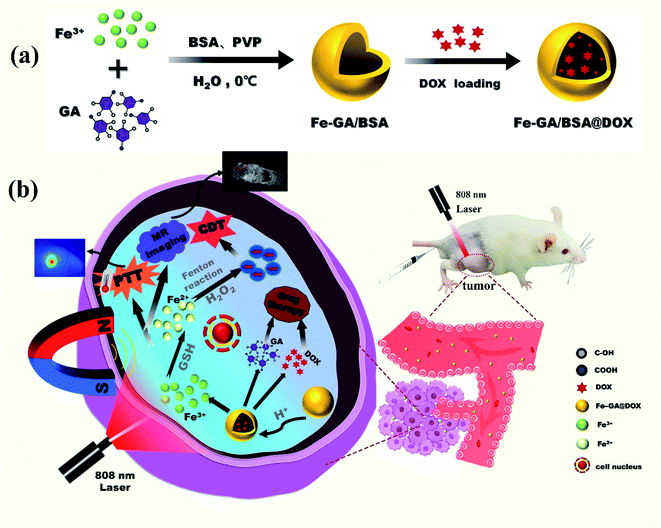
Herein, Fe–GA coordination polymers with a hollow nanosphere structure were prepared for the first time with bovine serum albumin (BSA) combination (denoted as Fe–GA/BSA) under mild reaction conditions. With the help of this hollow structure, the drug doxorubicin (DOX) for cancer treatment was physically embedded in the hollow Fe–GA/BSA. The prepared Fe–GA/BSA@DOX would be destroyed due to the unsaturated coordination structure in TME, releasing the drug DOX and Fe(III). The released Fe(III) was reduced by glutathione (GSH) into Fe(II) in TME and reacted with H2O2 to generate hydroxyl radicals (·OH) via the Fenton reaction for tumor CDT. Furthermore, the Fe–GA/BSA@DOX showed an excellent photothermal conversion effect and could kill cancer cells through PTT. In addition, the Fe–GA/BSA@DOX material also had a noticeable MRI biological imaging effect due to the existence of Fe element. All the above biological synergistic therapy and imaging diagnosis were evaluated by experiments in vitro and in vivo, suggesting the prepared hollow Fe–GA/BSA nanospheres as a potential image-guided synergistic therapy material, which can realize the integration of diagnosis and treatment (Scheme 1).
2. Results and discussion
2.1 Synthesis and characterization of Fe–GA/BSA@DOX nanospheres
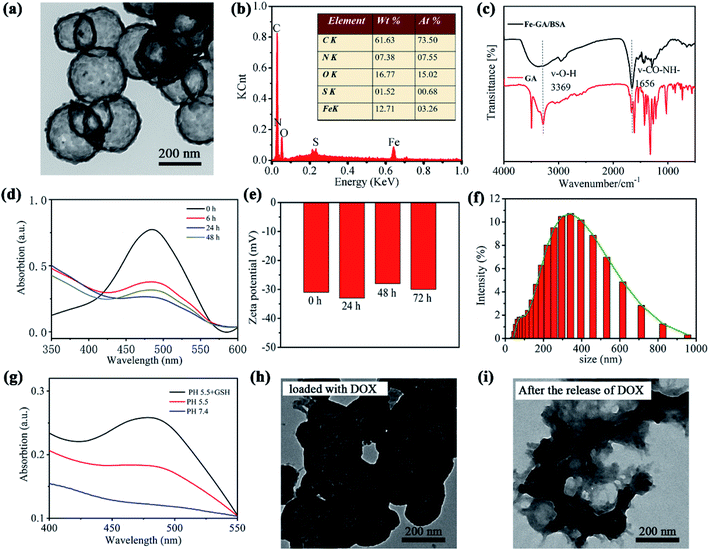
In the synthesis of hollow nanospheres of Fe–GA/BSA coordination polymer, FeCl3·6H2O and GA were chosen as the precursors and PVP as the surfactant template of the hollow structure; BSA was added in the reaction solution to increase the biocompatibility of the product (details in the ESI†). To obtain high-quality hollow nanospheres, we explored the reaction conditions, such as temperature, reaction time, and surfactant content as shown in Fig. S1 and S2.† Finally, high-quality Fe–GA/BSA hollow nanospheres with uniform size were obtained at 0 °C in 2 h.The prepared Fe–GA/BSA product was observed by TEM, which indicated that the hollow spherical structure had a diameter of about 200 nm and uniform shell thickness, as shown in Fig. 1a. Energy dispersive X-ray (EDX) spectrum (Fig. 1b) demonstrates the existence of Fe in the hollow spheres and the elements C, N, and S, which came from the GA ligand and BSA molecules. Fig. 1c is the FT-IR spectrum analysis of the prepared Fe–GA/BSA hollow nanospheres. It reveals that the O–H stretching vibration peak of Fe–GA/BSA at 3369 cm−1 disappeared, indicating the chelation of the phenols in GA with Fe(III). In addition, the BSA-amide I band vibration at 1650 cm−1 and the amide II band vibration at 1521 cm−1 suggest that BSA was present in the Fe–GA/BSA hollow nanospheres.
