Open Access Article
Open Access ArticleAdvances of bioactive tellurium nanomaterials in anti-cancer phototherapy
Can
Li
a,
Fucheng
Gao
a,
Yandong
Wang
a,
Li
Zhao
a,
Hui
Li
 *a and
Yanyan
Jiang
*a and
Yanyan
Jiang
 *ab
*ab
aKey Laboratory for Liquid-Solid Structural Evolution & Processing of Materials (Ministry of Education), School of Materials Science and Engineering, Shandong University, Jinan, Shandong 250061, P. R. China. E-mail: lihuilmy@sdu.edu.cn; yanyan.jiang@sdu.edu.cn
bShenzhen Research Institute of Shandong University, Shenzhen, Guangdong 518057, P. R. China
First published on 14th July 2022
Abstract
Tellurium nanomaterials show unique advantages, including high carrier mobility, excellent optical properties, and high specific surface areas. Although previous studies have confirmed the application of tellurium nanomaterials in the biological field, their biocompatibility, metabolism, and potential toxicity in organisms demand further exploration. This review systematically summarizes the application of tellurium nanomaterials in the field of tumor phototherapy. Firstly, the common synthesis methods of tellurium nanomaterials are elaborated, mainly including solid-phase deposition, liquid-phase exfoliation, the solution synthesis method, and molecular beam epitaxy. Secondly, the review summarizes the research progress of tellurium nanomaterials in tumor photothermal therapy, photodynamic therapy, photo-triggered molecule delivery, and synergetic therapy. Moreover, we emphasize some breakthroughs in these fields, such as surface-modification and size-control strategies of tellurium nanomaterials. Finally, future prospects are also discussed. The excellent characteristics of tellurium nanomaterials and their relative lack of exploration also suggest that they may have more application opportunities in the near future. This review aims to demonstrate the significance of tellurium nanomaterials in biomedicine and to provide strategies to expand their application in phototherapy.
1. Introduction
Cancer, namely malignant tumors, has become a major cause of death because of its uncontrollability and metastasis.1–3 Currently, the traditional methods of cancer treatment are mainly surgery, radiotherapy, and chemotherapy;4 however, existing drawbacks such as high risk of recurrence,5 limited therapeutic effectiveness, damage to normal tissues, and obvious toxicity and side effects6 demand the development of new methods of tumor treatment. Due to its unique advantages, including spatiotemporal addressability, minimal invasiveness, relative clinical safety, and high efficiency,7,8 phototherapy has rapidly developed into a new treatment for malignant tumors and a variety of skin diseases.9,10Nanoparticles belong to a mesoscopic scale, which is located in the transition region between atomic clusters and macro matter. The size of nanomaterials falls within 1–100 nm, which well matches macromolecules in organisms and provides a good crossing ability in tissue gaps. The appropriate size also creates an enhanced permeation and retention (EPR) effect,7 so some specific macromolecules such as nanoparticles and liposomes11 are more likely to penetrate and stay in tumor tissues for a long time, which has great advantages for long-term drug release. In addition, the surface of nanomaterials is easy to modify and has the ability to achieve targeted therapy.12 Therefore, the application of nanomaterials in tumor therapy has always been a research hotspot.
At present, the anticancer nanomaterials that have been clearly confirmed include metal nanoparticles,13 carbon nanomaterials,14 two-dimensional (2D) materials15–17 such as MXenes,18 inorganic semiconductor nanomaterials,19 and organic nanoparticles (like micelles or vesicles).20 The emergence of advanced nanomaterials has also greatly enhanced the development of phototherapies such as photoacoustic imaging,21 photothermal imaging,22 fluorescence imaging, and especially photosensitizer-enhanced photodynamic therapy (PDT) and photothermal therapy (PTT).23 In recent years, some semiconductor materials have been widely explored in the phototherapeutic field because of their excellent physical, chemical, electronic, and optical properties. Among them, as a semiconductor material, tellurium (Te) has attracted a great deal of attention because of several advantages, such as a medium band gap,24,25 an anisotropic crystal structure, low manufacturing costs, and better environmental stability.26 Te has great physicochemical reactivity27 and can generate a rapid response to external stimuli such as a near-infrared (NIR) laser, which is conducive to triggering the release of loaded molecules.28–30 Especially, the lamellar structure of 2D tellurene (Te nanomaterials with a 2D lamellar structure) provides a high loading capacity for therapeutic molecules and fluorescence factors.31 Te nanomaterials have various morphologies, such as zero-dimensional nanodots,32 one-dimensional (1D) nanowires33,34 or nanotubes, and 2D layered tellurene35 structures. Moreover, the existence of tellurene has been confirmed by theoretical calculations,36–38 and its significant light absorption performance has been predicted.28,31,39,40
The application of Te in the biomedical field is intriguing. For example, the microbial behavior of Te nanoparticles has been reviewed by Zannoni et al.41 Similarly, others have summarized the synthesis of Te nanomaterials and their application in tumor therapy.42 Moreover, there are few studies on the metabolism and toxicity mechanism of tellurene materials in organisms,43 which means there are still many obstacles that need to be overcome. In order to further promote the application of Te nanomaterials in the biomedical field and accelerate their clinical application, this review summarizes the synthetic methods of Te nanomaterials and the mechanism and application of Te nanomaterials in tumor phototherapy, as shown in Fig. 1. In light of the existing problems in application research, this review discusses different perspectives on Te nanomaterials in tumor treatment. Te nanomaterials have great application potential and require further research and exploration.
