Open Access Article
Open Access ArticleRational approach for an optimized formulation of silver-exchanged zeolites for iodine capture from first-principles calculations
Tarek
Ayadi
 a,
Michael
Badawi
a,
Michael
Badawi
 *a,
Laurent
Cantrel
b and
Sébastien
Lebègue
*a,
Laurent
Cantrel
b and
Sébastien
Lebègue
 a
a
aLaboratoire Physique et Chimie Théoriques (LPCT, UMR CNRS UL 7019), Institut Jean Barriol, Université de Lorraine, BP 239, Boulevard des Aiguillettes, 54506 Vandoeuvre-lès-Nancy Cedex, France. E-mail: michael.badawi@univ-lorraine.fr
bInstitut de Radioprotection et de Sûreté Nucléaire, PSN-RES, CEA Cadarache, F-13115 Saint Paul lez Durance, France
First published on 3rd February 2022
Abstract
Ab initio calculations have been carried out to investigate in detail the effect of potential inhibiting species (CO, H2O, CH3Cl and Cl2) on the adsorption of iodine species (I2 and CH3I) in silver-exchanged zeolites of different Si/Al ratios and structures (faujasite, mordenite, chabazite and clinoptilolite). We have found that the adsorption of iodine species becomes more favorable for a low Si/Al ratio. Indeed, a potentially strong inhibiting effect of CO for a high Si/Al ratio has been unravelled, while Cl2 appears as an inhibitor for a low Si/Al ratio. In addition, a spontaneous dissociation is observed for I2 and Cl2 at a low Si/Al ratio which greatly increases the interaction energy. With the addition of sodium cations together with the silver ones to compensate for the charge in the zeolite structures, an improvement in the performance of mordenite for the selective trapping of iodine compounds is observed, in contrast to chabazite and clinoptilolite. Interestingly, we found that the presence of the Na cations in faujasite and mordenite prevents the spontaneous dissociation of the Cl2 molecule, which leads to limitation of the strong inhibiting effect on the adsorption of iodine species, in particular CH3I. Also, an efficient comparison between different structures using radar charts has been performed, where not only the Si/Al ratio is taken into consideration but also the silver loading in the zeolite structure for a given Si/Al ratio. Finally, we found that Ag-chabazite with a Si/Al of 5 and AgNa-mordenite with a Si/Al of 11 are the best candidates for selective trapping of iodine species, noticeably for I2 trapping.
Design, System, ApplicationThe trapping of harmful iodine species as released during a nuclear accident can be achieved using silver zeolites. However, a large experimental study of many different zeolite structures and Si/Al ratios to find the best formulation would be extremely time consuming. Here, we have employed density functional theory to investigate systematically the performance of different silver-exchanged zeolites, with the aim to propose an optimized formulation that can be experimentally tested. |
1 Introduction
Since the Fukushima nuclear accident, great effort has been given by the scientific community to mitigate dangerous radioactive compounds before they reach the environment. In particular, iodine species attract specific interest among radiotoxic releases because the 131I isotope can contribute significantly to the radiological consequences in the short term.1–3 To confine these species and limit their effect in the environment, a specific filtration device on the CVS, selective towards iodine species, is recommended. Among the most efficient technologies that can be used to improve iodine filtration (I2 and CH3I) are those based on zeolites.4–9 Together with iodine species, different potential species are formed during severe nuclear accidents with different concentrations, which can inhibit iodine trapping. We mainly find large amounts of steam (40–90%) and other gaseous compounds such as CO, CH3Cl and Cl2.10,11 The CO compound comes from the molten core concrete interactions which start after vessel rupture, while chlorine compounds (CH3Cl and Cl2) are produced by fuel fission as well as possible thermal degradation of cables.12,13Zeolites are a family of porous adsorbent materials.14–17 Usually, zeolite structures are formed mainly by three dimensional microporous frameworks comprising channels and voids made up of tetrahedral silicon and aluminum atoms bonded to oxygen atoms. These crystals have interesting characteristics such as high thermal stability, the ability to exchange ions, high specific surface area, and large pores and cages. With these remarkable properties, zeolites proved their performance in various applications as cleaners, gas filters, catalysts and agents for treatment of nuclear wastes.5,7,18,19 Indeed, there are many types of zeolites with different structural frameworks such as faujasite, mordenite, chabazite and clinoptilolite.5,7 This diversity leads to different properties, which widens the field of applications.
Cation exchange is a result of the replacement of Si atoms by Al atoms in the zeolite structures where a negative charge is produced. This exchange is one of the most important parameters where the properties of the zeolite can vary depending on the type of cation used to compensate for the charge in the structure. Theoretical and experimental studies have shown that silver is the most efficient cation among various metals (Cu, Na, Pb, Tl, Cd) for iodine species trapping by the formation of AgI precipitates.6,17,20–24 For an in-depth understanding of this phenomenon, an experimental study has been carried out by Azambre and Chebbi24 on different Ag-exchanged zeolites (faujasite X and Y, mordenite, *BEA, MFI, FER) to understand the relationships between the structural and chemical parameters and the efficiency for iodine trapping (CH3I). They found that the formation of AgI for all Ag exchanged zeolites and the amount of AgI precipitates are directly related to the silver loading in the structure. Formation of silver clusters can also occur in zeolites25 but recent experimental studies have demonstrated that isolated silver cations can be well inserted into zeolites by cationic exchange even at high silver loading (up to 23%).24 For this cation loading which corresponds to a low Si/Al ratio, no blocking of channels has been observed.24
In order to find the best silver exchanged zeolitic formulation owing the good compromise between the selective trapping of iodine species towards inhibitor species (CO, H2O, CH3Cl and Cl2) and the silver amount, we have investigated the potential impact of CO, H2O, CH3Cl and Cl2 on iodine trapping for silver exchanged faujasite, mordenite, chabazite and clinoptilolite. During our study, we took into consideration not only the Si/Al ratio but also the silver content in the structure where the Na cations are introduced to compensate for the charge together with the Ag ones.
The paper is organized as follows: first, we detail our computational procedure, then the obtained results with Ag-faujasite, mordenite, chabazite and clinoptilolite for several Si/Al ratios are presented and discussed with a focus on the potential inhibiting effect of CO, H2O, CH3Cl and Cl2 on the trapping of iodine species, and finally we outline the main conclusions of our study.