To study the drug delivery of the prepared hollow Fe–GA/BSA nanospheres, we employed a chemotherapeutic drug (DOX) with anticancer effects as the drug model in our work. A 6 mL PBS solution of Fe–GA/BSA (7 mg) was mixed with DOX solution (1.5 mg, 0.25 mg mL−1), and the supernatant was monitored by UV-vis absorption at different time points. As shown in Fig. 1d, the DOX concentration of the supernatant decreased approximately to 48.8% at 6 h, indicating that 51.2% DOX had been loaded in the hollow Fe–GA/BSA nanospheres. The loading of the drug DOX reached the maximal value of 66.1% at 24 h according to UV-vis absorption. Therefore, we characterized the prepared hollow Fe–GA/BSA spheres with DOX loading for 24 h (defined as Fe–GA/BSA@DOX) for further therapy applications. To verify the stabilization in water, the Z potential and hydraulic dynamic size of the Fe–GA/BSA@DOX were measured. Fig. 1e shows around −30 mV during the tested 72 h, indicating high surface negative-electricity and satisfactory stabilization in aqueous solution. Simultaneously, the hydraulic dynamic size was distributed around 300 nm, as shown in Fig. 1f.42
Subsequently, we studied the Fe–GA/BSA@DOX to verify its targeted release performance in a simulated tumor microenvironment by UV-vis means. Fig. 1g shows that after the sample stayed in a weak alkaline PBS solution (pH = 7.4) for 24 h, its supernatant did not present the absorption peak of DOX. In contrast, after the sample stayed in a weakly acidic PBS solution (pH = 5.5) for 24 h, its supernatant indicated apparent characteristic absorption of DOX around 480 nm. The result suggested well that the Fe–GA/BSA@DOX release the drug for efficient tumor targeting due to the weakly acidic TME. Moreover, it is known that a high expression of GSH in TME is the characteristic of tumor. Therefore, we additionally tested the drug release performance of the Fe–GA/BSA@ DOX in a PBS solution containing GSH at pH 5.5. It shows a significantly enhanced DOX content as shown in Fig. 1g, demonstrating the higher release efficiency in the presence of GSH. This should be attributed to the reduction of Fe(III) by GSH, promoting the degradation of Fe–GA/BSA unsaturated-coordination polymer. The results indicate that the prepared material can be used as a drug delivery nanodrug, with a perfect targeted drug-release performance toward TME.