2. Synthesis of Te nanomaterials
2.1 Structure of Te nanomaterials
Te has many attractive properties, such as photoconductivity, thermoelectricity, and piezoelectricity,44,45 which makes it an attractive candidate for applications in sensors, optoelectronics, and energy equipment.46 As a representative element of the VIA family, Te is a material with a chain structure and anisotropic characteristics. Te atoms in the chain are connected by strong covalent bonds, as shown in Fig. 2a, while these chains are connected by van der Waals forces, which makes them assume a triangular helix and stack into a hexagonal array,47 as shown in Fig. 2b. A special chiral chain structure can be observed in tellurene. From Fig. 2c, Te atoms surround an axis parallel to the [0001] direction at the center and corner of the hexagonal basic unit.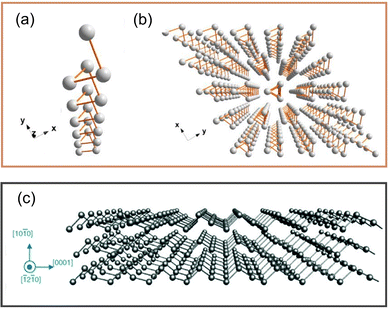 | ||
| Fig. 2 (a) Structure of single-molecule Te chain.47 (b) Crystal structure of Te.47 Copyright 2018, American Chemical Society. (c) Structure of tellurene.17 Copyright 2019, Royal Society of Chemistry. | ||
For nanomaterials, size and shape are two vital factors that affect their properties.48,49 Te has a strong tendency to grow as 1D nanostructures because of its chain structure.27 Therefore, there are numerous synthesis methods to manufacture 1D Te nanoparticles in various forms. However, studies on the synthesis of 2D tellurene nanomaterials are relatively rare. Therefore, exploring the reliable production methods of 2D tellurene materials is of great significance to widen their application in more fields.50 Taking the above-mentioned points into account, this section highlights the synthesis methods of zero-dimensional and 1D Te nanoparticles and mainly summarizes the synthesis methods of 2D Te nanomaterials.
2.2 Synthesis of zero-dimensional Te nanomaterials
Quantum dots are one of the leaders in the nano field,51 and they are usually considered zero-dimensional nanomaterials because of their ultra-small size of 1–10 nm;52–54 we address the synthesis methods of Te nanodots at this scale based on the size of existing quantum dots. Many works have successfully synthesized zero-dimensional Te nanodots with small sizes. Yang et al.55 used NaBH4 to reduce Na2TeO3 to produce Te nanodots, which were stabilized with albumin nanocages to yield diameters of 3.4 ± 0.6, 4.6 ± 0.6, and 5.9 ± 0.5 nm by controlling different reaction times, as shown in Fig. 3a–c; they successfully realized size control, which was critical for controlling the properties of the Te nanodots. He et al.56 synthesized Te nanoparticles at room temperature by using oleic acid to oxidize sodium telluride. In this way, an environmentally friendly synthetic strategy was developed for Te nanodots with a diameter centered at 1.5 ± 0.5 nm. The obtained Te nanodots could be evenly distributed as thin films via electrophoresis, which was also of great significance for the synthesis of 2D nanosheets.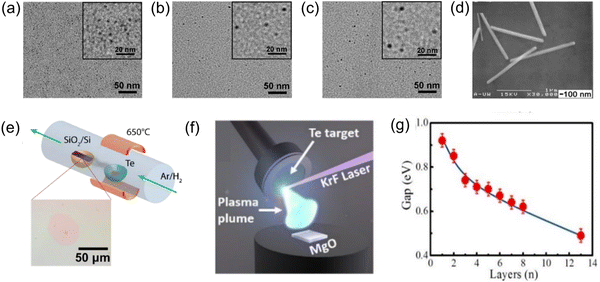 | ||
| Fig. 3 TEM images of Te nanodots with diameters of (a) 3.4 ± 0.6 nm, (b) 4.6 ± 0.6 nm, and (c) 5.9 ± 0.5 nm.55 The insets show the corresponding images at a higher magnification. Copyright 2017, American Chemical Society. (d) HR-TEM images of Te nanowires.34 Copyright 2002, Royal Society of Chemistry. (e) Experimental device diagram of ultrathin Te film prepared by PVD technology.66 (f) Schematic diagram of the synthesis of tellurene by PLD.66 Copyright 2019, IOP Publishing. (g) Relationship between the band gap and the thickness of Te films.75 Copyright 2017, American Chemical Society. | ||
2.3 Synthesis of 1D Te nanomaterials