2 Computational details
We have performed ab initio calculations by means of density functional theory (DFT) with the projector augmented wave (PAW) method26 as implemented in the Vienna ab initio simulation package (VASP).27 For the exchange and correlation contributions, the Perdew–Burke–Ernzerhof (GGA-PBE) functional28 was employed with a kinetic energy cutoff of 450 eV for the plane wave basis set. The correction of Tkatchenko–Scheffler with iterative Hirshfeld partitioning (TS/HI)29,30 was used to describe dispersion interactions. Recently, this method has proved its performance for describing adsorption phenomena on zeolites owing to its capacity to properly describe ionic systems.9,23,31 The calculations were performed with the Γ-point regarding the large size of the used unit cell. The convergence parameters were set to 10−6 eV for the total energy and 0.02 eV Å−1 for the atomic forces.The interaction energies between the adsorbate and the zeolite have been evaluated according to the following equation: Eint = Ezeolite+X − Ezeolite − EX, where Ezeolite+X is the energy of the zeolite with the adsorbed molecules; Ezeolite is the energy of the clean zeolite and EX represents the energy of the molecule, which is put in the same box as for the system (zeolite + X) obtained after optimization.
Also, the dispersion energy contribution to the interaction energy was determined using the following equation: Edisp = Edisp zeolite+X − Edisp zeolite − Edisp X, where Edisp zeolite+X, Edisp zeolite and Edisp X are the dispersion contributions to the interaction energies of the zeolite with the adsorbed molecules, the clean zeolite and the isolated molecule in the gaseous phase, respectively. The optimized geometries are obtained after two steps: first, we have optimized the unit cell (without adsorbates) where we took into consideration the shape, atomic position and volume, and second, we have put the adsorbates in the zeolite, and then optimized the cell by relaxing only the atomic positions.
3 Results
3.1 Faujasite structure
Faujasite (FAU) belongs to the family of zeolites with large pores and has great thermal stability. The three-dimensional framework of FAU is mainly composed of sodalite cages (named also β cages) and supercages (named also α cages). The β cages with a diameter of 6.6 Å are connected to each other by a hexagonal prism (D6R).The spherical α cages with a diameter of 12.4 Å are the source of the large pores in FAU and they are linked together via 12-membered ring windows with a diameter of 7.4 Å. Each supercage is connected to four other supercages and to four sodalite cages, and the access to the α cages from the β cages is possible via the hexagonal windows.17,32
Here, we have studied three faujasite structures with different Si/Al ratios of 47, 2.4 and 1.4, which correspond to silver loadings of 3.6 wt%, 34.5 wt% and 43 wt%, respectively. For the faujasite with a Si/Al ratio of 47 (the Ag-FAU(47) structure presented in Fig. 1), a Si atom is replaced by an Al atom and the charge produced from this change of atoms is compensated for by a Ag cation. For faujasite Y which corresponds to a Si/Al ratio of 2.4 (the Ag-Y structure presented in Fig. 1), in this case 14 Si atoms are replaced by 14 atoms of Al and to compensate for the charge induced in the Ag-Y model, 14 Ag cations are introduced. In the case of faujasite with a Si/Al ratio of 1.4 (named also X which corresponds to the Ag-X structure presented in Fig. 1), 20 cations of Ag are used to compensate for the charge induced from the replacement of 20 atoms of Si by 20 atoms of Al.
In Table 1, we present our computed interaction energies and the corresponding contribution of the dispersion energy of the different structures of faujasite. We found that the interactions between iodine species and the pure Ag-exchanged faujasite zeolites depend on the Si/Al ratios where the interaction energy of I2 (CH3I) is −107.7 kJ mol−1 (−117.7 kJ mol−1) for Ag-FAU(47), −96.4 kJ mol−1 (−105.7 kJ mol−1) in the case of Ag-Y, and −207.4 kJ mol−1 (−149.3 kJ mol−1) in the case of Ag-X. Also, we found that the interaction energy for I2 presents a significant increase in the case of Ag-X, which is related to a spontaneous dissociation of this molecule. Indeed, the CH3I molecule is more adsorbed than I2 by an energy amount (in terms of absolute value) of about 10 kJ mol−1 for FAU(47) and 9.3 kJ mol−1 for Ag-Y, while in the case of Ag-X, the observation is reversed where I2 is more adsorbed than CH3I by about 58.1 kJ mol−1. Besides, we found that the dispersion contribution of both I2 and CH3I to their total interaction energies presents almost the same values independent of the Si/Al ratios. For the CO, H2O and CH3Cl molecules, we found that they present a similar trend to CH3I where their interaction energies increase when the Si/Al ratio decreases to 1.4 (the case of Ag-X). In contrast, the molecule of Cl2 presents a similar trend to I2 where a spontaneous dissociation is obtained in the case of Ag-X, which leads to a considerable increase of the interaction energy.
| Si/Al = 47 | Si/Al = 2.4 (Y) | Si/Al = 1.4 (X) | ||||
|---|---|---|---|---|---|---|
| Ag-FAU(47) | Ag-Y | AgNa-Y | Ag-X | AgNa-X1 | AgNa-X2 | |
| CH3I | −117.7 (−26.9) | −105.7 (−27.3) | −100.8 (−26.2) | −149.3 (−28.5) | −149.4 (−28.2) | −149.5 (−33.0) |
| I2 | −107.7 (−32.9) | −96.4 (−34.3) | −91.5 (−32.0) | −207.4 (−30.5) | −202.6 (−35.9) | −195.3 (−30.5) |
| CO | −114.4 (−9.1) | −94.0 (−9.1) | −99.5 (−9.1) | −152.6 (−10.8) | −154.4 (−10.1) | −153.7 (−11.8) |
| H2O | −70.0 (−7.7) | −68.6 (−9.0) | −65.5 (−7.8) | −111.6 (−11.7) | −113.4 (−11.9) | −114.1 (−12.5) |
| CH3Cl | −82.7 (−21.7) | −72.0 (−21.3) | −74.11 (−21.32) | −114.4 (−22.6) | −114.4 (−22.5) | −113.1 (−25.7) |
| Cl2 | −59.6 (−19.6) | −45.0 (−18.3) | −45.5 (−18.0) | −190.8 (−18.2) | −192.5 (−13.1) | −159.1 (−17.6) |
For an efficient comparison of the performance of the different structures of faujasite, we used the radar charts presented in Fig. 2 where the best result is represented by the outermost ring.