2.2 Photothermal performances of the Fe–GA/BSA@DOX coordination polymer
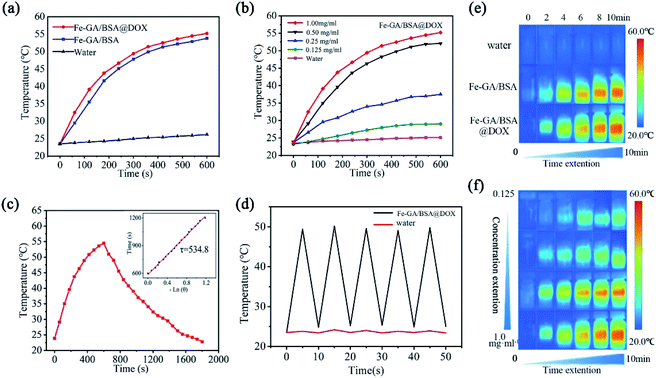
To preliminarily test the photothermal performance of the hollow Fe–GA/BSA nanodrugs, we conducted a series of photothermal conversion experiments by using 808 nm laser irradiation (0.7 W cm−2) and used an infrared thermal imager to monitor the temperature changes. Fig. 2a presents the temperature of pure water raised by about 3 °C (from 23 to 26 °C) after 808 nm laser irradiation for 10 min. In contrast, the solutions containing Fe–GA/BSA or Fe–GA/BSA@ DOX with the concentration of 1 mg mL−1 increased to above 50 °C from room temperature, indicating palpable photothermal performance, and the loading of the drug has no negative effect on the photothermal conversion. Fig. 2b shows that the temperature rise is well dependent on the solution concentration of Fe–GA/BSA@DOX from 0 to 1.0 mg mL−1, confirming that the photothermal performance came from the prepared nanodrugs indeed. According to Fig. 2c, the calculated photothermal conversion efficiency of Fe–GA/BSA@DOX is approximately 67.14% (details are provided in the ESI†).43 Compared with most iron-based photothermal nanodrugs, the prepared Fe–GA/BSA@DOX has a higher photothermal conversion efficiency (Table S2†).44–49 Furthermore, the prepared Fe–GA/BSA@DOX indicated favorable photothermal performance stability after 5 tested cycles (Fig. 2d). Fig. 2e and f display the color images of the irradiated part corresponding to the temperature curves in Fig. 2a and b, respectively. These results indicate that the Fe–GA/BSA@DOX nanodrug can effectively convert NIR light to local overheating, which provides a possibility for further application in the photothermal therapy of tumors in vivo.2.3 ROS generation of the hollow Fe– GA/BSA nanospheres for CDT
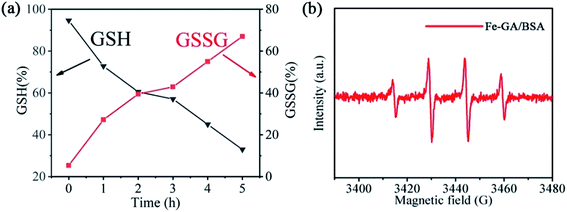
To confirm our deduction that the hollow Fe–GA/BSA nanospheres could play a potential role in CDT for cancer, we simulated the process of producing ˙OH through the Fenton reaction and examined it in an aqueous solution. As a control, the GSH mixed with DOX in PBS (pH = 5.5) rarely decreased and the oxidized GSSG did not increase, which were monitored using the GSH test kit (Fig. S7†). In contrast, as shown in Fig. 3a, the GSH percentage decreased gradually in the PBS (pH = 5.5) solution of the hollow Fe–GA/BSA nanospheres, and reduced to 33% after 5 hours. Correspondingly, the oxidation product of GSH (GSSG), increased from 0 to 81%. The results indicated that the GSH was oxidized by Fe(III) in Fe–GA/BSA, which was accordingly reduced into Fe(II). The produced Fe(II) was further able to catalyze the Fenton reaction in the presence of H2O2. Furthermore, as shown in Fig. 3b, a strong ROS signal of ˙OH radicals was detected by ESR analysis, confirming that the hollow Fe–GA/BSA nanospheres could be used for enhanced CDT toward the tumor.2.4 In vitro experiments of the prepared Fe–GA/BSA@DOX
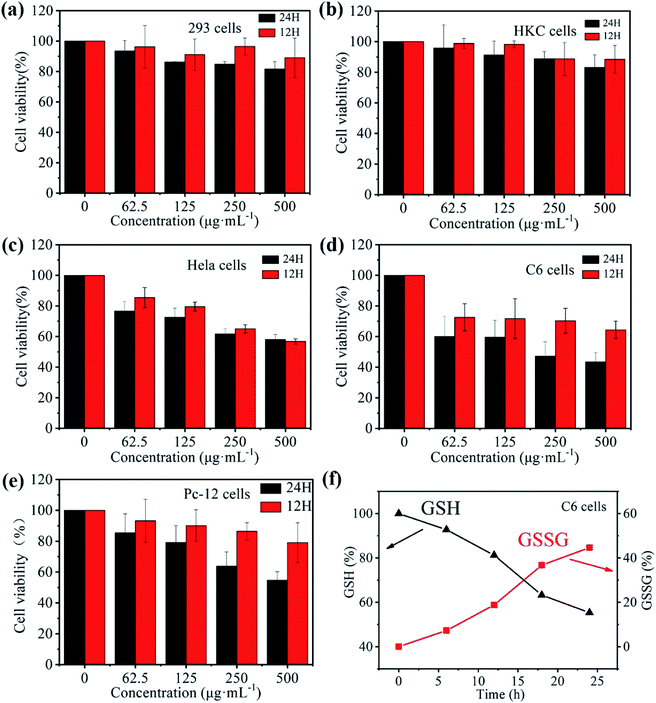
