Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photomemristive sensing via charge storage in 2D carbon nitrides†
Andreas
Gouder
 ab,
Alberto
Jiménez-Solano
ab,
Alberto
Jiménez-Solano
 a,
Nella M.
Vargas-Barbosa
a,
Nella M.
Vargas-Barbosa
 c,
Filip
Podjaski
c,
Filip
Podjaski
 *a and
Bettina V.
Lotsch
*a and
Bettina V.
Lotsch
 *ab
*ab
aDepartment Nanochemistry, Max Planck Institute for Solid State Research, Heisenbergstr. 1, 70569 Stuttgart, Germany. E-mail: f.podjaski@fkf.mpg.de; b.lotsch@fkf.mpg.de
bDepartment Chemistry, Ludwig-Maximilians-University, Butenandtstr. 5-13, 81377 Munich, Germany
cInstitute for Energy and Climate Research (IEK-12), Helmholtz Institute Münster, Forschungszentrum Jülich, Corrensstr. 46, 48148 Münster, Germany
First published on 26th April 2022
Abstract
Photomemristive sensors have the potential to innovate current photo-electrochemical sensors by incorporating new sensing capabilities including non-invasive, wireless and time-delayed (memory) readout. Here we report the charge storing 2D carbon nitride potassium poly(heptazine imide), K-PHI, as a direct photomemristive sensing platform by capitalizing on K-PHI's visible light bandgap, large oxidation potential, and intrinsic optoionic charge storage properties. Utilizing the light-induced charge storage function of K-PHI nanosheets, we demonstrate memory sensing via charge accumulation and present potentiometric, impedimetric and coulometric readouts to write/erase this information from the material, with no additional reagents required. Additionally, wireless colorimetric and fluorometric detection of the charging state of K-PHI nanoparticles is demonstrated, enabling the material's use as particle-based autonomous sensing probe in situ. The various readout options of K-PHI's response enable us to adapt the sensitivities and dynamic ranges without modifying the sensing platform, which is demonstrated using glucose as a model analyte over a wide range of concentrations (50 μM to 50 mM). Since K-PHI is earth abundant, biocompatible, chemically robust and responsive to visible light, we anticipate that the photomemristive sensing platform presented herein opens up memristive and neuromorphic functions.
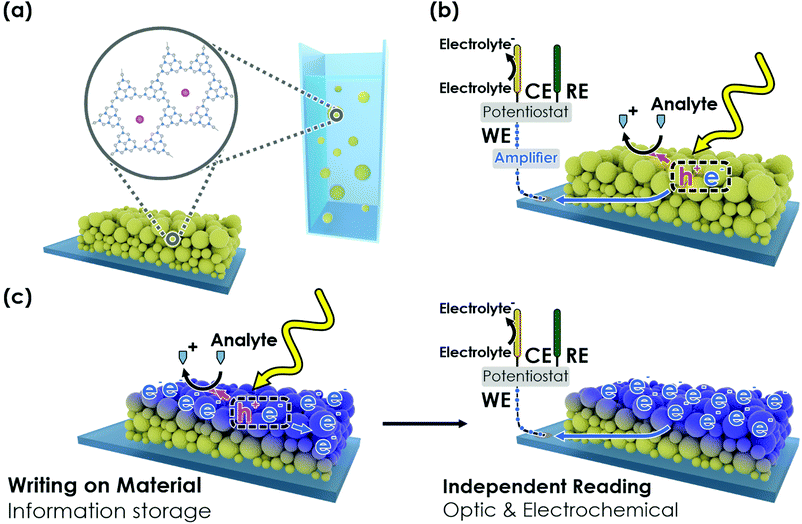
New conceptsWe demonstrate a multimodal photomemristive sensing concept that bridges the field of (photo)electrochemical (PEC) sensing and memristive organic electronics. The carbon nitride poly(heptazine imide) (K-PHI) is capable of “traditional” PEC sensing of a wide range of organic analytes and at the same time shows a characteristic bifunctionality of light absorption coupled to photocatalytic reactivity, and charge storage. Using these unique “optoionic” properties, the sensing information (i.e. the stored charge) can be written onto the sensor and read out based on a number of physical quantities such as photovoltage or color change. This concept allows us to access new functionalities for sensors: memory of the analyte concentration information, tuneable sensitivities and dynamic sensing ranges, as well as a diverse array of readout methods. Due to the facile sensor geometry – either as films or wireless particles – we circumvent conceptual and technical challenges of existing PEC- or memristive sensing concepts. This work underlines how functionalities from different fields (batteries, photocatalysts, memristors, sensors) can be merged to produce information storing devices with novel functionalities. While this concept accelerates the rapidly emerging research field of memristive sensors into a new direction, it also presents a toolkit to facilitate automated electronic signal processing. |
1. Introduction
The trend towards digitization and automation necessitates novel sensing and information storage concepts. Sensors are of critical importance for medical applications, in smart healthcare systems and environmental monitoring.1–3 Emerging neuromorphic sensing and monitoring concepts utilize memristive effects to enable exciting features such as memory sensing and low power data processing.4–7The traditional path to application of memristive devices are non-volatile memory applications with many distinctly addressable states.4 Artificial synapses are a class of bio-memristive devices which are cheap, offer biocompatibility and are investigated to develop synaptic electronics such as neural networks for neuromorphic computing.4,8–11 However, memristors have further potential. Coupling photovoltaics with memristors or transistors can drastically reduce computational energy consumption with photonic- or photomemristive devices, which have been dubbed as “solar brains”.12 The memristive sensing approach presented in this work relies on such a photomemristive behaviour. Most current memristive sensors are comprised of classical inorganic, memristive materials (e.g. Si-nanowires) which are functionalized with a receptor and produce a voltage gap in their memristive behaviour that can be linked to the analyte concentration.6 However, those two-terminal memristor devices suffer from complex device geometries, as they do not have a straightforward pathway for biomolecules to approach the device.13 To address this challenge, complex surface patterning14 or nanowire15 designs are necessary. While most memristive sensors so far have focused on the detection of complex biomolecules such as DNA aptamers,16 cancer markers17 or the ebola and dengue virus,18,19 sensing of easier biomolecules such as glucose was demonstrated but with a lack in both sensitivity (10–40 mM) and selectivity.20
Organic memristive devices have been designed with several different voltage thresholds or volatile switching mechanisms.4,5,21–23 The common denominator is the writing and reading of different conductance states, which can be utilized for information storage. Redox-based switching uses electrochemical redox reactions of polymers (e.g. PEDOT:PSS)4,23 accompanied by ion diffusion, which modifies the conductance of the material.