We note that the results are put to zero when the inhibitor compounds are more adsorbed than the iodine species. In Fig. 2(a), we present the performance for the selective trapping of iodine species towards H2O, CO, CH3Cl and Cl2 of the pure silver exchanged FAU with Si/Al ratios of 47, 2.4 and 1.4, which correspond to the FAU(47), Ag-Y and Ag-X structures, respectively. Globally, we can see that the area covered by Ag-X is larger than that covered by Ag-FAU(47) and Ag-Y. Also, we observe that the FAU(47) and Ag-Y structures offer very comparative results where we found that the I2(CH3I) molecule is more adsorbed (in terms of absolute value) than H2O, CH3Cl and Cl2 by about 33 kJ mol−1 (45 kJ mol−1), 25 kJ mol−1 (35 kJ mol−1) and 48 kJ mol−1 (56 kJ mol−1), respectively. On the contrary, a possible inhibiting effect is found with the CO molecule in the FAU(47) and Ag-Y zeolites, where the iodine species are weakly favored for adsorption by about 10 kJ mol−1. On the other hand, we found that the Ag-X zeolite presents a different profile from those of FAU(47) and Ag-Y where I2 is more adsorbed than H2O, CO and CH3Cl by an important amount of energy which can reach in absolute value: 95.8 kJ mol−1, 54.8 kJ mol−1 and 92.8 kJ mol−1, respectively. In contrast, I2 is weakly favored for adsorption compared with Cl2 by 16.6 kJ mol−1. Also, we found that CH3I is more adsorbed than the H2O and CH3Cl molecules by 37.7 kJ mol−1 and 34.9 kJ mol−1, respectively, while Cl2 and CO have similar interaction energies to CH3I and therefore could inhibit its adsorption. All in all, we found that the I2 molecule can be selectively adsorbed in faujasite X (see Fig. 2).
In order to use a smaller amount of silver which is an expensive metal, we have considered partially exchanged zeolites with Na cations in addition to Ag ones, to compensate for the charge in the Ag-Y and Ag-X structures. Previous experimental studies24,33 on the faujasite structure (exchanged with Na and Ag cations) revealed that Ag cations mainly occupy sites SII and, to a lesser extent, sites SIII, both being located in the supercages.34 Accordingly, we put the Ag cations in the supercages and the Na cations in the sodalite cages, which is the case of the AgNa-Y and AgNa-X1 structures. Then, we put the Na cations with the Ag cations in the supercages, while the sodalite cages are still occupied just by the Na cations, which corresponds to the AgNa-X2 model.
In these cases, the amount of silver decreases from 34.5 wt% to 21.5 wt% and from 43 wt% to 32 wt% in the cases of the Ag-Y and AgNa-X1(AgNa-X2) structures, respectively. The different structures are presented in Fig. 1.
Regarding the interaction energies and the corresponding contributions of the dispersion energy presented in Table 1, we can observe that the addition of the Na cations has a small effect on the interactions between the iodine species and the AgNa-faujasite structures. In the case of AgNa-Y, we found that the total interaction energy of I2(CH3I) increases slightly from −96.4 kJ mol−1(−105.7 kJ mol−1) to −91.5 kJ mol−1 (−100.8 kJ mol−1), and CH3I is still more adsorbed than I2 as in the Ag-Y zeolite. Moreover, we found that the total interaction energies of H2O and CH3Cl present a slight increase of about 5 kJ mol−1, while a decrease of about 5 kJ mol−1 and 0.5 kJ mol−1 is observed for CO and Cl2, respectively. For the AgNa-X1 and AgNa-X2 structures, we found that the total interaction energies for CH3I, CO, H2O and CH3Cl present very similar values, with and without Na, which are around −149 kJ mol−1, −153 kJ mol−1, −113 kJ mol−1 and −114 kJ mol−1, respectively. For I2, an increase in the total interaction energy of about 4.8 kJ mol−1 and 12.1 kJ mol−1 is obtained in the case of AgNa-X1 and AgNa-X2, respectively. Also, we observe that the addition of the Na cations has no significant effect on the adsorption of Cl2 in the case of AgNa-X1, where a decrease of about 2 kJ mol−1 is observed, but not in the case of AgNa-X2, where an increase in the total interaction energy value of about 31.5 kJ mol−1 is obtained which limits the effect of Cl2 on the adsorption of iodine species. With regard to the dispersion contribution, we observe that the addition of the Na cations in the Ag-Y and Ag-X structures has a very weak effect, where a variation in the values of around 5 kJ mol−1 is obtained in the extreme cases.
We turn now to the exploration of the effect of Na in the Ag-Y and Ag-X structures using the radar charts. In Fig. 2(b), we can observe that the profile obtained for AgNa-Y is very similar to that obtained for Ag-Y, but with a two-fold lower silver content (by about 12.5%). Also, we found that the presence of sodium in Ag-Y obstructs more strongly the adsorption of CH3I in the presence of CO. In Fig. 2(c), we observe that the Ag-X, AgNa-X1 and AgNa-X2 structures offer comparable performances for the selective trapping of iodine species towards H2O, CH3Cl and CO. Also, we can distinguish that CO has a similar interaction energy to CH3I, even with the addition of Na atoms in the faujasite X, and therefore could inhibit its adsorption. On the other hand, we found that the Cl2 molecule is being less adsorbed than I2 by about 30 kJ mol−1 in the case of AgNa-X2. Finally, we found that the presence of the Na atoms in the AgNa-X2 structure has a positive impact on the limitation of the spontaneous dissociation of Cl2 against I2 but not against CH3I.
3.2 Mordenite structure
Mordenite (MOR) is a three dimensional network which is formed by three different types of cavities: the main channel (MC), the side channel (SC), and the side pocket (SP). Along the c-axis, the MC is obtained from 12-membered rings stacked in a parallel array. The access to the SP from the MC is possible through 8-membered rings. The MC is characterized by a large aperture able to accommodate big molecules such as iodine molecules which makes this channel favored for the adsorption of this type of molecule compared to the SC and SP.16,23,35In this study, the Ag exchanged MOR structures based on the structure optimized by Bučko et al.36 are used, with the primitive monoclinic cell doubled in the c-direction, where its lattice parameters are a = b = 13.805 Å, c = 15.212 Å and β = 82.81. According to previous studies,23 we have selected the T1-E site which is the most energetically stable site of the MC. Depending on the Si/Al ratio, we have studied three Ag-MOR structures with Si/Al ratios of 47, 11 and 5, which correspond to the Ag-MOR(47), Ag-MOR(11) and Ag-MOR(5) structures, respectively. The silver loading is 3.6 wt% in the case of Ag-MOR(47), 13.1 wt% for Ag-MOR(11) and 23.1 wt% in the case of Ag-MOR(5). The different structures are presented in Fig. 3.