To test the possibility of medical application of the prepared Fe–GA/BSA@DOX, we used the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method to determine the cell toxicity in vitro. In our work, 293 cells (a human renal epithelial cell line), HKC cells (a human renal tubular epithelial cell line), HeLa cells (a human epithelioid cervix carcinoma cell line), C6 cells (rat glioma cells), and Pc-12 cells (pheochromocytoma cells of the rat adrenal medulla) were incubated respectively with the Fe–GA/BSA@DOX of various concentrations (0–500 μg mL−1) for 12 h and 24 h. As shown in Fig. 4a and b, the cell viability of 293 cells and HKC cells was not only retained up to 90% after 12 h of incubation but also up to 80% with 24 h of incubation. The results demonstrated that the Fe–GA/BSA@DOX nanodrug was less destructive and highly biologically safe to normal cells. However, Fig. 4c–e showed that the three kinds of cancer cells suffered palpable damage at the Fe–GA/BSA@DOX concentration of 500 μg mL−1. Especially, the viability of the incubated C6 cells was preserved less than 80% only at 125 μg mL−1 Fe–GA/BSA@DOX after 12 hours and decreased to less than 60% in 24 h of incubation. This confirmed that the prepared nanodrug could lead to the apoptosis of tumor cells. Further study showed that (Fig. 4f) the GSH in C6 cells was consumed obviously, which decreased ca. 50%; the oxidation product GSSG of GSH increased from 0 to 50% correspondingly. All results indicated that the released DOX in cancer cells and CDT effects caused by the Fenton reaction in the TME were responsible for cancer cell apoptosis.2.5 In vivo PTT and MRI performances of the prepared nanodrugs
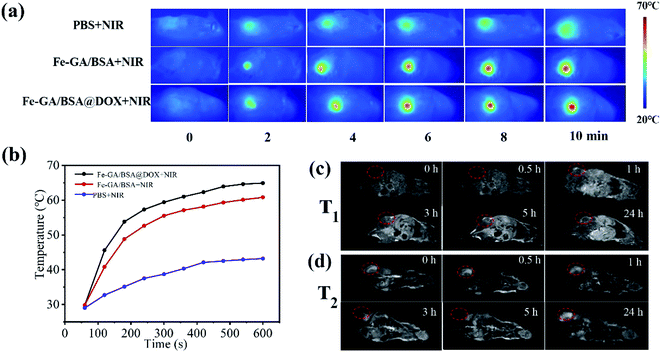
Inspired by the excellent photothermal conversion efficiency in vitro, we studied the PTT efficiency of the Fe–GA/BSA@DOX in vivo. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Capital Normal University and experiments were approved by the Animal Ethics Committee of Capital Normal University. To obtain a tumor mouse model, approximately 106 individual glioma cells were injected subcutaneously into male CD-1 experimental mice (6 weeks old, weighing 20–25 g) for 7 days of feeding. Then the glioma-bearing mice (tumor volume ∼100 mm3) were injected with 200 μL PBS, Fe–GA/BSA (1 mg mL−1), and Fe–GA/BSA@DOX (1 mg mL−1) solutions, respectively. Following this, an NIR laser with the wavelength of 808 nm (0.7 W cm−2) was used to irradiate mouse tumors for 10 min. During the irradiation, the temperature change data and photothermal photos of the tumor site were obtained through an NIR thermal imager. Fig. 5a is a thermal image of the whole body of a mouse taken at different time intervals. The results showed that under NIR irradiation, the temperature of the tumor site increased rapidly after the injection of Fe–GA/BSA and Fe–GA/BSA@DOX. In contrast, the mice injected with PBS detected a minimal temperature increase under NIR radiation. As shown in Fig. 5b, under NIR laser irradiation, the temperature of the tumor area containing Fe–GA/BSA or Fe–GA/BSA@DOX increased rapidly to above 60 °C within 10 minutes. The high temperature above 60 °C is sufficient to kill cancer cells, suggesting that the prepared hollow nanospheres are favorable for the PTT of the tumor.Based on the excellent performance of previous MRI tests in vitro (Fig. S8†), 200 μL Fe–GA/BSA@DOX in PBS solution (1 mg mL−1) was administered intravenously to the glioma-bearing mice at the dose of 10 mg kg−1. MRI system (0.47 T) was used to detect and record T1 and T2 signals before and after injection at various time points. Fig. 5c shows the T1-weighted MR images of the mouse with post-injection of Fe–GA/BSA@DOX from 0 h to 24 h. Before the injection (or at 0 h), the MR image of the tumor site showed considerable darkness (Fig. 5c). With the increase of time, the tumor area gradually changed from dark to bright, indicating the imaging effect of a representative T1 contrast agent. It displayed the brightest signal after the post-injection of 5 hours. Then, the T1-weighted image became dark gradually again, indicating the beneficial metabolic effect in the mouse body. On the other hand, Fig. 5d shows the T2-weighted images of the mouse for post-injection of the Fe–GA/BSA@DOX from 0 h to 24 h. The MR image of the tumor site showed considerable brightness before the injection (or at 0 h); then, the T2-weight images changed from bright to dark, which showed that the material had the imaging effect of a representative T2 contrast agent. The T2 signal of the tumor site reached the maximal value after the post-injection of 5 h. At the same time, the tumor site in Fig. 5d returned to bright after post-injection of 24 hours, also proving that the prepared Fe–GA/BSA@DOX had good metabolism and low toxicity.
2.6 In vivo combination therapy of PTT, CDT, and drug treatment
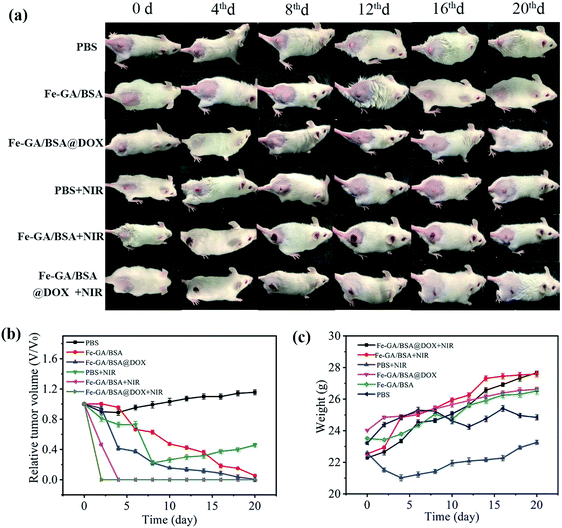
We further studied the combination therapy efficiency of the Fe–GA/BSA@DOX in vivo via the glioma-bearing mouse model. When the volume of the solid tumor grew to ∼100 mm3, we randomly divided the glioma-bearing mice into six groups for different treatments, including (1) only PBS (control group), (2) only hollow Fe–GA/BSA nanospheres, (3) Fe–GA/BSA@DOX, (4) PBS + NIR laser irradiation (10 minutes, 0.7 W cm−2), (5) Fe–GA/BSA + NIR laser irradiation (10 minutes, 0.7 W cm−2), and (6) Fe–GA/BSA@DOX + NIR laser irradiation (10 minutes, 0.7 W cm−2). The injection doses of the used PBS, Fe–GA/BSA (1 mg mL−1), and Fe–GA/BSA@DOX (1 mg mL−1) solution were 200 μL, which were all injected through the tail vein of the mice.After the next 20 days of injection, we recorded the growth of subcutaneous tumors in mice with different treatments. We took pictures of the tumors for each treated mouse every 4 days (Fig. 6a) and recorded the tumor volume every 2 days (Fig. 6b). As shown in Fig. 6a and b, the PBS group had no obvious antitumor effect on the mice in the monitored 20 days without NIR radiation. However, the Fe–GA/BSA group tumor dwindled gradually, and the tumor volume approached 0 on the 20th day. This suggests that the hollow Fe–GA/BSA nanospheres worked CDT against the tumor efficiently because the Fe ions catalyzed the Fenton reaction to produce ROS of ·OH. In addition, the Fe–GA/BSA@DOX group showed that the tumor volume depressed quickly in 10 days, and the tumor nearly vanished on the 18th day. This result demonstrated that the anticancer drug DOX delivered by Fe–GA/BSA could accelerate the tumor treatment.