Carbon nitrides are an interesting class of organic materials with potential applications in biosensing and memristive devices. They are layered molecular materials with one- (1D) or two-dimensional (2D) triazine- or heptazine backbones and a visible-light bandgap. Carbon nitrides have lately attracted interest in various research areas including photocatalysis,24–27 electrochemical and solar energy storage,24,28,29 molecular machines,30 environmental remediation,31 and non-enzymatic or ‘nanozyme’ sensing of e.g. glucose.3,32–35 Carbon nitride based sensors typically require heterostructures, mediators and/or redox indicator molecules. Potassium polyheptazine imide (K-PHI), a recently discovered 2D carbon nitride,26,36,37 has remarkable optoelectronic and optoionic properties38 that are derived from its dual functionality of light harvesting and charge storage.24,28,39,40 This versatile toolkit has led to the design of novel responsive and/or charge storing concepts, such as “dark photocatalysis”23 and a solar battery anode.28 K-PHI's large bandgap of ∼2.7 eV (see Fig. S1, ESI†) corresponding to an absorption edge in the blue region of the visible spectrum, together with the material's suitably positioned band edges (+2.2 and −0.5 V vs. NHE, for the valence band maximum and conduction band minimum, respectively), provides enough thermodynamic driving force for the oxidation and reduction of various chemical species,41 while being chemically robust.
Herein, we exploit the abovementioned property modifications of K-PHI upon photocharging (Fig. 1(a)) and propose a refined photomemristive sensor beyond the commonly used two-terminal design. We show that K-PHI simultaneously acts as a receptor unit for glucose as a model analyte and a transducer, which is coupled to a memristive amplifier, thereby combining all sensing components in the same material. This compact design is complemented by various readout methods, which are based on different physical quantities that encode K-PHI's charging state. Our concept of sensing via charge storage comes with a novel set of combined features, such as an intrinsic memory function with writing and erasing procedures, tuneable dynamic concentration ranges and adaptive sensitivities – properties which we explore, demonstrate and explain below. The manuscript is structured as follows: we first briefly show the direct photo-electrochemical (PEC) sensing ability of K-PHI (Fig. 1(b)) with different analytes to explain the interaction of the sensor with the analyte. This allows us to demonstrate in the following the K-PHI memsensing concept (Fig. 1(c)) via potentiometric, impedimetric and coulometric techniques that focus on the modified electronic material properties. Finally, we present a wireless, memristive sensing mode using optical readouts (colorimetric and fluorometric). Thus, we connect the field of memristive information storage to the well-established electrochemical sensor design by moving away from the more complex “classical” memristor design to a direct PEC sensor with built-in memristive functionalities. This is only possible due to the combination of a special set of photo-electrochemical properties of the carbon nitride K-PHI, namely light absorption, charge storage and a large oxidative driving force.
2. Results and discussion
2.1. PEC sensing interaction on K-PHI
The concept of photo-electrochemical (PEC) biosensors is briefly summarized in Fig. 1(b).44 To describe the direct memristive sensing mode discussed here, we start by summarizing the capability of K-PHI nanosheet films (see Fig. 1(a) and Fig. S2, S3 for details, ESI†) to perform direct PEC amperometric sensing, i.e., to simultaneously act as receptor and transducer while being illuminated in aqueous conditions (Fig. 1(b)). We use glucose as model analyte (see Section S2, ESI†), relating the analyte concentration to the photocurrent. Three linear concentration ranges are observed (0–1 mM, 1–10 mM, and 10–50 mM) and a limit of detection (LOD) of 11.4 μM (0.21 mg dL−1) can be determined from the lower concentration range.45 These observations are comparable to reported linear ranges and LOD's for metal-free amperometric carbon nitride glucose sensors (LOD of 11 μM and a linear range from 1 to 12 mM),34 which additionally require the mediator H2O2 in convolution with the enzyme glucose oxidase (GOx) as the receptor. To confirm the broad applicability and generality of K-PHI as a PEC sensor, we have also tested other sugars, alcohols, the physiologically relevant molecules uric acid and ascorbic acid, as well as typical electron donor molecules, which are widely used as sacrificial agents in photocatalytic applications (Fig. S4(d), ESI†). All these analytes show a strong photocurrent response and hence, can be detected with K-PHI sensors. At a concentration of 5 mM, sugars produce photocurrents between ∼0.6 and 1 μA cm−2 and the typical SED triethanolamine (TEoA) and 4-methylbenzyl alcohol (4-MBA) show photocurrents of 7.5 and 23 μA cm−2, respectively. The physiologically relevant molecules ascorbic acid (AA) and uric acid (UA) produce a photocurrent response of 0.38 and 0.35 μA cm−2, respectively, at a concentration of 0.1 mM, suggesting a viable monitoring strategy for these analytes as well. However, the strong oxidative driving force, which enables considerable photocurrents with many different species, makes it difficult to differentiate between organic substances in mixtures. Functionalization protocols for carbon nitrides developed by us46 and others47 are expected to further enhance the selectivity of K-PHI towards these analytes.2.2. Photo-memristive sensing with K-PHI films