In Table 2, we present the interaction energies and the corresponding contribution of the dispersion energy for CH3I, I2, CO, H2O, CH3Cl and Cl2 in Ag-MOR. Our results show that the interactions between I2 and the pure silver-MOR structures depend on the Si/Al ratio where the total interaction energy in absolute value increases from 132.6 kJ mol−1 for Si/Al = 47, to 183.8 kJ mol−1 in the case of Si/Al = 11, and then to 297.6 kJ mol−1 for Si/Al = 5. This increase obtained in the interaction energy values in the case of Si/Al ratios of 11 and 5, compared to the case of Si/Al = 47, is related to a spontaneous dissociation of I2 where the I–I bond length is about 3.06 Å when Si/Al = 11, while it is about 4.54 Å when Si/Al = 5. On the other hand, we found that the interaction energy of CH3I depends also on the Si/Al ratio where the values obtained are −152.1 kJ mol−1, −151.8 kJ mol−1 and −188.5 kJ mol−1 in the cases of a Si/Al ratio of 47, 11 and 5, respectively. For the CO and H2O molecules, we observe that the interaction energy values decrease with the decrease of Si/Al, while the values obtained for the CH3Cl molecule present an increase with the decrease of Si/Al. For Cl2, a similar observation to that for I2 is obtained where an increase in the interaction energies coupled with spontaneous dissociation is observed when the Si/Al ratio is 5 and 11. We can see that the capture mechanism of iodine species is more efficient with a low Si/Al ratio which is related to the size of molecules: the size of I2 and CH3I molecules is larger than the size of CO and H2O, which make them more favorable to interact with more than a single cation. In contrast, related to their small size, the CO and H2O molecules can interact just with a single cation.
| Si/Al = 47 | Si/Al = 11 | Si/Al = 5 | |||
|---|---|---|---|---|---|
| Ag-MOR(47) | Ag-MOR(11) | AgNa-MOR1(11) | AgNa-MOR2(11) | Ag-MOR(5) | |
| CH3I | −152.1 (−36.2) | −151.8 (−41.4) | −128.8 (−39.5) | −139.9 (−40.2) | −188.5 (−50.9) |
| I2 | −132.6 (−46.0) | −183.8 (−42.9) | −154.3 (−45.7) | −155.9 (−47.4) | −297.6 (−56.0) |
| CO | −127.0 (−12.2) | −119.7 (−16.1) | −116.4 (−14.4) | −110.6 (−15.5) | −115.1 (−15.6) |
| H2O | −111.5 (−10.4) | −90.3 (12.0) | −89.8 (−14.6) | −102.4 (−14.5) | −106.7 (−17.1) |
| CH3Cl | −103.8 (−30.4) | −89.6 (−32.0) | −90.4 (−32.0) | −80.3 (−31.6) | −126.8 (−36.4) |
| Cl2 | −76.9 (−26.3) | −159.9 (−23.5) | −97.2 (−25.2) | −81.3 (−27.2) | −308.5 (−28.3) |
In Fig. 4(a), we present the performance of the pure Ag-MOR(47), Ag-MOR(11) and Ag-MOR(5) for the selective trapping of iodine species towards H2O, CO, CH3Cl and Cl2. It is seen that Ag-MOR(5) presents the outermost ring compared to Ag-MOR(47) and Ag-MOR(11). In the case of Ag-MOR(47), we found that I2(CH3I) is more adsorbed than H2O, CH3Cl and Cl2 by about 21.1 kJ mol−1 (40.6 kJ mol−1), 28.8 kJ mol−1 (48.3 kJ mol−1) and 55.7 kJ mol−1 (75.2 kJ mol−1), respectively. Indeed, CH3I is also more adsorbed than CO by an important amount of energy of 25.1 kJ mol−1, while I2 is slightly more adsorbed than CO by about 5.6 kJ mol−1, which can inhibit its adsorption. On the other hand, the Ag-MOR(5) and Ag-MOR(11) structures present good results for the selective trapping of iodine species towards CO, H2O and CH3Cl. In contrast, we found that Cl2 can inhibit the adsorption of CH3I in both Ag-MOR(5) and Ag-MOR(11) zeolites.
Then, we have considered partially exchanged zeolites with Na cations in addition to the Ag ones, to compensate for the charge in the MOR structure with a Si/Al ratio of 11. We studied two structures named hereafter as AgNa-MOR1(11) and AgNa-MOR2(11), as presented in Fig. 3. The silver loading decreases from 13.1 wt% in the case of Ag-MOR(11) to 6.5 wt% in the cases of AgNa-MOR1(11) and AgNa-MOR2(11).
Our computed interaction energies and the corresponding contribution of the dispersion energy of iodine species (I2 and CH3I) and M molecules (M = CO, H2O, CH3Cl and Cl2) adsorbed on AgNa-MOR1(11) and AgNa-MOR2(11) are presented in Table 2. According to our results, the addition of the Na cations in Ag-MOR(11) decreases the total interaction energies (in absolute values) of CH3I by about 23 kJ mol−1 and 11.9 kJ mol−1 in the cases of AgNa-MOR1(11) and AgNa-MOR2(11), respectively. Indeed, for the CO, H2O and CH3Cl molecules, we found that the most important impact obtained by the addition of Na cations is a decrease/increase in their interaction energy of about 10 kJ mol−1 in the extreme case. Interestingly, we found that the addition of Na prevents the dissociation of both I2 and Cl2 molecules but its impact on the interaction energy of Cl2 is greater, where a decrease in the absolute values of the order of 62/78 kJ mol−1 is obtained, while a decrease in the order of 29.5/27.9 kJ mol−1 is obtained for I2, in the case of AgNa-MOR1(11)/AgNa-MOR2(11). Regarding the dispersion contribution, we found that they present almost the same values with and without Na, and independent of the Si/Al ratios.
To better understand the effect of the Na cations, we used the radar charts to compare the performance of Ag-MOR(11), AgNa-MOR1(11) and AgNa-MOR2(11) for the selective trapping of iodine species towards H2O, CO, CH3Cl and Cl2, see Fig. 4(b). We can clearly observe that the problem obtained during the adsorption of CH3I in the presence of Cl2 is resolved with the addition of the Na cations where CH3I is being more adsorbed than Cl2 by around 31.6 kJ mol−1 in the case of AgNa-MOR1(11) and by around 58.6 kJ mol−1 in the case of AgNa-MOR2(11). Also, we observe that CO has a similar interaction energy to CH3I and therefore could inhibit its adsorption in the case of AgNa-MOR1(11). Finally, we found that the presence of the Na cations presents a positive impact on the selective trapping of iodine species towards H2O, CO, CH3Cl and Cl2 on the AgNa-MOR(11) structures, especially in the case of AgNa-MOR2(11).
