Open Access Article
Open Access ArticleImmobilization of molecular complexes on graphitized carbon cloth as stable and hybrid electrocatalysts for enhanced hydrogen evolution reaction and oxygen evolution reaction†
Ram
Murthy
 and
Sundaresan
Chittor Neelakantan
and
Sundaresan
Chittor Neelakantan
 *
*
Department of Chemistry, Sri Sathya Sai Institute of Higher Learning, Brindavan Campus, Kadugodi, Bengaluru – 560067, India. E-mail: cnsundaresan@sssihl.edu.in
First published on 1st September 2022
Abstract
The search for an environment friendly, sustainable and cost-effective catalyst used in electrochemical water splitting via the hydrogen evolution reaction (HER) has generated considerable interest in renewable energy research worldwide. In the current study, we report cost-effective and easily synthesizable 2-thioureidobenzothiazole (BTT) metal complexes (M = Pd, Co, Ni) as dual and efficient electrocatalysts for HER and OER applications in acidic and alkaline conditions, respectively. The hybrid molecular catalysts were synthesized by integrating transition metal complexes with graphitized carbon cloth (GrCC) as the carbon support. The pristine Pd(II)BTT complex exhibited enhanced hydrogen evolution activity with an applied overpotential value of 178 mV (vs. RHE) and a significantly lower Tafel slope value of 73.74 mV per decade in 0.5 M H2SO4 solution. The high catalytic activity of the Pd(II)BTT complex can be ascribed to the strong affinity of the Pd metal ion towards H2. The Ni(II)BTT/GrCC hybrid catalyst for the HER exhibited an overpotential of 335 mV (vs. RHE) to drive a current density of 10 mA cm−2 and was found to be superior vis-a-vis the pristine nickel complex. The enhanced catalytic activity of the hybrid catalyst is due to the conducting and porous nature of the graphitized carbon cloth. For OER activity, the pristine Ni(II)BTT complex exhibited a very low overpotential value of 250 mV to drive a current density of 10 mA cm−2 and gave a low Tafel slope value of 93 mV per decade. The cost-effective and robust nickel complex as a novel electrocatalyst was found to be very effective for both HER and OER applications. This work paves the way for a new avenue to combine molecular catalysts with effective carbon supports for energy storage applications.
1 Introduction
Currently worldwide, there is an unprecedented awareness and there are efforts to reduce the carbon footprint by substituting fossil fuels with a very sustainable and economically viable green renewable energy source. Though wind and solar energy systems are evolving to provide viable and efficient alternative clean energy resources, they are beset with certain limitations. In this context, hydrogen has tremendous potential as a promising future energy currency and is prognosticated as a very prominent next-generation renewable energy source.1 The hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) have been recognised as clean and sustainable ways of generating green energy.2 Researchers have devoted significant and sustained effort to develop inexpensive, durable, and efficient electrocatalysts for water splitting reactions.Ideal electrocatalysts would involve small overpotential and possess fast reaction kinetics to produce hydrogen. To date, platinum (Pt)-based electrocatalysts are considered as the gold standard for the HER, owing to their strength of Pt–H bond and faster facile kinetics.3 Exorbitant cost and insufficient reserves of platinum have hindered industrial scalability. Palladium is emerging as a very promising alternative to platinum owing to its availability, relatively lower cost and good catalytic activity.4 Currently, palladium metal is extensively utilized in the automotive industry to lower the toxicity of emissions from the combustion engine.4 Many non-precious metals such as Co,5,6 Ni,7 and Fe are preferred for water electrolysis due to their affordable cost and abundance on the earth's crust.8,9 Various factors determine the efficiency of the electrocatalyst, i.e., electrode modification, nature of the electroactive material, presence of a carbon support, the porosity of the material, etc. Transition metals are preferred because of their ease of synthesis, broad applicability, and functionality in neutral, alkaline and acidic conditions.
2-Thioureidobenzheteroazoles (BTZs) undergo chelation with metal atoms through thiazole sulphur and thioureido sulphur atoms to give a stable metal complex. The synthesized metal complexes with the general formula [M(C8H8N6S6)2Cl2]+2 (M = Pd, Co, and Ni) have been used to evaluate HER & OER activity. The Pd complex has square planar geometry, while both Co and Ni metal complexes have tetrahedral geometry.10 In order to further enhance the activity of the molecular catalyst, the metal complexes were incorporated into a carbon support.
Various carbon supports, such as carbon nanotubes11 (MWCNTs, SWCNTs), fullerene,12 graphene,13 carbon fiber cloth,14 and porous carbon,15 are favored for electrocatalytic applications due to their excellent electrochemical stability, conductivity16 and favorable mass-transport properties. These carbon supports have shown remarkable electrochemical efficiency in numerous batteries and supercapacitors.17 The conventional strategy involves the modification of the electrode by drop casting the catalyst on the surface of the electrode. This strategy suffers from the problem of leaching due to the fragile linkage between the molecular catalyst and electrode surface. To overcome these limitations, molecular catalysts were integrated into carbon supports to enhance the HER activity (Fig. 2).