Next, we demonstrate the photomemristive operation of the K-PHI sensor (Fig. 1(c)) based on the PEC sensing interaction outlined above. The necessary double functionality of charge storage and light absorption is illustrated in Fig. 2(a). Electric charging via cyclic voltammetry (CV) measurement shows the charging and subsequent discharging of the material occurring dominantly at potentials negative of −0.7 V vs. Ag/AgCl. Upon illumination with 1 sun (AM1.5 G) at open circuit potential (OCP) and in the presence of an oxidizable analyte, a photopotential immediately develops due to electron accumulation in this potential range, which remains stable for ∼1 h after 20 min of illumination (Fig. 2(a) right). The photopotential decay rate is very slow (1.21 mV min−1) and can be attributed to self-discharge via miniscule amounts of oxygen leaking into the reactor and being reduced by K-PHI (e.g., ROS formation)30,48 or the FTO substrate promoting water reduction, since charge storage on K-PHI occurs at potentials more negative than the hydrogen reduction potential (ca. −0.61 V vs. Ag/AgCl at pH 7).28 Different analyte concentrations produce a different shift in this OCP (Fig. 2(c)). The inherent charge storage properties of K-PHI enable a quantitative comparison between the analyte concentration and the charging state of the material when the illumination time is fixed. The photomemristive behaviour integrates the analyte oxidation current and produces the stable change in the optoelectronic properties of K-PHI as the measurand. This accumulated charge is proportional to the charging current, which depends on both analyte concentration and illumination time. A longer illumination (or writing cycle) can improve the sensitivity for low analyte concentrations, as the system has more time to interact with the analyte and accumulate charges. In principle, there is no lower analyte detection limit in the absence of self-discharge (and other experimental limitations such as parasitic oxidation reactions due to a lack of selectivity), as the illumination time can be increased at will. On the other hand, a short light pulse allows for fast sensing of high analyte concentrations. Thus, K-PHI can act as receptor and transducer (as discussed in the previous paragraph) but also as a memristive amplifier due to its current integration mechanism, hence combining all sensor components on a single material. While related, it is important to contrast this sensing concept with persistent photoconductivity effects that have been reported in semiconductors like ZnO when exposed to gas molecules such as oxygen as the analyte.49,50 Whereas hole-induced desorption of chemisorbed oxygen leads to the accumulation of photogenerated electrons in the conduction band and their subsequent re-trapping by surface-adsorbed oxygen, electron trapping in K-PHI is mediated by the photointercalation of electrolyte ions into the bulk of the material, and is hence akin to memristors.38 The sole purpose of the analyte here is to act as a sacrificial electron donor (SED). In the following, three electrochemical readout methods of the sensor state are demonstrated and explained.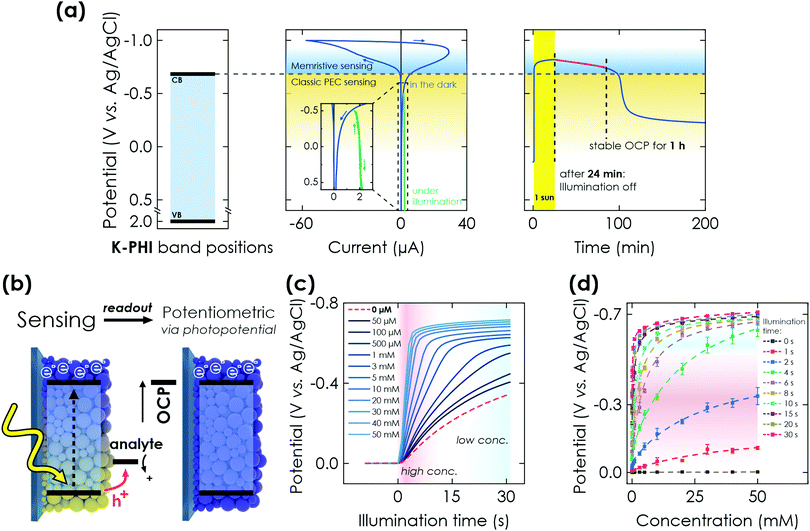 | ||
| Fig. 2 PEC properties of K-PHI and potentiometric memristive sensing via K-PHI films. (a) Left: Valence band (VB) and conduction band (CB) positions referenced to the Ag/AgCl reference electrode potential. Middle: Cyclic voltammetry (CV) measurement at 50 mV s−1, showing the behavior of K-PHI in the relevant potential window for sensing. At potentials more positive than −0.7 V vs. Ag/AgCl, a constant photocurrent is observed (green), which is used for direct PEC sensing. Charge storage occurs at potentials more negative than −0.7 V vs. Ag/AgCl. Right: Open circuit potential (OCP) evolution of K-PHI in the presence of glucose during illumination (photocharging) and its subsequent stability in the dark. (b) Scheme depicting memristive sensing and subsequent potentiometric readout via evaluation of the OCP. (c) Increase of the OCP during charging under illumination and in the presence of different glucose concentrations. With increasing concentrations, the OCP shift becomes more pronounced due to faster charging. (d) Correlation of the photopotential to the concentration of glucose. This concentration can be extracted at different illumination times independently, which allows to tune the sensitivity range. Dashed lines are non-linear fits of the sensor response over the entire dynamic. Fitting is discussed in Section S5.1 (ESI†). | ||