3.3 Chabazite structure
Chabazite (CHA) is a three dimensional framework formed mainly by double six-ring prisms (6DR), four-membered rings (4MR) and eight-membered rings (8MR). The double six-ring prisms (6DR) arranged in layers are connected to each other by the tilted four-membered rings (4MR). The access to the crystal is possible from the 8MR which function as highly selective doorways with a diameter of 3.8 Å.37–39 Here, we have studied three Ag-exchanged chabazite structures with Si/Al ratios of 23, 11 and 5, which correspond to the Ag-CHA(23), Ag-CHA(11) and Ag-CHA(5) structures (see Fig. 5), respectively. The silver loading is 7 wt% in the case of Ag-CHA(23), 23.1 wt% for Ag-CHA(11) and 23.1 wt% in the case of Ag-MOR(5).Regarding the interaction energy values presented in Table 3, we found that the interactions between I2(CH3I) and the CHA zeolites depend on the Si/Al ratio, where the interaction energy in absolute value increases from 124.2 kJ mol−1 (133.5 kJ mol−1) for Si/Al = 23, to 158.3 kJ mol−1 (134.4 kJ mol−1) in the case of Si/Al = 11, and to 190.6 kJ mol−1 (155.7 kJ mol−1) for Si/Al = 5. We observe that the impact of the Si/Al ratio is more important in the case of I2 than CH3I, which is in line with the increase of the silver amount (decrease of the Si/Al ratio) coupled with the increase of the I–I bond-length, which is 2.71 Å for Si/Al = 23 and 2.80 Å for both Si/Al = 11 and 5. Also, we found that the interaction energy in absolute value of Cl2 increases with the increase of the silver amount which is around 71 kJ mol−1 in the case of Si/Al = 23 and 11, and about 100.2 kJ mol−1 for Si/Al = 5. For CO, H2O and CH3Cl, a variation of about 5 kJ mol−1 is obtained in the interaction energy values as a function of the Si/Al ratio. Besides, we found that the dispersion contributions to the total interaction energies of the different molecules studied here present almost the same values, independent of the Si/Al ratio. For the chabazite structure, we found that the capture mechanism of iodine species depends on the Si/Al ratio where it is more efficient with the lower one.
| Si/Al = 23 | Si/Al = 11 | Si/Al = 5 | |||||
|---|---|---|---|---|---|---|---|
| Ag-CHA(23) | Ag-CHA(11) | AgNa-CHA(11) | Ag-CHA(5) | AgNa-CHA1(5) | AgNa-CHA2(5) | ||
| CH3I | −133.5 (−38.5) | −134.4 (−44.5) | −133.5 (−44.2) | −155.7 (−45.3) | −128.6 (−45.3) | −139.3 (−45.5) | |
| I2 | −124.2 (−47.0) | −158.3 (−44.4) | −136.7 (−46.0) | −190.6 (−52.2) | −131.1 (−49.5) | −149.7 (−47.8) | |
| CO | −126.5 (−16.1) | −124.7 (−16.5) | −124.3 (−16.4) | −129.8 (−16.8) | −118.5 (−15.5) | −119.1 (−47.8) | |
| H2O | −101.5 (−14.1) | −99.1 (−14.3) | −97.7 (−14.1) | −117.0 (−14.0) | −119.3 (−13.8) | −116.9 (−13.7) | |
| CH3Cl | −96.9 (−30.1) | −94.5 (−32.4) | −93.2 (−33) | −98.5 (−35.3) | −91.5 (−34.9) | −98.6 (−33.7) | |
| Cl2 | −71.7 (−27.2) | −70.3 (−27.7) | −69.6 (−27.5) | −100.2 (−28.0) | −67.9 (−27.9) | −81.5 (−29.7) | |
Using the radar charts presented in Fig. 6(a), we found that the Ag-CHA(5) zeolite presents the best performance for trapping iodine species towards the M molecule (M = CO, H2O, CH3Cl and Cl2) where it has the outermost ring compared to those of the Ag-CHA(23) and Ag-CHA(11) zeolites. We observe that Ag-CHA(11) and Ag-CHA(5) offer a good trapping of iodine species versus H2O, CH3Cl and Cl2. In contrast, we found that CO has a similar interaction energy to iodine species and therefore could inhibit their adsorption in the case of Ag-CHA(11). In the case of the Ag-CHA(23) zeolite, it is seen that the area occupied by this structure is the smallest compared to those of the Ag-CHA(5) and Ag-CHA(11) zeolites, where we found that the CO and H2O compounds can exhibit an inhibiting effect for the adsorption of iodine species.
With the addition of Na cations together with Ag ones, to compensate for the charge in the Ag-CHA(11) and Ag-CHA(5) zeolites, we studied three structures which are AgNa-CHA(11) with a Si/Al ratio of 11, and AgNa-CHA1(5) and AgNa-CHA2(5) with a Si/Al ratio of 5, as presented in Fig. 5. As per the results, the amount of silver decreases from 13.5 wt% to 6.5 wt% in the case of Si/Al = 11, and from 23.1 wt% to 12 wt% in the case of Si/Al = 5.
In Table 3, we present our computed interaction energies and the corresponding contribution of the dispersion energy for CH3I, I2, CO, H2O, CH3Cl and Cl2 in AgNa-CHA(11), AgNa-CHA1(5) and AgNa-CHA2(5). For AgNa-CHA(11), we found that the addition of Na has a small effect on the interaction between CH3I, CO, H2O, CH3Cl and Cl2, and the CHA zeolite, where a decrease/increase of around 3 kJ mol−1 in the interaction energy values is obtained, compared to those obtained in the case of pure exchanged CHA with Si/Al = 11. In contrast, we found that the interaction energy in absolute value of I2 decreases by about 21.6 kJ mol−1 with the decrease of silver loading, coupled with a weak decrease of the I–I bond length which is 2.8 Å for Ag-CHA(11) and 2.77 Å for AgNa-CHA(11). In the case of AgNa-CHA1(5) and AgNa-CHA2(5)s, we found that the interactions between I2(CH3I) are affected by the presence of Na atoms in the zeolites where a decrease in the interaction energy in absolute value of around 59.5 kJ mol−1 (27.1 kJ mol−1) and 40.9 kJ mol−1 (16.4 kJ mol−1) is obtained in the case of AgNa-CHA1(5) and AgNa-CHA2(5), respectively. For the CO, H2O and CH3Cl molecules, we found that the most important impact obtained by the addition of Na cations is a decrease in their interaction energy values of about 10 kJ mol−1, in the extreme case. In contrast, the effect of Na atoms is more important for the adsorption of the Cl2 molecule where it is less adsorbed by around 32.3 kJ mol−1 (18.7 kJ mol−1) in the case of AgNa-CHA1(5) (AgNa-CHA2(5)). Here, we observe that the addition of Na strongly influences the interaction energy of I2 and Cl2 compared to those of CH3I, CO, H2O and CH3Cl. With regard to the mode of adsorption, we found that CH3I, CO, H2O and CH3Cl interact only with the Ag atoms for the adsorption on AgNa-CHA(5), and they are not bound to the Na atoms; so here the main factor of the variation in the interaction energy for these molecules is the decrease in the silver loading. For I2 and Cl2, we found that the decrease in the interaction energy values is related not only to the silver loading, as for CH3I, but also to the mode of adsorption where we found that the I2(Cl2) molecule interacts with Ag atoms in the first side and with Na atoms in the second side, in some cases.