Considering the chain structure of Te, its 1D form is easy to synthesize. Te can form nanowires with helical atomic chains, and its current density exceeds that of most semiconductor nanowires.57 Similar to the widely explored carbon nanotubes,58 except for the third dimension, the other two dimensions remain between 0.1–100 nm, and nanomaterials58 with a high aspect ratio are considered to have a 1D nanostructure,59 such as nanowires, nanorods, and nanotubes. The nanowire structure is stable and easy to modify. The hollow structure inside nanotubes endows them with a higher loading efficiency. The synthesis methods of 1D Te nanomaterials have been explored and widely used. At present, many works have realized the synthesis of 1D Te nanostructures, mainly including solid-phase deposition and solution reaction synthesis.Solid-phase synthesis has been reported in many works. Geng et al.60 prepared 1D Te nanobelts with a length of 50–300 nm and a thickness of 10–20 nm by a simple chemical reaction deposition process of Al2Te3 powder and water. In order to realize the controllable growth of 1D Te nanowires, Safdar and colleagues61 systematically explored the fine structure of Te nanowires, synthesized ordered 1D Te nanostructures by vapor deposition, and proposed more possibilities for their new applications. However, impurities are easy to be mixed in the solid-phase deposition process, and it is still quite difficult to achieve high purity. Thus, solution synthesis provides a better idea.
The cost of solution synthesis is low, and the reaction conditions are relatively not harsh, which ensures the potential of large-scale synthesis. Mayers and Xia33,34 made a great effort towards the solution synthesis of 1D Te nanomaterials. In their work, Te nanorods with an average length of 1.80 ± 0.16 μm and a width of 98 ± 3 nm were realized (Fig. 3d), and they were able to synthesize nanoneedles, nanotubes, and nanorods with controllable lengths and controllable, uniform interfaces. It is worth noting that telluric acid needed to be decomposed into tellurium dioxide below 150 °C; otherwise, when the temperature was too high, tellurium dioxide would be directly reduced to Te nanorods. Moreover, the obtained samples were not physically degraded after more than one year in the dark. Wen et al.32 synthesized Te nanodot copolymers by the micro-emulsion method and controlled the photothermal and photodynamic effects by adjusting the ratio of pyrrole to tellurophene to achieve an excellent therapeutic effect. Prangya Bhol et al.62 also prepared Te nanowires as an excellent electrode material by solution reaction. In the work of Kang et al.,63 Te nanorods were grown in an ethylene glycol solvent system, and then some Te was replaced via a galvanic reaction to prepare spotted Ru–Te hollow nanorods with better stability, which improved the loading efficiency and realized combined phototherapy. According to the above work, the relatively abundant research on 1D Te nanostructures also lays a good foundation for their subsequent applications.
2.4 Synthesis of 2D tellurene nanomaterials
Research on 2D tellurene nanomaterials is relatively scarce, and their controllable synthesis plays an important role in promoting their application. At present, the existing synthesis methods mainly include physical vapor deposition (PVD),64–66 liquid-phase exfoliation,67 and solvothermal synthesis.35,47Apte et al.66 demonstrated two methods for the growth of ultrathin Te films by PVD. The first one was to use vacuum evaporation coating, where bulk Te was evaporated at 650 °C in an Ar/H2 atmosphere and then deposited on a Si/SiO2 substrate and cooled to obtain an ultrathin Te film, which had a typical thickness of 0.85 ± 0.1 nm, as shown in Fig. 3e. The second method utilized PLD technology. Apte et al.66 made use of PLD technology to obtain continuous Te films with a thickness of 2.7–6 nm on a 1 cm × 1 cm single-layer MgO substrate (Fig. 3f). The growth of Te film could be controlled by pulse parameters. In MBE technology,72 the vapor obtained by heating in an ultrahigh vacuum is directly sprayed onto the substrate, so that the atoms are arranged on the substrate according to the crystal to form a thin film. The use of MBE in the synthesis of tellurene has also been confirmed. Chen et al.45 used MBE to grow Te film on highly oriented pyrolytic graphite.