| Method | Useful range (glucose sensitivity in reported conditions) | Illumination time | Readout time | Sensing characteristics |
|---|---|---|---|---|
| Amperometric | 0.1 mM to 10 mM (11 μM) | Continuous | 60 s | – Direct sensing |
| Potentiometric | 0.05 mM to 50 mM (50 μM) | <1 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >10 s >10 s |
Instant | – Non-invasive |
1–10 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4–10 s 4–10 s |
– Memory sensing | |||
>10 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <4 s <4 s |
||||
| Impedimetric | 0.05 mM to 20 mM (50 μM) | Only 30 s illumination analysed | <10 s to 100 s | – Non-invasive |
| – Memory sensing | ||||
| – No fitting required | ||||
| Coulometric | 5 mM to 50 mM (100 μM) | Only 30 s illumination analysed | <300 s | – Memory sensing |
| – Sensor reset (invasive) | ||||
| Colorimetric | 0.2 mM to 50 mM (200 μM) | Only 200 s illumination analysed | <1 s | – Minimum-invasive |
| – Memory sensing | ||||
| – Wireless sensing | ||||
| – Visible colour change | ||||
| Fluorometric | 0.2 mM to 50 mM (200 μM) | Only 200 s illumination analysed | <1 s | – Minimum-invasive |
| – Memory sensing | ||||
| – Wireless sensing |
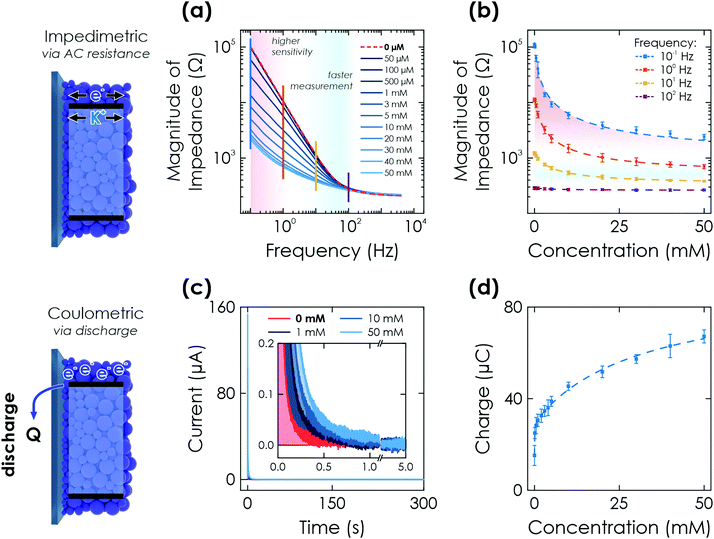 | ||
| Fig. 3 Further electrochemical readout methods via K-PHI thin films. (a) Impedimetric readout. Bode plot of the impedance response after illumination of the sensor for 60 s at OCP conditions and in presence of different glucose concentrations. (b) A change in magnitude of impedance can be correlated to the analyte concentration due to conductivity changes upon memristive charging. This effect has a varying signal amplitude at different time scales (i.e., frequencies; shown with vertical lines in (a)) and concentrations. (c) Coulometric readout. Evolution of discharge current with time when applying a bias of +0.2 V vs. Ag/AgCl after illumination of the sensor for 60 s at OCP conditions and in presence of different glucose concentrations (inset shows a zoom of low currents). (d) The extracted charge Q correlates monotonously with the glucose concentration. Dashed lines in (b) and (d) are non-linear fits of the sensor response over the entire dynamic range. Fitting is discussed in Sections S5.2 and S5.3 (ESI†). | ||
We perform fitting of the relationship between impedance magnitude and analyte concentration with either a non-linear phenomenological fit over the entire dynamic range (Fig. 3(d) dashed lines and Fig. S10, ESI†) or linear with two linear ranges (0–1 mM and 10–50 mM, see Fig. S11, ESI†), albeit with significantly reduced fitting quality. A detailed discussion of the fitting is given in Section S5.2 and summarized in Table S1 (ESI†).
Note that this experiment also acts as a reset of the sensor for all memristive cases so that it can afterwards be reused for the next sensing experiment, i.e., it deletes all previously stored information. This underlines that the change in memristive state is only dependent on the charged state and fully reversible, an important characteristic of a memristor.52
Combining this sensing method with a possible long charge retention time (when illuminating for 20 min, more than 1 h is possible; see Fig. 2(a)) allows us to demonstrate the delayed sensor readout, i.e., ‘memory’ sensing. When charging the sensor for 60 s and performing the discharge after delays of 60 and 300 s, a decrease in charge is observed when increasing the delay time (see Section S6.1 for details, ESI†). We attribute this to self-discharge via uncovered parts of the FTO substrate due to water reduction from the aqueous electrolyte and oxygen reduction from oxygen leaking into the reactor. This self-discharge is also responsible for larger error bars in the signal to concentration correlation compared to the other electrochemical readout methods as due to the integrating mechanism, small differences among samples such as coating degree and density play a much more significant role. However, since sensor fabrication can be scaled easily (dip coating) and sample batches can be calibrated with an offset factor, these effects are of little practical concern (see Section S4, ESI†).
Fitting of the measurement to extract the concentration information is done with the same fit function as for the potentiometric readout over the entire concentration range (Fig. 3(d) dashed line) or by a linear fit that requires two linear ranges (0.1–5 mM and 10–50 mM, see Fig. S13, ESI†) with a much reduced fit quality. The delay times for memory sensing produce a systematic feature by either changing fit contributions or an offset for non-linear and linear fitting, respectively. An offset factor can thus be calculated to account for the self-discharge losses. Details of the fitting are given in Section S5.3 and Table S1 (ESI†).