On the other hand, NIR irradiation of 808 nm was further applied to the tumor-bearing mice with injections of PBS, Fe–GA/BSA (1 mg mL−1), and Fe–GA/BSA@DOX (1 mg mL−1), respectively. As suggested in Fig. 6a and b, the tumor growth was hardly blocked in the PBS + NIR group. However, the tumors in the Fe–GA/BSA + NIR group and Fe–GA/BSA@DOX + NIR group clearly showed a scab, indicating the PTT effect of the prepared hollow Fe–GA/BSA nanospheres. Significantly, the tumor growth in the Fe–GA/BSA@DOX + NIR group was rapidly inhibited after 2 days of NIR irradiation, which was shorter than that of Fe–GA/BSA + NIR (4 days). All results show that the Fe–GA/BSA@DOX + NIR group had the most substantial antitumor effect due to the combination treatment of PTT, drugs, and CDT. Moreover, no recurrence was detected within the next two months.
At the same time, Fig. 6c showed that the bodyweight of each group kept increasing, and the mice survival-rate was 100%, proving that our combined treatment had fewer side effects. Finally, the experimental mice were sacrificed after the observation of 20 days, and the possibility of toxicity to major organs was studied by hematoxylin and eosin staining (Fig. S10†). No obvious tissue abnormalities or damage was found in the mice's heart, liver, spleen, lung, and kidney in all groups, which indicated that the Fe–GA/BSA@DOX has no apparent systemic toxicity to major organs during tumor treatment. All experimental results showed that the Fe–GA/BSA@DOX has excellent tumor inhibitory effects via drug delivery, PTT, and CDT with good biocompatibility and minimal side effects.
3. Conclusion
In summary, we prepared hollow Fe–GA/BSA nanospheres as a framework for drug delivery of DOX. The Fe–GA/BSA@DOX could be disrupted, leading to the nanospheres' cleavage and thus releasing the DOX encapsulated inside for drug treatment of tumor tissues. At the same time, the released Fe(III) consumed the overexpressed GSH in tumor cells to obtain Fe(II), which triggered the Fenton reaction to produce ˙OH. The hydroxyl radicals can kill tumor cells in the CDT of cancer. In addition, the Fe–GA/BSA@DOX showed a high photothermal conversion efficiency of 67.14%, making it an excellent agent of PTT. Meanwhile, this material can also be used as a T1 and T2 contrast agent. Overall, the Fe–GA/BSA@DOX has achieved the simultaneous action of three therapies (drug therapy, PTT, and CDT) and cancer diagnosis. We expect that this therapeutic strategy will provide new insights into the future of cancer treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 52172096) and classification development at Capital Normal University (No. 2155091, 2155160, 2155161).References
- R. van der Meel, E. Sulheim, Y. Shi, F. Kiessling, W. J. Mulder and T. Lammers, Nat. Nanotechnol., 2019, 14, 1007–1017 CrossRef CAS PubMed.
- R. G. Kenny and C. Marmion, Chem. Rev., 2019, 119, 1058–1137 CrossRef CAS.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- I. de Lázaro and D. J. Mooney, Nat. Mater., 2021, 1–11 Search PubMed.