As a promotion, van der Waals epitaxy (vdWE) combines the substrate and film through weak van der Waals forces, which makes the material fall off more easily and provides a simpler synthesis technology.73,74 Wang et al.64 used vdWE to grow 2D Te nanosheets on inert mica substrates. After that, Huang et al.75 prepared tellurene on the surface of graphene grown on a 6H–SiC substrate and concluded that the band gap was related to the thickness; that is to say, the band gap decreased monotonically with an increase in Te film thickness, as shown in Fig. 3g. It is worth mentioning that the adjustable band gap (0.49–0.92 eV) covered the mid to NIR spectral range. This was similar to the interaction between MoS2 and graphene65 and will lead to the accumulation of local holes, which means that the electronic and optical properties of 2D Te films can be adjusted, a fact that is worthy of our in-depth exploration.
We summarized the above works to show that PVD experiences no chemical reactions in the stacking process and to demonstrate its advantages of less consumption of substrate, uniform and fine film formation, and fast speed.70,71 Therefore, PVD has strong potential for the preparation of 2D tellurene nanomaterials for biomedical applications, especially uniform and fine Te films expected for tumor treatment.
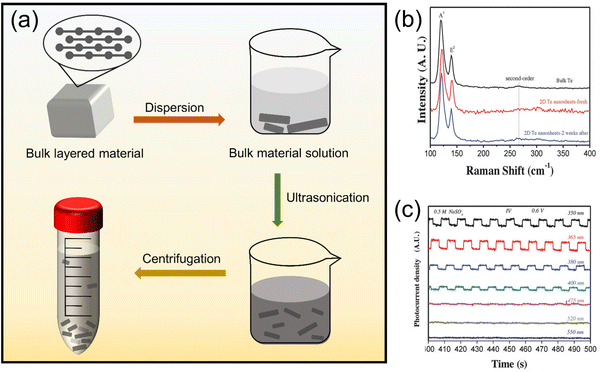 | ||
| Fig. 4 (a) Schematic of ultrasonic exfoliation to obtain nano-layered materials. (b) and (c) show the photocurrent behavior of 2D Te nanosheets under light at wavelengths of 350, 365, 380, 400, 475, 520, and 550 nm.67 Copyright 2018, Wiley-VCH. | ||
Liu et al.78 obtained large-area 2D tellurene nanosheets in ethanol solvent after ultrasonication and centrifugation. With continuous efforts, Zhang's team67 prepared 2D layered Te nanostructures with a width of 41.5–177.5 nm and a thickness of 5.1–6.4 nm by LPE. In addition, they also found that 2D Te nanosheets showed excellent optical response under light at wavelengths of 350, 365, 380, and 400 nm, as seen in Fig. 4b and c, which inspires the application of tumor phototherapy in organisms. The use of 2D Te nanosheets prepared by LPE for PDT is reflected in the work of Lin et al.,23 who sonicated raw Te powder in a dimethylformamide bath to break the bonds of large-size Te layers, and a peeled tellurene nanosheet was obtained.
Compared with the strict requirements of PVD, LPE has the advantages of low cost, simple operation, and low environmental impact,79 and the latter has the potential to realize mass production. N-Methylpyrrolidinone (NMP) has been proven to be one of the successful solvents in LPE technology.80 Manna et al.79 used a water–NMP mixed solvent system to significantly increase the stripping yield and stabilize the synthesized nanosheets over a long time. Their exploration inspires us to develop a solvent system with good biocompatibility to overcome the obstacles of 2D tellurene applications in the biomedical field, which also opens up a broader scientific perspective for the preparation of nanosheets in more specific biomedical fields. However, controlling the size uniformity of nanomaterials by LPE is still a choke point that limits its application to a certain extent,81 so further exploration and breakthroughs are needed to solve the existing problems.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the average size of tellurene was the smallest, and the width decreased monotonously with a decrease in PVP. This method was also employed in the work of Du et al.82 Amani et al.47 also obtained Te nanosheets through this method, and their thickness was characterized by optical microscopy, as seen in Fig. 5d.
1, the average size of tellurene was the smallest, and the width decreased monotonously with a decrease in PVP. This method was also employed in the work of Du et al.82 Amani et al.47 also obtained Te nanosheets through this method, and their thickness was characterized by optical microscopy, as seen in Fig. 5d.