2.3. Photomemristive sensing with K-PHI wireless particles
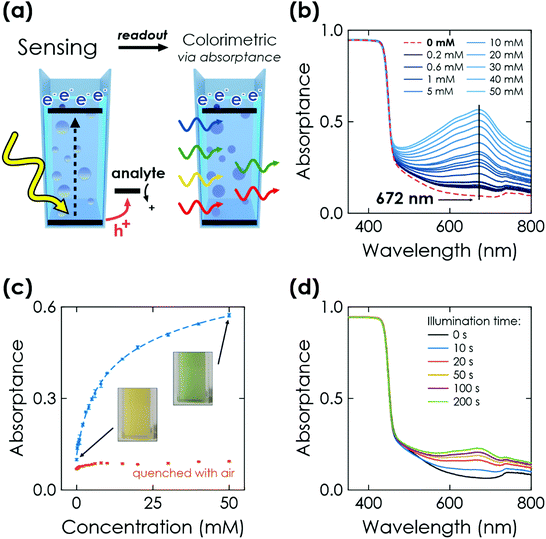
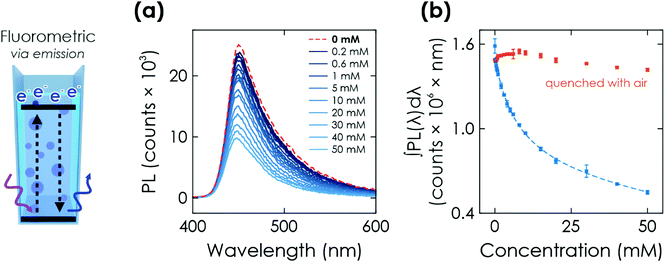
Finally, we study the optical response of K-PHI nanoparticles suspended in an aqueous suspension. This sensing mode is more direct and its implementation is potentially simpler compared to the previous ones, as no wires or electrode substrates are needed for in situ probing. The memristive charge accumulation is caused by the same light-induced oxidative charging process and reflects the accumulated sensing information via a change in photophysical properties.Similar to the above-discussed electrochemical readout methods, illumination time can be used to tune the response towards a more specific sensitivity range (Fig. 4(d) and Section S7.2, ESI†). Furthermore, a time-delayed readout of the sensing information is easily possible, with a signal decay of ∼30% after a delay of 20 min (see Section S7.3, ESI†). This enables for example in situ measurements with ex situ readout in environments where in situ optical sensing is not possible. Note that the colour change is also clearly visible by eye (Fig. 4(c), inset), which makes this readout method useful for label-free colorimetric applications and qualitative analysis even without instrumentation. In comparison to previously reported colorimetric carbon nitride sensors that rely on colour changes of external species such as 3,3′,5,5′-tetramethylbenzidine (TMB), our approach does not require any additional external signal molecules to achieve a visible colour change, minimizing fabrication cost and time while simultaneously improving the design simplicity and recyclability.33 The sensing information can be erased by opening the cuvette and enabling quenching of the reduced state by oxygen contained in air.28,30 Notably, the quenched state always restores the initial absorptance value (Fig. 4(c), red dots) and no degradation of the material's optical properties was observed. This resetting method is more practical than washing the sensor to reset the concentration of external species (such as TMB). The relationships between absorptance signal and concentration can be fit analogous to the above discussed electrochemical readout methods. We find three linear ranges (0.2 to 2 mM, 3 to 10 mM, 15 to 50 mM, see Fig. S15, ESI†) with a limited fitting quality. Phenomenological non-linear fitting works much better and over the entire dynamic concentration range (Fig. 4(c) dashed line and Fig. S14, ESI†). A more detailed discussion of the fitting is given in Section S5.4 and summarized in Table S1 (ESI†).
3. Conclusion
We have introduced the biocompatible, environmentally friendly K-PHI as a photomemristive sensing platform enabling multimodal analyte sensing. Building on the classical PEC amperometric sensing approach, in which an oxidative photocurrent can be correlated to the concentration of various analytes, we develop a photomemristive sensing concept, which utilizes the material's inherent bifunctionality of light absorption and charge storage to photo-oxidatively sense and quantify analytes by accumulating the generated electrons on the material. The change in the material's optoelectronic properties upon photocharging translates into various electrochemical and optical readout methods: (i) potentiometric and (ii) impedimetric sensing (non-invasive), (iii) coulometric sensing (invasive and method to reset), as well as (iv) colorimetric and (v) fluorometric sensing (minimally invasive). The latter two optical methods enable wireless analyte quantification for in situ applications as particulate systems and a facile qualitative visual readout.30,48,53 Contrary to most other sensors, the sensing methods are all direct, i.e., we combine receptor, transducer and current integrating memristive amplifier on the same material, while no additional intermediate species (e.g., mediators) are required. In comparison to state-of-the-art memristive sensors requiring tailored nanostructures, the K-PHI sensor is simple (only a degassed standard electrochemical cell is required), flexible (nanosheet film electrodes or wireless sensing of nanoparticles) and easily scalable (cheap and easy material synthesis, film preparation via solution process). Moreover, the sensor can be reset and easily reused. Input parameters for sensing such as illumination time and intensity can be used to tune the sensitivity and measuring ranges. A time-delayed readout is possible. The main practical advantage of our sensing approach is its great flexibility for different applications, which enable fast (<1 s) and facile readout, as well as the combination of different techniques for adapting sensitivity ranges without modification of the sensor. A summary of the key advantages and measurement properties of the different analysis methods is given in Table 1. The organic nature of K-PHI with functionalizable groups at its edges was further shown to enable a targeted surface property engineering by chemical modifications, which can lead to much enhanced selectivity.46 Similarly, the chemical attachment of dedicated receptors appears possible,54 which would allow to engineer selectivity and responsivity further, according to the field of application. Besides sensing, the concepts described here are useful for evaluating SED strengths and thereby, to provide a better understanding of photocatalytic process parameters, while also being of interest as a characterization tool for light-driven charge storage properties of ‘solar battery’ materials. At the same time, these novel sensing approaches can be applied to other photocatalytic and especially light-storing materials, including both organic and inorganic semiconductors. Since the memristive sensor generates an electrical signal upon charging (OCP), which can be used to drive a current by discharging (coulometric measurements), it can be coupled to other (bio)electronic devices in feedback loops, which can be triggered by using voltage or current thresholds. With this, true neuromorphic applications that facilitate automated electronic signal processing would be directly enabled.4,5,12,55,564. Experimental