In this study, graphitized carbon cloth (CC) has been used as a carbon support owing to graphene's excellent electronic conducting nature and the porous nature of the carbon cloth for HER activity. The current study reports a method to synthesize low-cost transition metal complexes as dual electrocatalysts for HER and OER applications. We demonstrate a simple method of integrating transition metal complexes with graphitized carbon cloth as a carbon support for enhanced hydrogen and oxygen evolution in acidic and alkaline conditions, respectively. With this motive, Co, Ni, and Pd metal complexes of 2-thioureidobenzothiazoles were chosen as electroactive materials for studying hydrogen evolution reactions and oxygen evolution reactions.
2 Results and discussion
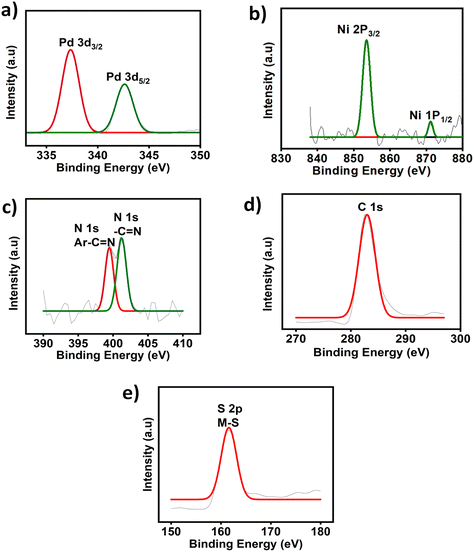
Pd, Co, and Ni coordination complexes were prepared under alkaline conditions (Fig. 1). The procedure detailing the synthesis and characterization have been described in S2 and S3 (ESI†). The metal complexes were immobilized on carbon cloth by a simple drop-casting method and dried at room temperature (Fig. 2). To confirm the successful immobilization of the complexes on the graphitized carbon cloth, X-ray photoelectron spectroscopy (XPS) measurements were performed (shown in Fig. 3). The XPS spectrum of the electrocatalyst immobilized graphitized carbon cloth exhibits two characteristic peaks of 335.5 eV and 337.8 eV corresponding to Pd 3d5/2 and Pd 3d3/2 metal (Table 1). The peak at 284.5 eV can be attributed to the sp2 hybridized carbon atoms.18 In Fig. 3 and 4, the peak at 335.5 ± 0.2 eV can be attributed to Pd2+, which can be quickly bonded to an oxygen atom.18 Comparing the XPS pattern of carbon cloth before and after the HER reaction, Fig. 3(a and b) show the used Pd(II)BTT complex, which is observed to show a decreased intensity at 343.88 eV (Table 1). In Fig. 4b, the Ni complex shows a characteristic peak at 853.7 eV, which can be attributed to Ni 2p3/2 (Ni2+) present on the carbon cloth surface, and other peaks remain constant (Fig. S1, ESI†) (Table 1).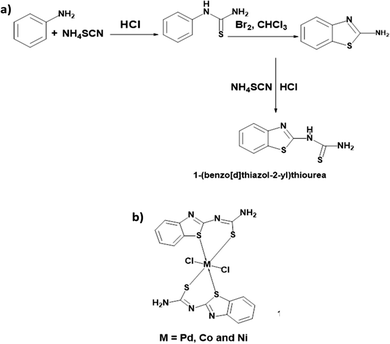 | ||
| Fig. 1 (a) Synthetic route of the ligand 2-thioureidobenzothiazole and (b) general structure of the 2-thioureidobenzothiazole metal complex. | ||
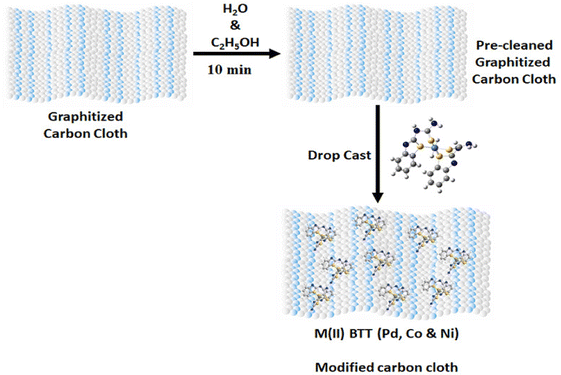 | ||
| Fig. 2 Schematic representation of immobilization of M(II)BTT complexes onto graphitized carbon cloth (GrCC) at 298 K. | ||
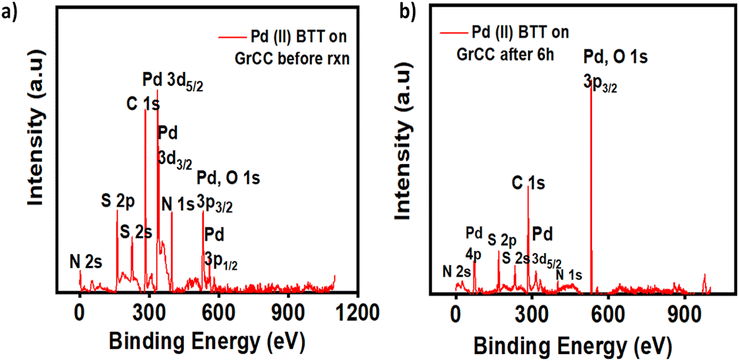 | ||
| Fig. 3 XPS spectra of the Pd(II)BTT complexes. (a) Pd(II)BTT/GrCC hybrid electrocatalyst before the LSV test. (b) PdBTT/GrCC after the test (LSV 6 h stability). | ||
| Complex | Pd 3d5/2 | Pd 3d3/2 | Ni 2p | N 1s | S 4s | C 1s |
|---|---|---|---|---|---|---|
[Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (BTT)/GrCC] before rxn (BTT)/GrCC] before rxn |
335.2 | 337.5 | — | 400 | 164 | 284.5 |
[Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (BTT)/GrCC] after 6 h (BTT)/GrCC] after 6 h |
343.88 | 338.72 | — | 400.74 | 162.3 | 284.5 |
| [Ni(BTT)/GrCC] | — | — | 853.7 | 400 | 164 | 284.5 |
The FT-IR spectra of the metal complexes Pd(II)BTT, Co(II)BTT, and Ni(II)BTT are shown in Section S3 (ESI†). The bands at 1585 cm−1 and 755 cm−1 are due to thioamide stretching and bending vibrations, respectively. The band at 1629 cm−1 corresponds to the ν(N–H) bending of the ligand. The bands at 1132 cm−1, 1184 cm−1, and 996 cm−1 can be attributed to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) for Pd(II), Co(II) and Ni(II) complexes, respectively.10 The bands in the range of 740–755 cm−1 are attributed to ν(C–S–C) of the thiazole ring in all three complexes.10 Complexation of the ligand with metals leads to a shift in the band to lower frequency, implying that the thiocarbonyl group is involved in complexing with the metal ion.