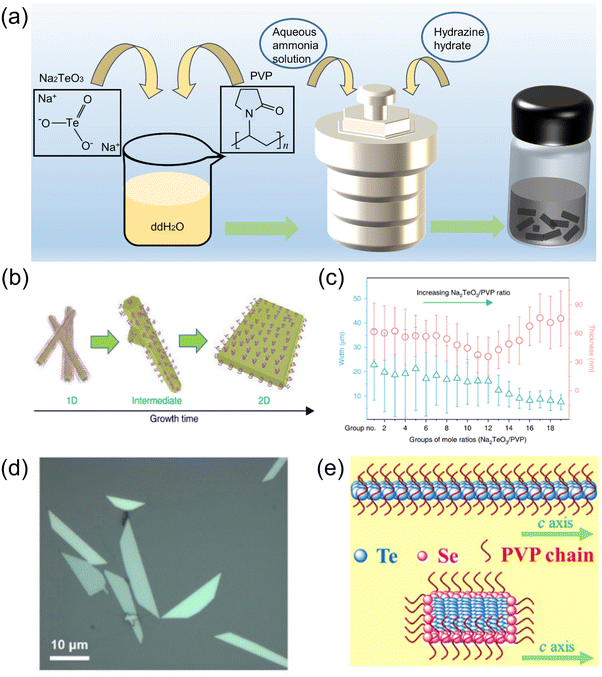 | ||
| Fig. 5 (a) Schematic diagram of the solvothermal reaction for the synthesis of 2D tellurene. (b) Changes in growth morphology from a 1D Te structure to a 2D Te structure.35 (c) The growth of 2D tellurene width and thickness for different groups, where each group has a different ratio of Na2TeO3/PVP.35 Copyright 2018, Springer Nature. (d) Optical image of 2D Te nanosheets.47 Copyright 2018, American Chemical Society. (e) Structure of TeSe nano heterojunction.83 Copyright 2020, American Association for the Advancement of Science. | ||
Inspired by the solvothermal synthesis of 2D tellurene, Chen et al.83 prepared selenium-coated Te nano-heterojunctions with a Te phase as the “core” and a Se phase as the “shell.” The presence of Se causes the Te nanowires to turn into 2D nanosheets (Fig. 5e), with a similar layer structure to tellurene. The solvothermal synthesis method is relatively flexible, and the size and structure of the material can be controlled by the reaction conditions. In addition, some works on the preparation of other 2D nanomaterials provide other possibilities for the synthesis of 2D tellurene, which require further verification. For example, a bismuthene nano-heterojunction was prepared by solvothermal reaction,84 and antimony was prepared by ultrasonication,85 which inspired us to think about the preparation of 2D tellurene with a similar structure.
The advantages and disadvantages of these synthesis methods described above are summarized in Table 1. PVD can control the area and thickness of nanosheets through the size of the substrate and the deposition time; the tellurene prepared by this method is of high quality and high purity, and it is likely to be used in fields with precision requirements, but it requires harsh environment. LPE peels large layered materials to yield nanoflakes through ultrasonication or mechanical shear, when the energy of ultrasonic crushing is large, it will be more conducive to the separation of materials. This method is relatively insensitive to the environment, simple, easy to implement, and universal. In the solvothermal strategy, different precursors and the proportion of each component in the reaction are the key factors that control the synthesis size of nanomaterials, it is also possible to synthesize materials with various morphologies and properties, so there are many possibilities for upgrading.
| Advantage | Disadvantage | |
|---|---|---|
| PVD | • Thin, uniform and continuous films | • Harsh environment usually under ultra-high vacuum |
| • High accuracy | • The size and thickness of the film can be adjusted by changing the deposition conditions | |
| • Fast speed | ||
| • Strong condition controllability | ||
| LPE | • Simple operation and easy implementation | • Difficult to control the size uniformity of materials |
| • Low cost | • Difficult to separate | |
| • Insensitive to environmental conditions | ||
| • Suitable for mass production | ||
| Solvothermal | • High stability | • Slow method |
| • High flexibility |
3. Application and breakthrough of Te nanomaterials in tumor phototherapy
Currently, traditional cancer treatment methods such as surgery, radiotherapy, and chemotherapy are still widely used in clinics, which have limited treatment efficacy and serious side effects.86–88 Therefore, it is necessary to develop a targeted, effective, and efficient treatment method to eliminate cancer cells while causing negligible damage to normal tissues. In recent years, phototherapies such as PTT and PDT have attracted significant attention because of their advantages of fewer traumas, lower toxicity, and better selectivity.8,89 Phototherapy is a treatment method that gathers phototherapeutic reagents at the tumor tissue and irradiates the target area with a light source, especially an NIR light source.In phototherapy, in addition to the excitation light, the conversion ability of a photosensitizer under radiation is also a pivotal factor for effectiveness, like common inorganic and organic materials90 such as zinc phthalocyanine, chlorin e6, indocyanine green, graphene quantum dots, gold nanorods,13 and copper sulfide nanosystems.91 At present, there are many gaps in Te nanomaterials; for example, the controllable synthesis of 2D tellurene and the potential toxicity of Te nanomaterials. Generally, Te nanomaterials have many applications because of their good electrical conductivity. Especially, the thin layer structure gives 2D tellurene a high specific surface area and high drug loading. Te nanomaterials can also produce heat or cytotoxic reactive oxygen species (ROS) under laser irradiation,23,92 indicating that they have great potential applications in tumor phototherapy.