4.1. Synthesis of the carbon nitride modification K-PHI as nanoparticles
The carbon nitride materials were synthesized as described in literature.24,26,36 The precursor material melamine as well as potassium thiocyanate were acquired from Sigma Aldrich in reagent grade purity. Exfoliation was carried out in 2-propanol (IPA) via sonication in an ice bath for 2 h (300 mg K-PHI in 100 mL IPA). Subsequently the nanosheets were separated via two centrifugation steps at 353 RCF for 20 min and 795 RCF for 40 min in a centrifuge (3–30k, Sigma) to ensure a uniform small particle size, akin to a reported procedure.27 To reach the desired concentration, density was first evaluated by drying 1 mL of suspension and measuring the weight of the dried residue on a quartz crystal microbalance. To increase the particle concentration to 0.2 mg mL−1 excess IPA was then removed using a rotary evaporator (Hei-Vap Value Digital G3B, Heidolph) at a pressure of 137 mbar and a water bath temperature of 50 °C.4.2. Material characterization
ATR-IR spectra of K-PHI bulk suspensions and nanosheets were collected with an IR spectrometer (UATR TWO, PerkinElmer), which was equipped with a diamond crystal. The optical bandgap of K-PHI bulk suspensions and nanosheets was characterized with an UV-VIS spectrometer (Cary 5000, Agilent), equipped with an integrating sphere. Films were characterized via AFM (MFP-3D, Asylum Research), SEM (ZEISS Merlin electron microscope), and TEM (CM 30 ST (300 kV, LaB6 cathode, Philips)).4.3. Preparation of the sensor films and suspensions
Thin films of K-PHI nanoparticles were deposited onto FTO substrates (Sigma Aldrich, surface resistivity of 7 Ω cm−2) via dip coating with 400 dips, 100 mm min−1 extraction speed and 120 s drying time at ambient temperature between the dips (ND-R Rotary Dip Coater, Nadetech). A subsequent annealing at 70 °C for 2 days was performed to ensure removal of all leftover solvent. The sample was then cut into 10 × 12 mm and a small part of the film was scratched off for contacting. This was performed by gluing a wire to an uncovered part of the FTO using conductive silver paste (Silver Conductive RS 186-3600, RS-Pro). The contact was then sealed with epoxy glue (DP410, 3 M Scotch-Weld) to provide a rigid connection and prevent both the silver paste and uncovered FTO to influence the measurements. Last, an electrochemical self-cleaning of the finished samples was performed according to a procedure described in Section S3 (ESI†). For measurements, which utilize particles in a suspension, dried as synthesized bulk K-PHI was suspended in water (3 mg mL−1) and vortexed for 120 s to ensure a proper distribution of particles.4.4. Electrochemical measurements
All electrochemical measurements were performed in an aqueous electrolyte which contained a 10 mM KCl (Sigma Aldrich) background electrolyte. The electrolyte was purged with >99% argon for at least 20 min through a porous glass frit before every measurement. An oxygen content of <100 ppb during measurements was ensured by measuring trace oxygen with a trace optical oxygen meter (PSt6 sensor spot and Fibox 3 trace, Presens). All analytes (Sigma Aldrich) were dissolved in deionized (DI) water and added to the electrolyte in respective concentrations. An Ag/AgCl electrode with saturated KCl electrolyte (RE-1CP, ALS Japan) was used as the reference electrode and a gold foil (Sigma Aldrich) as counter electrode. Measurements were carried out with a multichannel potentiostat (Autolab M204, Metrohm) in a glass reactor equipped with a quartz window for illumination. Impedance measurements were carried out with a single-channel potentiostat (CompactStat, Ivium). Impedance fitting was performed with the RelaxIS 3 software, rhd Instruments.Illumination (1 sun) was generated by a calibrated Sciencetech LightLine A4 solar simulator, which provides simulated sunlight with class AAA quality (AM1.5G). Light was turned on and off using a ThorLabs SHB1T shutter.
4.5. Optical measurements
All optical measurements were performed in a quartz cuvette (Hellma Analytics). The suspension (K-PHI in DI water, 3 mg mL−1) was purged with >99% argon before every measurement for 300 s and during charging illumination. Glucose (Sigma Aldrich) was dissolved in DI water and added into the cuvette in respective concentrations. The sample was illuminated (AM1.5 G) using a solar simulator (IEC/JIS/ASTM, Newport). Fluorescence was measured using a spectrofluorometer (FLS980, Edinburgh Instruments). Absorptance spectra were measured using a spectrophotometer equipped with an integrating sphere (Cary 5000 UV-VIS, Agilent). The sample was positioned in the centre of the sphere on an angle to obtain both total transmission and total reflection signals.Author contributions
AG, AJS, FP and BVL conceived the project. AG performed the electrochemical measurements. AG and AJS performed the optical measurements. AG and AJS, with assistance of FP, analysed the data. AJS, NVB, FP and BL supervised the research. AG, AJS and FP wrote the manuscript with assistance of all authors.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge J. Kröger for insightful discussions. We thank L. Yao for SEM imaging and V. Duppel for TEM imaging. Financial support is gratefully acknowledged by the Max Planck Society, the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Program (grant agreement no. 639233, COFLeaf), the Deutsche Forschungsgemeinschaft (DFG) via the cluster of excellence ‘e-conversion’ (project number EXC2089/1-390776260) and by the Center for NanoScience (CENS). A. J. S. gratefully acknowledges a postdoctoral scholarship from the Max Planck Society. Open Access funding provided by the Max Planck Society.References
- N. Wongkaew, M. Simsek, C. Griesche and A. J. Baeumner, Chem. Rev., 2019, 119, 120–194 CrossRef CAS PubMed.