S) for Pd(II), Co(II) and Ni(II) complexes, respectively.10 The bands in the range of 740–755 cm−1 are attributed to ν(C–S–C) of the thiazole ring in all three complexes.10 Complexation of the ligand with metals leads to a shift in the band to lower frequency, implying that the thiocarbonyl group is involved in complexing with the metal ion.
The UV-Vis absorbance spectra of 2-BTT and its complexes are provided in Fig. S2 (ESI†). From Fig. S2 (ESI†), the ligand shows a strong peak at 306 nm, but in the case of all the three complexes, there is a hypsochromic shift in the wavelength of the complex, revealing the complex formation. The electron transition observed is π–π* for the complex. In the case of all the three complexes, a hypsochromic shift from 306 nm to 290 nm is observed, depicted in Fig. S2 of the ESI.†
The electronic spectrum of the Pd complex exhibited two broad absorption bands at 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 089 cm−1 and 34
089 cm−1 and 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 482 cm−1 corresponding to charge transfer bands. The peaks observed in the UV region reflect π–π* transition. The energy absorption of 32
482 cm−1 corresponding to charge transfer bands. The peaks observed in the UV region reflect π–π* transition. The energy absorption of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 cm−1 (306 nm) is due to the thiazole moiety of the ligand. The energy absorption band moves to further higher energy of 34
679 cm−1 (306 nm) is due to the thiazole moiety of the ligand. The energy absorption band moves to further higher energy of 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 482 cm−1 implying complexation. The crystal field splitting energy (CFSE) values increase from 390 kJ mol−1 (306 nm) to 412.47 kJ mol−1 (290 nm) reinforcing complex formation.19
482 cm−1 implying complexation. The crystal field splitting energy (CFSE) values increase from 390 kJ mol−1 (306 nm) to 412.47 kJ mol−1 (290 nm) reinforcing complex formation.19
The thiocarbonyl moiety of the ligand undergoes strong chelation with the metal atom. The occupied spx orbitals of the ligand interact with dx2−y2 orbitals of the metal to form a σ bond. Furthermore, the metal donates an electron back to the ligand through π-back bonding. The back bonding is observed between the dxz orbital of the metal and pz of the ligand. This π-back bonding interaction results in increased mixing of the metal and ligand orbitals resulting in stable molecular complexes.20
2-Thioureidobenzothiazole metal complexes were subjected to morphological analysis by scanning electron microscopy as shown in Fig. 5(a–d). The SEM images revealed the crystalline nature of the complexes. The palladium complex possesses a rod-like morphology while the nickel and cobalt complexes showed flaky morphology. The SEM images show increased space in the complex compared to the 2-thioureidobenzothiazole ligand aiding in better adsorption and evolution of hydrogen in the process.
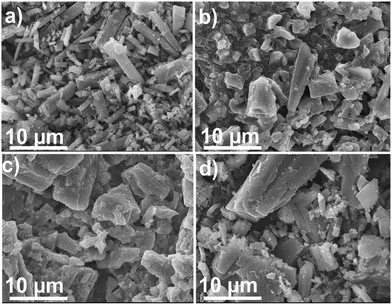 | ||
| Fig. 5 SEM images depicting the morphology of the M(II)BTT complexes. (a) Pd(II)BTT, (b) Co(II)BTT, (c) Ni(II)BTT, and (d) 2-thioureidobenzothiazole ligand. | ||
The thermal stability of the Pd, Co, and Ni(II) complexes of the 2-thioureidobenzothiazoles was investigated by thermogravimetric (TGA) analysis. Three prominent stages of decomposition were observed for each of the complexes (Fig. S5, ESI†). At temperatures ranging from 170 °C to 320 °C, the loss of HCl was observed.10 Subsequently, the loss of the thioureido moiety was observed at a slightly higher temperature between 280 °C and 620 °C. A significant mass loss of the complex was observed at temperatures above 350 °C, and exo-effects with maxima at 370–390 °C were observed for the above decomposition. In the third stage of degradation, the ligand completely decomposes at the temperature range of 760–870 °C; the endo effect is observed, and no further weight loss was observed with metal oxide (MOx) as the final product10 (Fig. S5, ESI†).
