Design, synthesis, and biological evaluation of C6-difluoromethylenated epoxymorphinan Mu opioid receptor antagonists†
Abstract
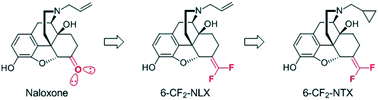
The recent widespread abuse of high potency synthetic opioids, such as fentanyl, presents a serious threat to individuals affected by substance use disorder. Synthetic opioids generally exhibit prolonged in vivo circulatory half-lives that can outlast the reversal effects of conventional naloxone-based overdose antidotes leading to a life-threatening relapse of opioid toxicity known as renarcotization. In this manuscript, we present our efforts to combat the threat of renarcotization by attempting to extend the half-life of traditional MOR antagonists through the design of novel, fluorinated 4,5-epoxymorphinans possessing increased lipophilicity. Analogues were prepared via a concise synthetic strategy highlighted by decarboxylative Wittig olefination of the C6 ketone to install a bioisosteric 1,1-difluoromethylene unit. C6-difluoromethylenated compounds successfully maintained in vitro potency against an EC90 challenge of fentanyl and were predicted to have enhanced circulatory half-life compared to the current standard of care, naloxone. Subsequent in vivo studies demonstrated the effective blockade of fentanyl-induced anti-nociception in mice.


 Please wait while we load your content...
Please wait while we load your content...