- H. Teymourian, A. Barfidokht and J. Wang, Chem. Soc. Rev., 2020, 49, 7671–7709 RSC.
- M. Wei, Y. Qiao, H. Zhao, J. Liang, T. Li, Y. Luo, S. Lu, X. Shi, W. Lu and X. Sun, Chem. Commun., 2020, 56, 14553–14569 RSC.
- Y. Van De Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Nat. Electron., 2018, 1, 386–397 CrossRef.
- E. R. W. Van Doremaele, P. Gkoupidenis and Y. Van De Burgt, J. Mater. Chem. C, 2019, 7, 12754–12760 RSC.
- S. Carrara, IEEE Sens. J., 2021, 21, 12370–12378 CAS.
- K. Sun, J. Chen and X. Yan, Adv. Funct. Mater., 2021, 31, 2006773 CrossRef CAS.
- K. Lu, X. Li, Q. Sun, X. Pang, J. Chen, T. Minari, X. Liu and Y. Song, Mater. Horiz., 2021, 8, 447–470 RSC.
- Y. Y. Zhao, W. J. Sun, J. Wang, J. H. He, H. Li, Q. F. Xu, N. J. Li, D. Y. Chen and J. M. Lu, Adv. Funct. Mater., 2020, 30, 2004245 CrossRef CAS.
- H. Ling, D. A. Koutsouras, S. Kazemzadeh, Y. Van De Burgt, F. Yan and P. Gkoupidenis, Appl. Phys. Rev., 2020, 7, 011307 CAS.
- Y. Park and J. S. Lee, ACS Nano, 2017, 11, 8962–8969 CrossRef CAS PubMed.
- A. Pérez-Tomás, Adv. Mater. Interfaces, 2019, 6, 1–52 Search PubMed.
- A. Vahl, J. Carstensen, S. Kaps, O. Lupan, T. Strunskus, R. Adelung and F. Faupel, Sci. Rep., 2019, 9, 1–9 Search PubMed.
- N. S. Mohamad Hadis, A. A. Manaf and S. H. Herman, Microsyst. Technol., 2013, 19, 1889–1896 CrossRef CAS.
- I. Tzouvadaki, N. Madaboosi, I. Taurino, V. Chu, J. P. Conde, G. De Micheli and S. Carrara, J. Mater. Chem. B, 2016, 4, 2153–2162 RSC.
- I. Tzouvadaki, P. Jolly, X. Lu, S. Ingebrandt, G. De Micheli, P. Estrela and S. Carrara, Nano Lett., 2016, 16, 4472–4476 CrossRef CAS PubMed.
- P. Torino, C. Duca, J. Delaloye, I. Xenarios, S. Carrara and M. Doucey, Br. J. Cancer Res., 2020, 3, 341–348 Search PubMed.
- B. Ibarlucea, T. F. Akbar, K. Kim, T. Rim, C. Baek and A. Ascoli, Nano Res., 2018, 11, 1057–1068 CrossRef CAS.
- N. S. M. Hadis, A. A. Manaf, S. H. Ngalim, S. H. Herman, K. Sawada and N. A. Fauzi, EEE Asia Pac. Conf. Postgrad. Res. Microelectron. Electron., 2017, 97, 105–108 Search PubMed.
- N. S. M. Hadis, A. A. Manaf and S. H. Herman, 2015 IEEE International Circuits and Systems Symposium, 2015, pp. 36–39 Search PubMed.
- Z. Xiao and J. Huang, Adv. Electron. Mater., 2016, 2, 1600100 CrossRef.
- R. Wang, J.-Q. Yang, J.-Y. Mao, Z.-P. Wang, S. Wu, M. Zhou, T. Chen, Y. Zhou and S.-T. Han, Adv. Intell. Syst., 2020, 2, 2000055 CrossRef.
- L. Yuan, S. Liu, W. Chen, F. Fan and G. Liu, Adv. Electron. Mater., 2021, 7, 2100432 CrossRef CAS.
- V. W. H. Lau, D. Klose, H. Kasap, F. Podjaski, M. C. Pignie, E. Reisner, G. Jeschke and B. V. Lotsch, Angew. Chem., Int. Ed., 2017, 56, 510–514 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- H. Schlomberg, J. Kröger, G. Savasci, M. W. Terban, S. Bette, I. Moudrakovski, V. Duppel, F. Podjaski, R. Siegel, J. Senker, R. E. Dinnebier, C. Ochsenfeld and B. V. Lotsch, Chem. Mater., 2019, 31, 7478–7486 CrossRef CAS PubMed.
- A. Savateev and M. Antonietti, ChemCatChem, 2019, 11, 6166–6176 CrossRef CAS.
- F. Podjaski, J. Kroger and B. V. Lotsch, Adv. Mater., 2018, 30, 1705477 CrossRef PubMed.
- Y. Luo, Y. Yan, S. Zheng, H. Xue and H. Pang, J. Mater. Chem. A, 2019, 7, 901–924 RSC.