The magnetic properties of the 2-thioureidobenzothiazole metal complexes (Pd, Co, and Ni) were investigated using the vibrating sample magnetometer (VSM) technique. From Fig. S4 (ESI†), it was evident that the Pd(II)BTT and Ni(II)BTT complexes behave as diamagnetic due to the effective bonding of the unpaired electrons with the sulfur atoms of the ligand. In the case of the Co(II)BTT complex, ferromagnetic behavior is reflected in Fig. S4 (ESI†).
2.1 Electrochemical reaction
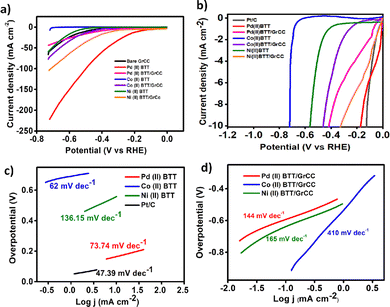
The 2-thioureidobenzothiazole metal complexes were evaluated for the hydrogen evolution reaction. The complexes were directly immobilized on graphitized carbon cloth (GrCC) by a simple drop-casting method and were dried at room temperature. The catalyst loading of 0.04 mg cm−2 and metal loading of 0.011 mg cm−2 were achieved on the carbon cloth. In the case of the pristine metal complexes, a catalyst loading of 0.282 mg cm−2 was attained on the electrode surface. The catalytic activity of the hybrid catalyst (M(II)BTT/GrCC, M = Pd, Co, and Ni) was compared to that of the pristine metal complexes (M(II)BTT) in 0.5 M H2SO4. The electrochemical measurements were obtained using a three-electrode system configuration with a glassy carbon electrode (GCE) as the working electrode, graphite rod as a counter electrode to avoid possible contamination by redeposition of Pt, and a saturated calomel electrode (SCE) as the reference electrode under continuous N2 flow during the experiment. Graphitized carbon cloth (0.5 × 1 cm) of area 0.5 cm2 was pretreated with deionized water and ethanol for 10 min sequentially. The pretreated GrCC was used to obtain a hybrid electrocatalyst for the HER.The Pd(II)BTT/GrCC, Co(II)BTT/GrCC and Ni(II)BTT/GrCC showed a maximum current density of 44 mA cm−2, 63 mA cm−2 and 110 mA cm−2 at 720 mV, respectively (Fig. 6a). In the case of the pristine complexes, Pd(II)BTT showed the maximum current density of 247 mA cm−2, and the Co(II) and Ni(II) complexes of BTT showed a cumulative current density of 18 mA cm−2, and 56 mA cm−2 at the potential of 720 mV (Fig. 6a). In the case of Ni and Co complexes, graphitized carbon cloth enhances the catalytic performance due to the carbon cloth's conducting and porous nature. The porosity determines the ease of accessibility (pore dimensions) of the active sites and affects the mass transport.23 Current density (j) is a measure of rate of the reaction, which in turn is proportional to the number of active sites on the electrode surface.24 The porousness of the hybrid electrocatalyst on carbon cloth has a pore diameter of 37 ± 5 μm, while bare carbon cloth has a pore diameter of 42 ± 5 μm. The results implied that the porous electrode shows much higher (j) compared to planar systems of similar dimensions.25 The enhanced electrocatalytic activity exhibited by the hybrid catalyst complex can be ascribed to the synergistic effect of the M(II)BTT complex and graphitized carbon cloth. The weaved textile-like property with graphite exposes a large fraction of active surface area, in turn enlarging the contact area with the electrolyte accelerating the diffusion. These factors favor excellent electrocatalytic activity for the HER.
Tafel slopes were obtained from linear sweep voltammetry measurements by plotting potential as a function of log j using the relation η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j.26 The Tafel slope values reveal the kinetics of the HER reaction. The Tafel slope value of 73.74 mV dec−1 of Pd(II)BTT is comparable to the Tafel slope value of Pt/C (47.34 mV dec−1) (Fig. 6c). The slope values reveal that the HER process involves the Volmer–Tafel mechanism,27,28 where hydrogen on the electrode surface recombines with a hydronium ion to produce H2. The Tafel mechanism of Pd(II)BTT/GrCC, Co(II)BTT/GrCC, and Ni(II)BTT/GrCC exhibited high Tafel slope values of 144 mV dec−1, 410 mV dec−1, and 165 mV dec−1, respectively (Fig. 6d) indicating sluggish reaction kinetics and obey Volmer–Heyrovsky as the possible mechanistic pathway.29,30 To understand the high catalytic activity of the Pd, Co, and Ni complexes, the double-layer capacitance was measured. Cyclic voltammetry was performed at different scan rates ranging from 20 to 200 mV and the plot of Δj(ja − jc) against the scan rate yielded the slope value (Cdl). The hybrid catalyst showed a Cdl value of 12.06 mF cm−2, 26.19 mF cm−2, and 8.94 mF cm−2 for the Pd, Co, and Ni complexes on GrCC, respectively (Fig. 7b). The electrochemical active surface area (ESCA) is a good descriptor of the electrochemical activity toward the hydrogen evolution reaction. The ECSA was determined from eqn (2), and the double-layer capacitance current is directly proportional to the electrochemical active surface area (ECSA).31 The linearity obtained from the plot of geometrical current density and scan rate revealed a diffusion-controlled electrocatalytic process. The facile electron flow in the process is due to diffusion.