- R. Kumari, D. Sunil and R. Ningthoujam, J. Controlled Release, 2019, 319, 135–136 CrossRef PubMed.
- Y. Bae and K. Park, Adv. Drug Delivery Rev., 2020, 158, 4–16 CrossRef CAS.
- C. Liu, Y. Cao, Y. Cheng, D. Wang, T. Xu, L. Su, X. Zhang and H. Dong, Nat. Commun., 2020, 11, 1–9 Search PubMed.
- A. Shetty and S. Chandra, Mater. Today Chem., 2020, 18, 100381 CrossRef CAS.
- N. Fernandes, C. F. Rodrigues, A. Moreira and I. Correia, Biomater. Sci., 2020, 8, 2990–3020 RSC.
- Y.-f. Zhang, F. Fang, L. Li and J. Zhang, ACS Biomater. Sci. Eng., 2020, 6, 4816–4833 CrossRef CAS.
- H. Yin, P. Cao, X.-Y. Wang, Y. Li and X. Yin, Computed Tomography Imaging-Guided Tandem Catalysis-Enhanced Photodynamic Therapy with Gold Nanoparticle Functional Covalent Organic Polymers, 2020, pp. 2534–2542 Search PubMed.
- H. J. Won, B. Ryplida, S.-G. Kim, G. Lee, J. Ryu and S. Y. Park, ACS Nano, 2020, 14, 8409–8420 CrossRef CAS PubMed.
- Y. Xiao, Y. Gu, L. Qin, L. Chen, X. Chen, W. Cui, F. Li, N. Xiang and X. He, Colloids Surf., B, 2021, 200, 111581 CrossRef CAS.
- C. Wang, N. Zhao and W. Yuan, ACS Appl. Mater. Interfaces, 2020, 12, 9118–9131 CrossRef CAS.
- Y. Liu, S. Lv, D. Liu and F. Song, Acta Biomater., 2020, 116, 16–31 CrossRef CAS.
- X. Li, S. Lu, X. Mu, T. Li, S. Sun, Y. Zhao, J. Hai and B. Wang, Biosens. Bioelectron., 2021, 190, 113417 CrossRef CAS.
- W. Meng, J. Hao, Z. Tong, H. Liu, Y. Li and X. Zhang, Inorg. Chim. Acta, 2020, 120108 Search PubMed.
- L. Ni, S.-L. Wang, J. Zhao, G. Wu and L.-Y. Lang, J. Mol. Struct., 2020, 1227, 129364 CrossRef.
- X. Yu, T. Jin, F. Lu, Z.-J. Luo, C. Liu and W.-X. Jin, Arabian J. Chem., 2021, 14, 102998 CrossRef CAS.
- Y. Wang, Z. Li and Q. Hu, Nano Today, 2021, 38, 101127 CrossRef CAS.
- Z. Liao, S. W. Wong, H. L. Yeo and Y. Zhao, NanoImpact, 2020, 20, 100253 CrossRef.
- W. Wang, X. Liu, X. Zheng, H. Jin and X. Li, Adv. Healthcare Mater., 2020, 9, e2001117 CrossRef PubMed.
- H. Zheng, B. Ma, Y. Shi, Q. Dai, D. Li, E. Ren, J. Zhu, J. Liu, H. Chen, Z. Yin, C. Chu, X. Wang and G. Liu, Chem. Eng. J., 2021, 406, 126888 CrossRef CAS.
- X. Mu, C. Yan, Q. Tian, J. Lin and S. Yang, Int. J. Nanomed., 2017, 12, 7207 CrossRef CAS.
- Q. Tian, Y. Cai, N. Li, Q. Liu, B. Gu, Z.-G. Chen and S. Song, Nanomedicine, 2020, 28, 102219 CrossRef CAS.
- P. Zhang, Y. Hou, J. Zeng, Y. Li, Z. Wang, R. Zhu, T. Ma and M. Gao, Angew. Chem., Int. Ed., 2019, 58, 2–11 CrossRef.
- C. Zhang, J. Li, C. Yang, S. Gong, H. Jiang, M. Sun and C. Qian, Nanomedicine, 2020, 23, 102071 CrossRef CAS PubMed.