3.1 Properties of Te nanomaterials
Te is a p-type semiconductor material93 with an unusual anisotropic crystal structure and a high melting point of ∼450 °C;64,94 under certain radiation conditions, it will produce active electrons and vacancies, react with the surrounding environment, and cause oxidative stress,95 which is very similar to the death caused by intracellular ROS during PDT. At the same time, 2D tellurene has great advantages in loading other substances in the application of active catalysis and drug-loaded therapy. The traditional drug delivery platform based on nanoparticles usually only has a 10–30% (w/w%) drug loading capacity,96 while the high surface area of tellurene will produce strong surface interactions with therapeutic or diagnostic molecules, and the loading rate of chemotherapy drugs even reaches 162%.97 Therefore, Te materials have great potential in PDT98 and broad application prospects in the biomedical field.3.2 Application and mechanism of Te nanomaterials in tumor phototherapy
Te-nanomaterial-mediated anticancer phototherapy mainly refers to PTT, PDT, photo-triggered molecule delivery, and their synergetic strategies. Compared with ultraviolet-visible (UV-Vis) light, NIR light used in phototherapy has a larger penetration depth and is a better choice for superficial and deep malignant tissues.89As a thickness-dependent bandgap (0.35–1.2 eV) semiconductor nanomaterial,101,102 Te will generate and relax electron–hole pairs103 as a narrow bandgap semiconductors such as CuS and MoS2,104,105 as shown in Fig. 6a. Therefore, Te nanomaterials can generate a rapid response to NIR lasers,28–30 which helps to achieve deeper tissue penetration. Wu et al.37 explored the strong light absorption (Fig. 6b) of a few layers of Te. In addition, similar to Mxenes, the layered structure of tellurene causes light waves to reflect between layers and greatly improves the absorption of light energy.106 Studies have shown that the photothermal conversion efficiency (η) of Te nanosheets is calculated to be 55% under an 808 nm laser,97 which is significantly higher than bismuthene (19.4%)107 and traditional graphene oxide (22%)108 at the same wavelength. These studies have confirmed that Te nanomaterials have a good light absorption effect and high photothermal conversion performance under NIR, which is undoubtedly very attractive in the application of PTT.28,109,110
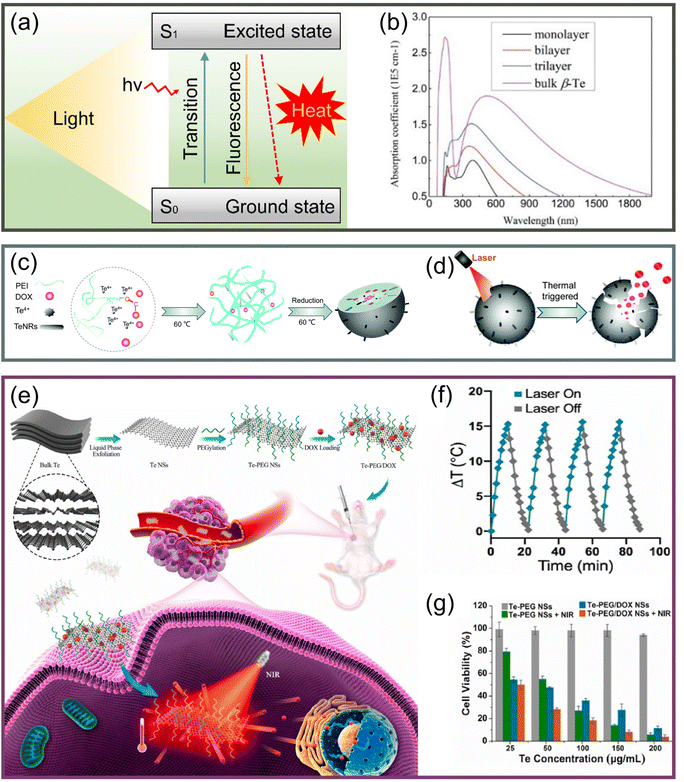 | ||
| Fig. 6 (a) Schematic of the generation and relaxation of electron–hole pairs due to the absorption of photon energy by nanoparticles and the realization of the photothermal effect. (b) Strong light absorption of Te from UV to visible.37 Copyright 2017, IOP Publishing. (c) Schematic diagram of DOX/PEI@TeNPs formation process.112 (d) Light-controlled release mechanism of DOX/PEI@TeNPs.112 Copyright 2021, Royal Society of Chemistry. (e) Schematic diagram of synthesis and cancer therapy of Te–PEG-NSs.97 (f) Stability of Te–PEG-NSs in the heating–cooling cycle process.97 (g) Relative survival rate of 4T1 cells incubated for 24 hours with different treatments.97 Copyright 2021, Elsevier. | ||
The mushroom polysaccharide–protein complex modified Te nanorods prepared by Huang et al.111 realized high stability in the physiological environment, high selectivity to tumor cells, and efficient tumor ablation. In addition, a high-yield synthesis was achieved by a simple redox method, which was a great impetus to the subsequent development of multifunctional nanosystems. Duan et al.112 developed a functionalized Te nanosystem (DOX/PEI@TeNPs) with good photothermal effectiveness in the NIR region, as shown in Fig. 6c. The DOX/PEI@TeNPs arrived directly at the tumor tissue, and then the anticancer drug doxorubicin hydrochloride (DOX) was released under light control (Fig. 6d), which greatly reduced the damage to normal tissue. This study provided an effective strategy for the design of nanodrug systems and realized precise chemotherapy/PTT synergistic tumor treatment.