j.26 The Tafel slope values reveal the kinetics of the HER reaction. The Tafel slope value of 73.74 mV dec−1 of Pd(II)BTT is comparable to the Tafel slope value of Pt/C (47.34 mV dec−1) (Fig. 6c). The slope values reveal that the HER process involves the Volmer–Tafel mechanism,27,28 where hydrogen on the electrode surface recombines with a hydronium ion to produce H2. The Tafel mechanism of Pd(II)BTT/GrCC, Co(II)BTT/GrCC, and Ni(II)BTT/GrCC exhibited high Tafel slope values of 144 mV dec−1, 410 mV dec−1, and 165 mV dec−1, respectively (Fig. 6d) indicating sluggish reaction kinetics and obey Volmer–Heyrovsky as the possible mechanistic pathway.29,30 To understand the high catalytic activity of the Pd, Co, and Ni complexes, the double-layer capacitance was measured. Cyclic voltammetry was performed at different scan rates ranging from 20 to 200 mV and the plot of Δj(ja − jc) against the scan rate yielded the slope value (Cdl). The hybrid catalyst showed a Cdl value of 12.06 mF cm−2, 26.19 mF cm−2, and 8.94 mF cm−2 for the Pd, Co, and Ni complexes on GrCC, respectively (Fig. 7b). The electrochemical active surface area (ESCA) is a good descriptor of the electrochemical activity toward the hydrogen evolution reaction. The ECSA was determined from eqn (2), and the double-layer capacitance current is directly proportional to the electrochemical active surface area (ECSA).31 The linearity obtained from the plot of geometrical current density and scan rate revealed a diffusion-controlled electrocatalytic process. The facile electron flow in the process is due to diffusion.
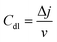 | (1) |
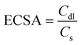 | (2) |
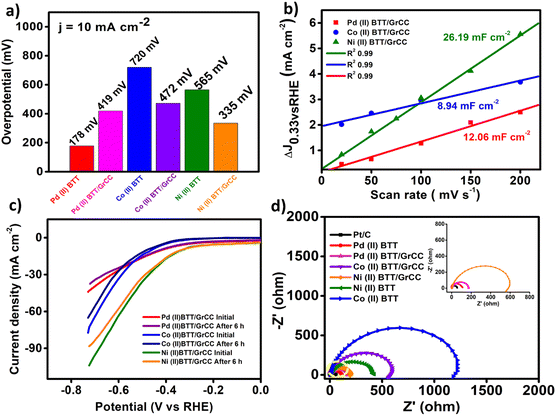
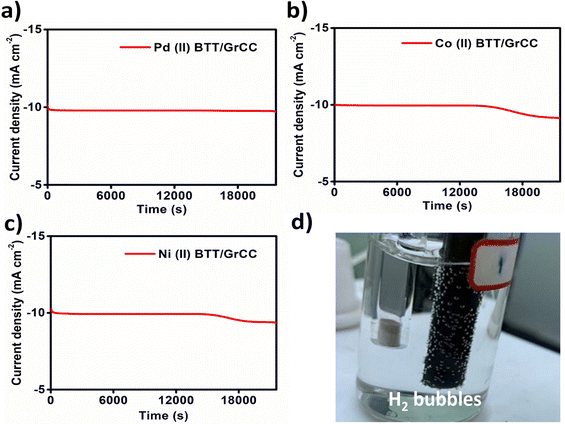
Carbon supports have been extensively used to enhance the durability of electrocatalysts.33 The metal complexes were subjected to linear sweep voltammetry in N2 saturated 0.5 M H2SO4 before and after 6 h of chronoamperometry (Fig. 7c). The durability of the electrodes during the HER process was investigated using chronoamperometry. A constant potential was applied to the electrode for 6 h. As can be seen from Fig. 9(a–c), the hybrid catalysts showed good durability with the current density decreasing after 4 h. From Fig. 9a, the Pd complex (η = 0.410 V) showed excellent stability for 6 h with 85% current density retention activity compared to the Co and Ni complex, which showed a drop in the current density value after 4 h (Fig. 9b and c). In contrast, the Co(II) and Ni(II) hybrid catalyst activity gradually diminished after 4 h (Fig. 8b and c). The durability has been one of the prominent limitations with respect to molecular complexes. The durability of the complex can be improved further with better carbon support materials.34
2.2 Oxygen evolution reaction
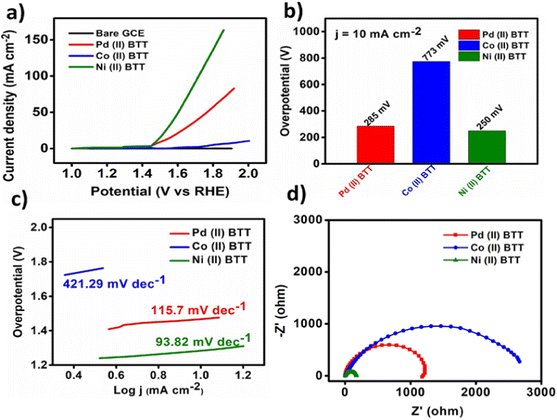