- E. Hwang and H. S. Jung, Chem. Commun., 2020, 56, 8332–8341 RSC.
- K. Han, W. Y. Zhang, J. Zhang, Z. Y. Ma and H. Y. Han, Adv. Healthcare Mater., 2017, 6, 1700470 CrossRef.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- Q. Chen, Q. Hu, E. Dukhovlinova, G. Chen, S. Ahn, C. Wang, E. A. Ogunnaike, F. S. Ligler, G. Dotti and Z. Gu, Adv. Mater., 2019, 31, 1900192 CrossRef.
- X. Li, J. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 1–18 CrossRef PubMed.
- S. Liu, X. Pan and H. Liu, Angew. Chem., 2020, 132, 5943–5953 CrossRef.
- Z. Zhang, D. Ni, F. Wang, X. Yin, S. Goel, L. N. German, Y. Wang, J. Li, W. Cai and X. Wang, Nano Res., 2020, 13, 3217–3223 CrossRef CAS PubMed.
- Q. Tian, F. Xue, Y. Wang, Y. Cheng, L. An, S. Yang, X. Chen and G. Huang, Nano Today, 2021, 39, 101162 CrossRef CAS.
- X. Liu, Y. Jin, T. Liu, S. Yang, M. Zhou, W. Wang and H. Yu, ACS Biomater. Sci. Eng., 2020, 6, 4834–4845 CrossRef CAS PubMed.
- S. Gao, Y. Jin, K. Ge, Z. Li, H. Liu, X. Dai, Y. Zhang, S. Chen, X. Liang and J. Zhang, Adv. Sci., 2019, 6, 1902137 CrossRef CAS.
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS PubMed.
- Y. Shi, J. Zhang, H. Huang, C. Cao, J. Yin, W. Xu, W. Wang, X. Song, Y. Zhang and X. Dong, Adv. Healthcare Mater., 2020, 9, 2000005 CrossRef CAS.
- X. Wang, X. Zhong, Z. Liu and L. Cheng, Nano Today, 2020, 35, 100946 CrossRef CAS.
- Y. Cheng, H. Lu, F. Yang, Y. Zhang and H. Dong, Nanoscale, 2021, 13, 3049–3060 RSC.
- Y. Wang, Y. Wu, Y. Liu, J. Shen, L. Lv, L. Li, L. Yang, J. Zeng, Y. Wang and L. W. Zhang, Adv. Funct. Mater., 2016, 26, 5335–5344 CrossRef CAS.
- Q. Tian, J. Hu, Y. Zhu, R. Zou, Z. Chen, S. Yang, R. Li, Q. Su, Y. Han and X. Liu, J. Am. Chem. Soc., 2013, 135, 8571–8577 CrossRef CAS PubMed.
- L.-S. Lin, Z.-X. Cong, J.-B. Cao, K.-M. Ke, Q.-L. Peng, J. Gao, H.-H. Yang, G. Liu and X. Chen, ACS Nano, 2014, 8, 3876–3883 CrossRef CAS PubMed.
- Y. Liu, S. Liu, C. Hu, Y. Li and M. Pang, Dalton Trans., 2019, 48, 16848–16852 RSC.
- Y. Wang, X. Liu, G. Deng, Q. Wang, L. Zhang, Q. Wang and J. Lu, J. Mater. Chem. B, 2017, 5, 4221–4232 RSC.
- S. Zhou, X. Jiao, Y. Jiang, Y. Zhao, P. Xue, Y. Liu and J. Liu, Appl. Surf. Sci., 2021, 552, 149498 CrossRef CAS.
- Y. Meng, J. Yang, R. Jiang, S. Wang, L. Zheng, G. Wang, X. Tian, H. Zhu, D. Yan and C. Liu, Appl. Surf. Sci., 2021, 562, 150189 CrossRef CAS.
- J. Wang, H. Zhao, Z. Zhou, P. Zhou, Y. Yan, M. Wang, H. Yang, Y.-j. Zhang and S. Yang, ACS Appl. Mater. Interfaces, 2016, 8 31, 19872–19882 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00721a |
| This journal is © The Royal Society of Chemistry 2022 |