To better understand the effect of the addition of Na cations in the chabazite structures, we show in Fig. 6 the results obtained for selective trapping of iodine species in Ag-CHA(11) and AgNa-CHA(11) in the form of radar charts. In Fig. 6(b), we see that the Ag-CHA(11) and AgNa-CHA(5) zeolites offer a good performance for trapping I2 and CH3I towards H2O, CH3Cl and Cl2. In contrast, CO presents an inhibiting effect on the adsorption of iodine compounds in Ag-CHA(11) even with the presence of Na atoms.
In Fig. 6(c), it is seen that the addition of Na cations decreases the area occupied by Ag-CHA(5). Despite the decrease in the area occupied by AgNa-CHA1(5) compared to that occupied by Ag-CHA(5), we found that AgNa-CHA1(5) offers a good trapping of iodine species towards H2O, CO, CH3Cl and Cl2, with a lower silver content of around 11.1 wt%. On the other hand, we observe in the case of AgNa-CHA2(5) that H2O and CO have similar interaction energies with iodine compounds and therefore could inhibit their adsorption.
3.4 Clinoptilolite structure
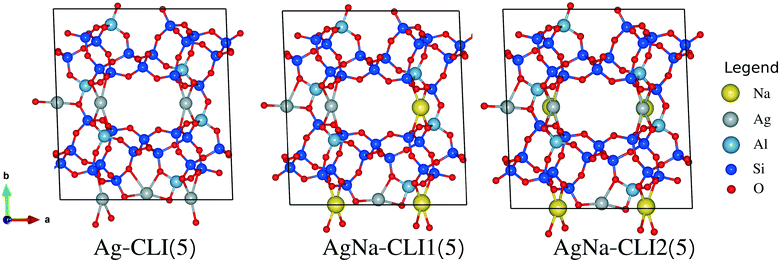
Clinoptilolite (CLINO) is characterized by a monoclinic structure and is one of the most common natural zeolites which is easily obtained from mines. CLINO is basically made up of three main channels A, B and C, where A and B run parallel to the c-axis, while C runs parallel to the a-axis and the [100] direction. Channels A and B are occupied by six cations at positions M1 and M2.38,40–42 In this study, we used the unit cell suggested by Ruiz-Salvador et al.,43 where its lattice parameters are a = 17.619 Å, b = 17.805 Å and c = 7.374 Å, and its cell angle β is 116.21°. In order to lodge the iodine compounds, our calculations are performed in the monoclinic supercell doubled in the c-direction. With a Si/Al ratio of 5, we have studied the pure Ag-exchanged CLINO structure, where the silver loading is about 23.1 wt%. In order to decrease the amount of silver, we introduced the Na cations in parallel with the Ag ones in the CLINO zeolite where we have studied two structures, which are AgNa-CLINO1(5) and AgNa-CLINO2(5), with an amount of silver of about 12.7 wt%, see Fig. 7.Regarding the interaction energy values presented in Table 4, we found that the total interaction energies of I2 and CH3I are about −105.3 kJ mol−1 and −104.4 kJ mol−1, respectively. On the other hand, H2O, CO, CH3Cl and Cl2 are less adsorbed than the iodine species where their interaction energy values are −81.6, −88.3, −72.4 and −58.4 kJ mol−1, respectively. With the addition of Na cations, we found that I2, CH3I, CO, CH3Cl and Cl2 are less adsorbed by around 5 kJ mol−1 in the case of AgNa-CLI(5) compared to Ag-CLI(5). In contrast, H2O is more adsorbed by about 20 kJ mol−1 with the presence of Na cations, which makes it more adsorbed than iodine species. In regard to the contributions of dispersion energy to the interaction energies, we found that they are not significantly influenced by the addition of Na cations.
| Ag-CLI(5) | AgNa-CLI1(5) | AgNa-CLI2(5) | |
|---|---|---|---|
| CH3I | −104.4 (−65.8) | −99.9 (−65.8) | −100.6 (−66.0) |
| I2 | −105.3 (−70.7) | −90.5 (−70.3) | −90.3 (−70.4) |
| CO | −88.3 (−26.0) | −84.9 (−26.6) | −84.1 (−26.6) |
| H2O | −81.6 (−19.6) | −101.5 (−21.4) | −101.3 (−20.4) |
| CH3Cl | −72.4 (−53.0) | −70.9 (−51.3) | −69.8 (−51.6) |
| Cl2 | −58.4 (−47.2) | −55.0 (−45.7) | −45.9 (−45.9) |
In Fig. 8, we observe that the Ag-CHA(5) structure offers the best performance for trapping iodine species, compared to AgNa-CLI1(5) and AgNa-CLI2(5), which is presented by the larger area. On the other hand, we see that the profiles of AgNa-CLI1(5) and AgNa-CLI2(5) are similar, where we found that H2O and CO have a similar interaction energy to the iodine species, which can inhibit their adsorption.