- V. Sridhar, F. Podjaski, J. Kröger, A. Jiménez-Solano, B.-W. Park, B. V. Lotsch and M. Sitti, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 202007362 Search PubMed.
- Q. Cao, J. Barrio, M. Antonietti, B. Kumru, M. Shalom and B. V. K. J. Schmidt, ACS Appl. Polym. Mater., 2020, 2, 3346–3354 CrossRef CAS.
- M. Xiong, Q. Rong, H. M. Meng and X. B. Zhang, Biosens. Bioelectron., 2017, 89, 212–223 CrossRef CAS PubMed.
- P. Zhang, D. Sun, A. Cho, S. Weon, S. Lee, J. Lee, J. W. Han, D. P. Kim and W. Choi, Nat. Commun., 2019, 10, 1–14 CrossRef PubMed.
- J. Tian, Q. Liu, C. Ge, Z. Xing, A. M. Asiri, A. O. Al-Youbi and X. Sun, Nanoscale, 2013, 5, 8921–8924 RSC.
- J. Tian, Q. Liu, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2013, 85, 5595–5599 CrossRef CAS PubMed.
- V. W. H. Lau, I. Moudrakovski, T. Botari, S. Weinberger, M. B. Mesch, V. Duppel, J. Senker, V. Blum and B. V. Lotsch, Nat. Commun., 2016, 7, 10 Search PubMed.
- J. Kröger, A. Jiménez-Solano, G. Savasci, V. W. Lau, V. Duppel, I. Moudrakovski, K. Küster, T. Scholz, A. Gouder, M. L. Schreiter, F. Podjaski, C. Ochsenfeld and B. Lotsch, Adv. Funct. Mater., 2021, 31, 2102468 CrossRef.
- F. Podjaski and B. V. Lotsch, Adv. Energy Mater., 2020, 11, 2003049 CrossRef.
- J. Kröger, F. Podjaski, G. Savasci, I. Moudrakovski, A. Jiménez-Solano, M. W. Terban, S. Bette, V. Duppel, M. Joos, A. Senocrate, R. Dinnebier, C. Ochsenfeld and B. V. Lotsch, Adv. Mater., 2022, 34, 2107061 CrossRef PubMed.
- H. Kasap, C. A. Caputo, B. C. M. Martindale, R. Godin, V. W. H. Lau, B. V. Lotsch, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2016, 138, 9183–9192 CrossRef CAS PubMed.
- N. L. Weinberg and H. R. Weinberg, Chem. Rev., 1968, 68, 449–523 CrossRef CAS.
- F. J. Knorr, C. C. Mercado and J. L. McHale, J. Phys. Chem. C, 2008, 112, 12786–12794 CrossRef CAS.
- C. Adler, S. Selim, I. Krivtsov, C. Li and D. Mitoraj, Adv. Funct. Mater., 2021, 31, 2105369 CrossRef CAS.
- A. Devadoss, P. Sudhagar, C. Terashima, K. Nakata and A. Fujishima, J. Photochem. Photobiol. C: Photochem. Rev., 2015, 24, 43–63 CrossRef CAS.
- G. Gauglitz, Anal. Bioanal. Chem., 2018, 410, 5–13 CrossRef CAS PubMed.
- J. Kröger, A. Jiménez-Solano, G. Savasci, P. Rovó, I. Moudrakovski, K. Küster, H. Schlomberg, H. A. Vignolo-González, V. Duppel, L. Grunenberg, C. B. Dayan, M. Sitti, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, Adv. Energy Mater., 2021, 11, 2003016 CrossRef.
- T. Fidan, M. Torabfam, Q. Saleem, C. Wang, H. Kurt, M. Yüce, J. Tang and M. K. Bayazit, Adv. Energy Sustainable Res., 2021, 2, 2000073 CrossRef.
- V. Sridhar, F. Podjaski, Y. Alapan, J. Kröger, L. Grunenberg, V. Kishore, B. V. Lotsch and M. Sitti, Sci. Robot., 2022, 7, eabm1421 CrossRef PubMed.
- S. Hullavarad, N. Hullavarad, D. Look and B. Claflin, Nanoscale Res. Lett., 2009, 4, 1421–1427 CrossRef CAS PubMed.
- I. M. Costa, E. P. de Araújo, A. N. Arantes, M. A. Zaghete and A. J. Chiquito, Nanotechnology, 2020, 32, 015702 CrossRef CAS PubMed.
- C. Costentin and J. M. Savéant, Chem. Sci., 2019, 10, 5656–5666 RSC.
- Y. V. Pershin and M. Di Ventra, J. Phys. D: Appl. Phys., 2019, 52, 01LT01 CrossRef.
- A. C. H. Tsang, E. Demir, Y. Ding and O. S. Pak, Adv. Intell. Syst., 2020, 2, 1900137 CrossRef.
- M. M. Xavier, P. R. Nair and S. Mathew, Analyst, 2019, 144, 1475–1491 RSC.
- Z. Wang, S. Joshi, S. Savel’Ev, W. Song, R. Midya, Y. Li, M. Rao, P. Yan, S. Asapu, Y. Zhuo, H. Jiang, P. Lin, C. Li, J. H. Yoon, N. K. Upadhyay, J. Zhang, M. Hu, J. P. Strachan, M. Barnell, Q. Wu, H. Wu, R. S. Williams, Q. Xia and J. J. Yang, Nat. Electron., 2018, 1, 137–145 CrossRef.
- Y. Van De Burgt, E. Lubberman, E. J. Fuller, S. T. Keene, G. C. Faria, S. Agarwal, M. J. Marinella, A. Alec Talin and A. Salleo, Nat. Mater., 2017, 16, 414–418 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2mh00069e |
| This journal is © The Royal Society of Chemistry 2022 |