The three complexes were also evaluated for their oxygen evolution reaction (OER) activity using a standard three-electrode cell with a rotating disk electrode (RDE) as the working electrode, graphite rod as an auxiliary electrode, and mercury/mercury oxide as the reference electrode. The OER reaction was carried out in 0.1 M KOH solution with a rotation rate of 1600 rpm in oxygen saturated environments. The LSV curves obtained were all iR corrected with a scan rate of 50 mV s−1 (Fig. 10a). Fig. 10a displays the linear sweep voltammetry polarization curves. The Ni(II)BTT complex exhibited remarkably high OER performance with an overpotential of 250 mV to achieve a current density of 10 mA cm−2 at 1.48 V (Fig. 10a). The Co(II)BTT and Pd(II)BTT complexes exhibited an overpotential of 773 and 285 mV, respectively, to drive a current density of 10 mA cm−2 (Fig. 10b). The Co(II)BTT and Pd(II)BTT complexes exhibited a maximum current density of 17.12 mA cm−2 and 63 mA cm−2 at 1.98 V and 1.92 V, respectively. The Ni(II)BTT complex exhibited a maximum current density of 163 mA cm−2 at 1.92 V (Fig. 10a). The observed catalytic activity is due to the charge transfer from ligand to metal atoms (LMCT). From Fig. 8b, Ni(II) contains oxygen bound to a high valent metal center leading to (Ni(II)–O). (Ni(II)–O) is a key intermediate in the oxygen evolution reaction process, which in turn reacts with H2O through a water nucleophilic attack mechanism to give O–O bond formation, and the intermediate formation is the rate determining step. The reaction is aided by a hydroxide ion coupled electron transfer reaction resulting in an oxygen evolution reaction (Fig. 8b).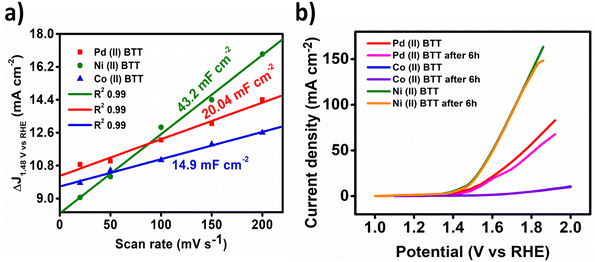 | ||
| Fig. 10 (a) Determination of the double-layer capacitance value of the M(II)BTT complexes. (b) Stability study of the M(II)BTT complexes by linear polarization curves for 6 h in 0.1 M KOH. | ||
The Tafel slopes for all the three complexes are shown in Fig. 9c, where Ni(II)BTT showed a slope value of 93.82 mV dec−1, Co(II)BTT showed 421.21 mV dec−1, and the Pd(II)BTT complex showed 182.6 mV dec−1 (Fig. 9c). The Tafel slope values are the lowest compared to many nanocomposites1 and other transition metal nanosheets.35 The metal complex on the modified glassy carbon surface undergoes oxidation from Ni(0) to Ni(II), leading to reduction of the hydroxide ion to an oxygen molecule. Hence, the Ni(II) complex showed better OER catalytic activity compared to Co(II) and Pd(II). However, in the case of the HER, the Pd(II) complex exhibited enhanced hydrogen evolution reaction activity due to the affinity of palladium towards hydrogen. The Ni and Pd complexes exhibit promising activity in terms of overpotential towards both the hydrogen and oxygen evolution reaction.
The kinetics of the oxygen evolution reaction were evaluated by impedance analysis under alkaline conditions. All three complexes' onset potential were used to obtain impedance plots. The lower the semicircle diameter, the better the electrocatalytic efficiency of the catalyst. The Ni(II) complex showed good catalytic activity with a charge transfer resistance value of 246 ohms (Fig. 9d). Pd(II) and Co(II) exhibited a charge transfer resistance of 1600 ohms and 2700 ohms, respectively, as shown in Fig. 9d. The Ni(II)BTT complex showed the least charge transfer resistance compared to the Co and Pd complexes. The catalysts showed a Cdl value of 20.04 mF cm−2, 14.9 mF cm−2, and 43.2 mF cm−2 for the Pd, Co, and Ni complexes, respectively (Fig. 11a), thus reinforcing the Ni complex as an excellent electrocatalyst towards the oxygen evolution reaction.