 | ||
| Fig. 8 Radar charts comparing the difference in interaction energies between iodine species (I2 and CH3I) and M molecules (M = CO, H2O, CH3Cl and Cl2) adsorbed on Ag- and AgNa-clinoptilolite. | ||
4 Discussion
As described in previous sections, the spontaneous dissociation of the molecules I2 and Cl2 considerably increases their interaction energies with zeolite. In fact, this increase in energy is very important in the case of I2 which makes it privileged in adsorption versus most of the molecules studied here. In contrast, the spontaneous dissociation of Cl2 has a negative impact since it favours the adsorption of this molecule against the iodine species, in particular CH3I. Also, we found that the dissociation of I2 and Cl2 depends on the distance between the Ag atoms interacting with the I(Cl) atom on each side of their molecule. In Fig. 9, the interaction energy is presented as a function of the average distance ‘d’ between Ag cations which interact with I2(Cl2) during the adsorption. Here, we aim to decouple the spontaneous dissociation observed in parallel for I2 and Cl2 where we are looking to find a Ag–Ag distance which increases the adsorption of I2 and at the same time avoids the spontaneous dissociation of Cl2. We can observe that I2 and Cl2 have similar interaction energy values where a spontaneous dissociation is seen in the cases of Ag-X, Ag-MOR(11) and Ag-MOR(5). On the other hand, we found that I2 is more adsorbed than Cl2 by an important amount of energy, which is around 90.4 kJ mol−1, 88.7 kJ mol−1 and 46.9 kJ mol−1 for Ag-CHA(5), Ag-CHA(11) and Ag-CLI(5), respectively. In these last cases, we note that no spontaneous dissociation of I2 and Cl2 is obtained but an increase in the I–I (Cl–Cl) bond length is observed which is 2.8 Å(2.25 Å) for Ag-CHA(5), 2.8 Å(2.03 Å) in the case of Ag-CHA(11) and 2.7 Å(2.0 Å) for Ag-CLI(5). We note that our optimized I–I (Cl–Cl) bond length in the gas phase with TS/HI is 2.68 Å(1.99 Å). As per the results, we found that the suitable average Ag–Ag distances are about 7.04 Å and 9.53 Å, which are able to limit the dissociation of Cl2 and improve the adsorption of I2.In Fig. 10, we present the radar charts to compare the results obtained for the Ag-CLI(5), AgNa-Y, AgNa-MOR2(11) and Ag-CHA(5) zeolites. The Ag-CHA(5) and AgNa-MOR2(11) zeolites offer the best results for the selective trapping of I2 towards CO, H2O, CH3Cl and Cl2 compared to others; AgNa-Y and Ag-CLI(5), this trend is less marked for CH3I. Regarding the silver amount in structures, we found that AgNa-MOR2(11) with 6.5 without 10 wt% of silver loading is required to get good performance.
 | ||
| Fig. 10 Radar charts comparing the difference in interaction energies between iodine species (I2 and CH3I) and M molecules (M = CO, H2O, CH3Cl and Cl2) adsorbed on Ag- and AgNa-zeolites. | ||
Here, we discuss the effect of the adsorption of a second iodine species, and the co-adsorption of inhibitor compounds (CO and H2O) with iodine species (I2 and CH3I) for the less efficient case AgNa-Y (see Fig. 10). In Table 5, we present the calculated interaction energies ΔEint (kJ mol−1) and distances (Ag–I) for 2 I2, 2 CH3I, I2 + CO/H2O and CH3I + CO/H2O in the AgNa-Y structure. We found that the adsorption of a second iodine species has almost no effect on the adsorption of the first iodine molecule on a specific cation. Indeed, the interaction energy of 2 I2 together is equivalent to the sum of the interaction energies of 2 I2 alone. For the co-adsorption mechanism, we found that indeed there is a significant effect with a variation of the interaction energy values, depending on the molecule. Water has a small effect where a decrease in the interaction energy of about 17.3 kJ mol−1 and about 3.5 kJ mol−1 is obtained upon adsorption with I2 and CH3I, respectively (see Table 5). Together with this small decrease in the interaction energy, a small increase in the Ag–I distance of about 0.1 Å is observed upon the adsorption of H2O with iodine molecules. In contrast, we found that CO presents a significant effect where a decrease in the interaction energy of about 27.7 kJ mol−1 and 60.8 kJ mol−1 is obtained upon adsorption with I2 and CH3I, respectively. Also, this strong effect of CO appears in the Ag–I distance where an increase of about 0.2 Å is observed in both cases. Finally, we see that the adsorption of CH3I is more perturbed than I2 by the presence of CO.
| E int (kJ mol−1) | d(Ag–I) (Å) | |
|---|---|---|
| 2 I2 | −187.1 (−183.0) | 2.62 |
| I2 +H2O | −139.7 (−157.0) | 2.72 |
| I2 + CO | −163.3 (−191.0) | 2.83 |
| 2 CH3I | −173.7 (−201.6) | 2.64 |
| CH3I + H2O | −162.8 (−166.3) | 2.67 |
| CH3I + CO | −139.2 (−200.3) | 2.88 |
5 Conclusion
In summary, we have investigated the selective trapping of iodine species versus H2O, CO, CH3Cl and Cl2 for four different zeolites which are faujasite, mordenite, chabazite and clinoptilolite. For the faujasite structure, we found that the results obtained depend on the Si/Al ratio where a better selective trapping of iodine compounds in comparison with H2O and CH3Cl is obtained with Si/Al = 1.4 (X structure), while a better selective trapping of iodine compounds in comparison with Cl2 is obtained with Si/Al = 47 and 2.4 (Y structure). In contrast, we found that faujasite is not suitable for the selective trapping of iodine species towards CO, independent of the Si/Al ratio. In the case of mordenite, CO is expected to present an inhibiting effect on the adsorption of iodine compounds with a Si/Al ratio of 47. Going from a Si/Al ratio of 47 to 11 and 5, the CO inhibitory effect has disappeared but we found that Cl2 is expected to present an inhibiting effect. On the other hand, we found that the addition of the Na cations in mordenite with Si/Al = 11 improved its performance in the selective trapping of iodine compounds and a positive impact is observed in the limitation of the spontaneous dissociation of Cl2. For chabazite, it has been shown that the pure Ag-exchanged structure with a Si/Al of 5 presents the best results for selective trapping of iodine species where an inhibiting effect is observed. In contrast, CO is expected to present an inhibiting effect on the adsorption of CH3I for Si/Al = 23 and 11. In the case of clinoptilolite, we found that the pure Ag-exchanged structure offers the best performance compared with the other AgNa-exchanged structures. These results could be confirmed by experiments to get relevant data considering representative molar ratios of the gas involved and the shape of the potential porous material in considering the filtering efficiency requirement.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was granted access to the HPC resources of TGCC under the allocation 2021-A0100810433 by GENCI -EDARI project, and was supported by the French research program ANR-11-RSNR-0013-01 called MiRE (Mitigation of Iodine Releases in the Environment).References
- J. R. Brian, D. V. John, M. S. Denis, S. M. John and L. J. James, J. Nucl. Mater., 2016, 470, 307–326 CrossRef
.
- A. J. González, Health Phys., 2007, 93, 571 CrossRef PubMed
.
- M. Chebbi, B. Azambre, C. Monsanglant-Louvet, B. Marcillaud, A. Roynette and L. Cantrel, J. Hazard. Mater., 2021, 409, 124947 CrossRef CAS PubMed
.
- J. Jolley and H. Tompkins, Appl. Surf. Sci., 1985, 21, 288–296 CrossRef CAS
.
- K. W. Chapman, P. J. Chupas and T. M. Nenoff, J. Am. Chem. Soc., 2010, 132, 8897–8899 CrossRef CAS PubMed
.
- L. N. Rastunov, E. P. Magomedbekov, A. V. Obruchikov and L. A. Lomazova, At. Energy, 2011, 110, 68 CrossRef CAS
.