Some studies have also explored 2D tellurene. Zhang's team prepared a TeSe nano-heterojunction similar to tellurene,83 finding it to produce an extremely high photothermal effect and provide efficient cancer treatment. Then, Pan et al.97 prepared and characterized tellurene; modifying with polyethylene glycol (PEG), they obtained Te–PEG-NSs with an average size of approximately 90 nm by using LPE and realizing the application of PTT/chemotherapy, as shown in Fig. 6e. Under the irradiation of an 808 nm laser, the Te–PEG-NSs showed good photothermal stability (Fig. 6f) and achieved a conversion efficiency of 55%, which was significantly higher than some other forms of Te nanomaterials. The significant death of 4T1 cells shown in Fig. 6g and the obvious inhibition of tumor volume both validated the synergistic therapeutic effect of PTT and chemotherapy, which confirmed the superiority of Te nanomaterials as phototherapeutic agents.
The relatively simple synthesis method and adjustable size of Te nanomaterials are very important for the design of nano drug-delivery platforms. Zhang's team found that Te nanoneedles could induce mitochondrial dysfunction and enhance the antioxidant activity of scavenging free radicals with an NIR laser,113 which provided a new breakthrough for Te nanomaterials in the field of cancer phototherapy.
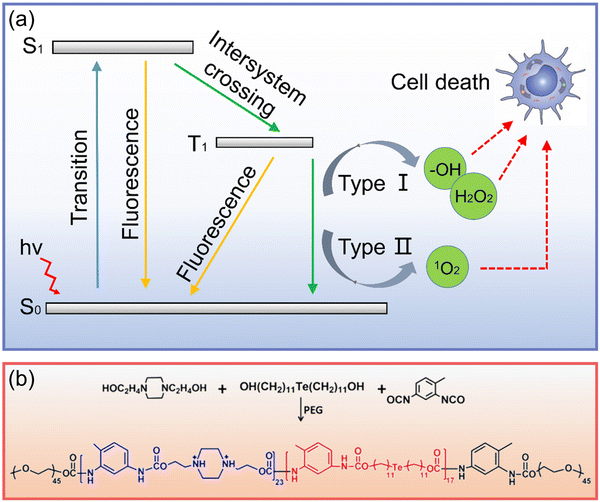 | ||
| Fig. 7 (a) Schematic diagram of PDT anticancer process (S0: ground state, S1: singlet excited state, T1: excited triplet state). (b) Synthesis of Te-containing polymer.119 Copyright 2016, American Chemical Society. | ||
Xu et al.120 realized the PDT of organic phosphorescent materials by exploring the bonding between π-conjugated scaffolds and chalcogenide elements, providing a new platform for the next generation of efficient photosensitizers. In terms of Te nanomaterials, Te nanorods synthesized by Kang et al.63 showed excellent nano enzyme activity, “catalyzed the production of oxygen from hydrogen dioxide, and alleviated the hypoxia condition of tumor tissue, which greatly improved the effect of phototherapy”. Fan et al.119 assembled a multilayer structure comprising a Te-containing polymer (Fig. 7b), indocyanine green, and poly(styrenesulfonate) layer-by-layer. Under laser irradiation, the generated ROS could damage cancer cells and oxidize Te to generate the Te![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group to achieve the controlled release, which provided an interesting insight into Te nanomaterials in phototherapy.
O group to achieve the controlled release, which provided an interesting insight into Te nanomaterials in phototherapy.