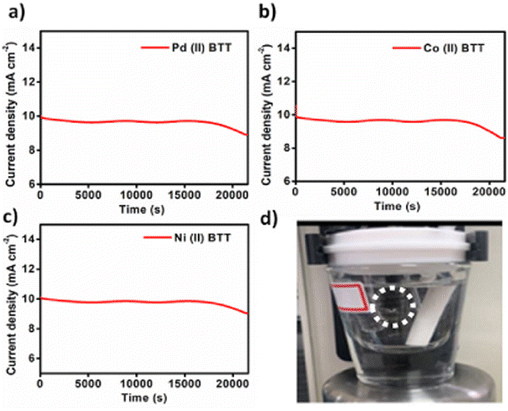
The durability of the complexes was tested using chronoamperometric i–t curves (Fig. 12). From Fig. 12a–c, in the case of the Ni(II)BTT complex, it can be seen that the current density gradually decreases at 1.48 V (vs. RHE) after 4 h demonstrating good durability for all the three complexes. Linear sweep voltammetry was performed before and after 6 h of chronoamperometry, and the catalysts showed 85% of current density retention capability (Fig. 11b).
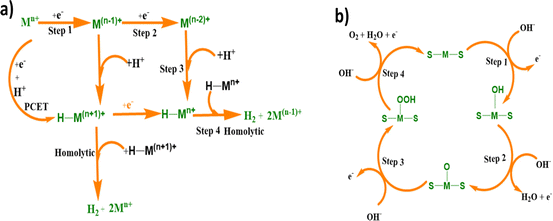 | ||
| Fig. 12 (a) Mechanistic pathway of the hydrogen evolution reaction of the M(II)BTT complexes (Pd, Co and Ni). (b) Mechanism of the oxygen evolution reaction of the M(II)BTT complexes (Pd, Co and Ni). | ||
3 Conclusions
In conclusion, M(II)BTT/GrCC hybrid electrocatalysts were synthesized using 2-thioureidobenzheteroazole metal complexes and graphitized carbon cloth. The hybrid catalysts were utilized for the HER and the pristine metal complexes were used for the OER. The Pd(II)BTT complex showed an outstanding overpotential value of 178 mV to achieve a current density of 10 mA cm−2 in 0.5 M H2SO4. In contrast, the Ni(II)BTT/GrCC hybrid catalyst showed an overpotential value of 335 mV to drive a current density of 10 mA cm−2. The enhanced catalytic activity is due to the conducting and porous nature of the graphitized carbon cloth. Furthermore, the pristine Ni(II) complex exhibited an overpotential value of 250 mV to achieve 10 mA cm−2 for OER activity. The Ni(II) metal has strong affinity for O2 molecules, leading to better oxygen evolution activity the other two metal complexes. The Ni(II)BTT complex is preferred over the other two metal complexes due to its low cost and abundance on the earth's crust. The Ni(II)BTT complex exhibited good durability with 85% current density retention capability. This work paves a new avenue to design efficient electrocatalysts in combination with more effective carbon supports for clean energy storage and conversion.Author contributions
Ram Murthy – original manuscript writing, data curation, analysis, data interpretation revision. C. N. Sundaresan – conceptualization, ideation, review and editing, supervision.Conflicts of interest
The authors declare there is no conflict of interest to report.Acknowledgements
The authors express their deep sense of gratitude to their founder, chancellor Bhagawan Sri Sathya Sai Baba, for being a constant source of inspiration and motivation for the work. RM is grateful to Prof. Noriyoshi Matsumi and Dr Rajashekar Badam at ‘Japan Advanced Institute of Science Technology’ (JAIST) and Sakura Science Exchange Program for providing hands on training with the instruments. The authors thank Dr Raman Vedarajan from ARCI for his insightful suggestions and discussions. RM is thankful to Dr B. V. V. S. Pavan Kumar, IIT Roorkee, for the XPS characterization facility. The authors acknowledge Central Research Instrumentation Facility (CRIF) for the characterization facility. The authors thank SSSIHL for the financial support for carrying out the work.References
- H. He, Y. Zhang, W. Zhang, Y. Li, X. Zhu, P. Wang and D. Hu, ACS Appl. Mater. Interfaces, 2022, 14, 834–849 CrossRef CAS PubMed.
- A. P. Murthy, J. Madhavan and K. Murugan, J. Power Sources, 2018, 398, 9–26 CrossRef CAS.
- L. Jia, P. Wagner and J. Chen, Inorganics, 2022, 10, 53 CrossRef CAS.
- M. Shao, J. Power Sources, 2011, 196, 2433–2444 CrossRef CAS.
- H. Lin, M. S. Hossain, S.-Z. Zhan, H.-Y. Liu and L.-P. Si, Appl. Organomet. Chem., 2020, 34, e5583 CAS.
- S. Shen, Z. Wang, Z. Lin, K. Song, Q. Zhang, F. Meng, L. Gu and W. Zhong, Crystalline-Amorphous Interfaces Coupling of CoSe2/CoP with Optimized d-Band Center and Boosted Electrocatalytic Hydrogen Evolution, 2022 Search PubMed.
- M. Yu, E. Budiyanto and H. Tüysüz, Angew. Chem., Int. Ed., 2022, 61, e202103824 CAS.