- T. M. Nenoff, M. A. Rodriguez, N. R. Soelberg and K. W. Chapman, Microporous Mesoporous Mater., 2014, 200, 297–303 CrossRef CAS
.
- S. U. Nandanwar, K. Coldsnow, V. Utgikar, P. Sabharwall and D. Eric Aston, Chem. Eng. Sci., 2016, 306, 369–381 CrossRef CAS
.
- H. Jabraoui, E. Hessou, S. Chibani, L. Cantrel, S. Lebègue and M. Badawi, Appl. Surf. Sci., 2019, 485, 56–63 CrossRef CAS
.
- L. E. Herranz, T. Lind, K. Dieschbourg, E. Riera, S. Morandi, P. Rantanen, M. Chebbi and N. Losch, OECD REPORTRN: 47070575, 2013, p. 2846 Search PubMed.
- D. Jacquemain, S. Guentay, S. Basu, M. Sonnenkalb, L. Lebel, H.-J. Allelein, B. Liebana, B. Eckardt and L. Ammirabile, OECD Report-RN:45089842, 2014, p. 174 Search PubMed.
- J. Foit, Nucl. Eng. Des., 1997, 170, 73–79 CrossRef CAS
.
- A. Auvinen, R. Zilliacus and J. Jokiniemi, Nucl. Technol., 2005, 149, 232–241 CrossRef CAS
.
- M. Kitamura, K. Funabashi, M. Kikuchi, H. Yusa, Y. Fukushima and S. Horiuchi, Silver-impregnated alumina for removal of radioactive methyl iodide, Trans. Am. Nucl. Soc., 1978, vol. 30, https://www.osti.gov/biblio/6537164 Search PubMed.
- D. F. Sava, M. A. Rodriguez, K. W. Chapman, P. J. Chupas, J. A. Greathouse, P. S. Crozier and T. M. Nenoff, J. Am. Chem. Soc., 2011, 133, 12398–12401 CrossRef CAS PubMed
.
- S. Chibani, M. Chebbi, S. Lebègue, L. Cantrel and M. Badawi, Phys. Chem. Chem. Phys., 2016, 18, 25574–25581 RSC
.
- M. Chebbi, S. Chibani, J.-F. Paul, L. Cantrel and M. Badawi, Microporous Mesoporous Mater., 2017, 239, 111–122 CrossRef CAS
.
- V. Van Speybroeck, K. Hemelsoet, L. Joos, M. Waroquier, R. G. Bell and C. R. A. Catlow, Chem. Soc. Rev., 2015, 44, 7044–7111 RSC
.
- E. R. Vance and D. K. Agrawal, J. Mater. Sci., 1982, 17, 1889 CrossRef CAS
.
- R. T. Jubin, Airborne waste management technology applicable for use in reprocessing plants for control of iodine and other off-gas constituents, Report Number: ORNL/TM-10477, 1988, DOI:10.2172/5169490.
- B. S. Choi, G. I. Park, J. H. Kim, J. W. Lee and S. K. Ryu, Adsorption, 2001, 7, 91 CrossRef CAS
.
- Q. Cheng, Z. Li, T. Chu, W. Yang, Q. Zhu, D. He and C. Fang, J. Radioanal. Nucl. Chem., 2015, 303, 1883 CAS
.
- S. Chibani, M. Chebbi, S. Lebègue, T. Bucko and M. Badawi, J. Chem. Phys., 2016, 144, 244705 CrossRef PubMed
.
- B. Azambre and M. Chebbi, ACS Appl. Mater. Interfaces, 2017, 9, 25194–25203 CrossRef CAS PubMed
.
- T. Sun and K. Seff, Chem. Rev., 1994, 94, 857–870 CrossRef CAS
.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS
.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- F. Göltl, A. Grüneis, T. Bučko and J. Hafner, J. Chem. Phys., 2012, 137, 114111 CrossRef PubMed
.
- T. Bučko, S. Lebègue, J. Hafner and J. G. Ángyàn, J. Chem. Theory Comput., 2013, 9, 4293–4299 CrossRef PubMed
.
- T. Bučko, S. Lebègue, J. G. Ángyán and J. Hafner, J. Chem. Phys., 2014, 141, 034114 CrossRef PubMed
.
- E. Dempsey, G. H. Kuehl and D. H. Olson, J. Phys. Chem., 1969, 73, 387–390 CrossRef CAS
.
- B. Azambre, M. Chebbi and A. Hijazi, Chem. Eng. J., 2020, 379, 122308 CrossRef CAS
.
- T. Frising and P. Leflaive, Microporous Mesoporous Mater., 2008, 114, 27–63 CrossRef CAS
.
- A. Alberti, P. Davoli and G. Vezzalini, Z. Kristallogr. - Cryst. Mater., 1986, 175, 249–256 CrossRef CAS
.
- T. Bučko and J. Hafner, J. Catal., 2015, 329, 32–48 CrossRef
.
- C. Paolucci, A. A. Parekh, I. Khurana, J. R. Di Iorio, H. Li, J. D. Albarracin Caballero, A. J. Shih, T. Anggara, W. N. Delgass, J. T. Miller, F. H. Ribeiro, R. Gounder and W. F. Schneider, J. Am. Chem. Soc., 2016, 138, 6028–6048 CrossRef CAS PubMed
.
- M. Abatal, A. R. Ruiz-Salvador and C. H. Norge, Microporous Mesoporous Mater., 2020, 294, 109885 CrossRef CAS
.
- Y. Guo, T. Sun, Y. Gu, X. Liu, Q. Ke, X. Wei and S. Wang, Chem. – Asian J., 2018, 13, 3222–3230 CrossRef CAS PubMed
.
- A. R. Ruiz-Salvador, A. Gómez, D. W. Lewis, C. R. A. Catlow, L. Marleny Rodríguez-Albelo, L. Montero and G. Rodríguez-Fuentes, Phys. Chem. Chem. Phys., 2000, 2, 1803–1813 RSC
.
- A. Rivera, T. Farías, L. C. de Ménorval, M. Autié-Pérez and A. Lam, J. Phys. Chem. C, 2013, 117, 4079 CrossRef CAS
.
- O. Korkuna, R. Leboda, J. Skubiszewska-Zieba, T. Vrublevska, V. Gunko and J. Ryczkowski, Microporous Mesoporous Mater., 2006, 87, 243–254 CrossRef CAS
.
- A. R. Ruiz-Salvador, D. W. Lewis, J. Rubayo-Soneira, G. Rodríguez-Fuentes, L. R. Sierra and C. R. A. Catlow, J. Phys. Chem. B, 1998, 102, 8417–8425 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2022 |