- M. Rajakumar, M. Manickam, N. N. Gandhi and K. Muthukumar, Int. J. Hydrogen Energy, 2020, 45, 3905–3915 CrossRef CAS.
- V. Vij, S. Sultan, A. M. Harzandi, A. Meena, J. N. Tiwari, W.-G. Lee, T. Yoon and K. S. Kim, ACS Catal., 2017, 7, 7196–7225 CrossRef CAS.
- H. Yapati, S. R. Devineni, S. Chirumamilla and S. Kalluru, J. Chem. Sci., 2016, 128, 43–51 CrossRef CAS.
- B. Huang, L. Chen, Y. Wang, L. Ouyang and J. Ye, Chem. – Eur. J., 2017, 23, 7710–7718 CrossRef CAS PubMed.
- M. Chhetri, U. Gupta, L. Yadgarov, R. Rosentsveig, R. Tenne and C. Rao, ChemElectroChem, 2016, 3, 1937–1943 CrossRef CAS.
- J. Zhang and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 13296–13300 CrossRef CAS PubMed.
- J.-X. Wu, C.-T. He, G.-R. Li and J.-P. Zhang, J. Mater. Chem. A, 2018, 6, 19176–19181 RSC.
- D. K. Singh, R. N. Jenjeti, S. Sampath and M. Eswaramoorthy, J. Mater. Chem. A, 2017, 5, 6025–6031 RSC.
- W. Zhou, J. Jia, J. Lu, L. Yang, D. Hou, G. Li and S. Chen, Nano Energy, 2016, 28, 29–43 CrossRef CAS.
- H. Shi, G. Wen, Y. Nie, G. Zhang and H. Duan, Nanoscale, 2020, 12, 5261–5285 RSC.
- S. Niu, W. Guo, T.-W. Lin, W. Yu, Y. Wu, X. Ji and L. Shao, RSC Adv., 2017, 7, 25885–25890 RSC.
- A. K. El-Sawaf, F. El-Essawy, A. A. Nassar and E.-S. A. El-Samanody, J. Mol. Struct., 2018, 1157, 381–394 CrossRef CAS.
- M. Drosou, F. Kamatsos and C. A. Mitsopoulou, Inorg. Chem. Front., 2020, 7, 37–71 RSC.
- Z. Zong, K. Xu, D. Li, Z. Tang, W. He, Z. Liu, X. Wang and Y. Tian, Peptide templated Au@Pd core-shell structures as efficient bi-functional electrocatalysts for both oxygen reduction and hydrogen evolution reactions, 2018 Search PubMed.
- T. Wang, D. Gao, J. Zhuo, Z. Zhu, P. Papakonstantinou, Y. Li and M. Li, Chem. – Eur. J., 2013, 19, 11939–11948 CrossRef CAS PubMed.
- P. Adler, Porous media: geometry and transports, Elsevier, 2013 Search PubMed.
- J. Haverkort, Electrochim. Acta, 2019, 295, 846–860 CrossRef CAS.
- A. J. Bard, L. R. Faulkner and H. S. White, Electrochemical methods: fundamentals and applications, John Wiley & Sons, 2022 Search PubMed.
- Q. Yin, Z. Xu, T. Lian, D. G. Musaev, C. L. Hill and Y. V. Geletii, Catalysts, 2021, 11, 87 CrossRef CAS.
- J. Chen, H. Wang, Y. Gong and Y. Wang, J. Mater. Chem. A, 2019, 7, 11038–11043 RSC.
- Z. Lin, B. Xiao, M. Huang, L. Yan, Z. Wang, Y. Huang, S. Shen, Q. Zhang, L. Gu and W. Zhong, Adv. Energy Mater., 2022, 2200855 CrossRef CAS.
- L. Yang, Y. Lv and D. Cao, J. Mater. Chem. A, 2018, 6, 3926–3932 RSC.
- S. Shen, Z. Hu, H. Zhang, K. Song, Z. Wang, Z. Lin, Q. Zhang, L. Gu and W. Zhong, Angew. Chem., Int. Ed., 2022, 61, e202206460, DOI:10.1002/ange.202206460.
- Y.-H. Choi, Nanomaterials, 2022, 12, 939 CrossRef CAS PubMed.
- V.-T. Nguyen, H. Ha, N.-A. Nguyen, H. An, H. Y. Kim and H.-S. Choi, ACS Appl. Mater. Interfaces, 2020, 12, 15500–15506 CrossRef CAS.
- Z. Lin, B. Xiao, M. Huang, L. Yan, Z. Wang, Y. Huang, S. Shen, Q. Zhang, L. Gu and W. Zhong, Adv. Energy Mater., 2013, 12, 2200855 CrossRef.
- S. Manzoor, S. V. Trukhanov, M. N. Ansari, M. Abdullah, A. Alruwaili, A. V. Trukhanov, M. U. Khandaker, A. M. Idris, K. S. El-Nasser and T. A. Taha, Flowery ln2MnSe4 Novel Electrocatalyst Developed via Anion Exchange Strategy for Efficient Water Splitting, 2022 Search PubMed.
- R. Elakkiya and G. Maduraiveeran, Langmuir, 2020, 36, 4728–4736 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00764a |
| This journal is © The Royal Society of Chemistry 2022 |