Lin and colleagues further verified the application of 2D tellurene materials in PDT.23 They synthesized Te nanosheets through LPE and functionalized them with glutathione (GSH) to improve their stability and biocompatibility (Fig. 8a). Fig. 8b shows the strong light absorption of the Te nanosheets@GSH. The change of the trilinear signal in Fig. 8c confirmed the ability of the Te nanosheets@GSH to produce 1O2. Then, dichlorofluorescin-diacetate (DCFH-DA) was used to observe the production of ROS. DCFH-DA is non-fluorescent, but it can be oxidized by ROS and show green fluorescence.121 The cells incubated with Te nanosheets@GSH had almost no fluorescence, while, after 670 nm light irradiation, they exhibited strong green fluorescence, as shown in Fig. 8d. Further, from Fig. 8e, the absorption peak intensity of 1,3-diphenylisobenzofuran (DPBF)122 at 410 nm gradually decreased with an increase in illumination time, which also confirmed that the Te nanosheets@GSH produced ROS under light. In vitro experiments, as shown in Fig. 8g, showed that the cell survival rate did not change significantly under dark conditions, while it decreased with an increase in Te nanosheets@GSH concentration under light, which proved that the production of ROS was sufficient to kill cancer cells. The tumor growth of mice was observed after different treatment methods (Fig. 8f and h). The tumor growth of mice treated with Te nanosheets@GSH and light was significantly inhibited, and the tumor tissue was seriously damaged, as shown in Fig. 8i. Both in vivo and in vitro results showed that 2D tellurene can be used as an attractive photosensitizer for PDT and has broad prospects in biomedical applications.
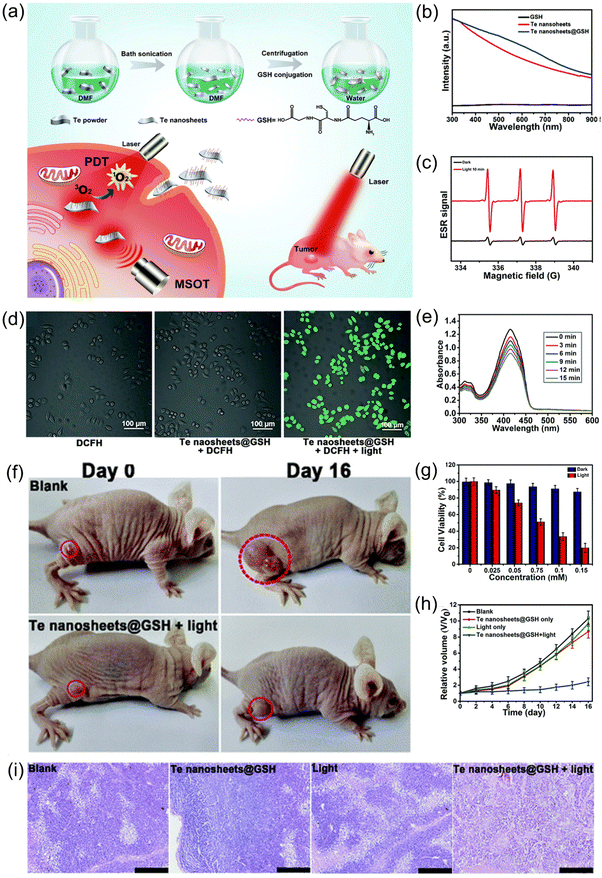 | ||
| Fig. 8 (a) Schematic diagram of preparation process of Te nanosheets@GSH and their potential applications.23 (b) UV-Vis absorption spectra of Te nanosheets@GSH, Te nanosheets, and GSH.23 (c) ESR signal diagram of Te nanosheets in the presence of TEM under light and dark conditions.23 (d) Merged confocal fluorescence microscopy images of HeLa cells incubated with DCFH, Te nanosheets@GSH + DCFH, and Te nanosheets@GSH + DCFH + light.23 (Scale bar = 100 μm) (e) UV-Vis absorption spectra of DPBF probe molecules with increasing radiation time.23 (f) Tumor growth in mice after treatment with a blank and Te nanosheets@GSH + light at Day 0 and Day 16.23 (g) Cell viability at different concentrations of Te nanosheets@GSH under dark and light conditions.23 (h) Changes in tumor volume in four groups under different treatment conditions.23 (i) H&E staining histological images of tumor sections after four groups of different treatment conditions.23 (Scale bar = 200 μm) (Laser wavelength = 670 nm, 160 mW cm−2) Copyright 2018, Royal Society of Chemistry. | ||
A 2D Bi/BiOX lateral nano-heterostructure with similar structure to tellurene was shown to exhibit effective tumor ablation caused by ROS at 660 nm,123 and antimonene realized PDT under 660 nm and PTT under 808 nm,124 Jiang et al.63 found that Te nanomaterials exhibit photodynamic effects at 473, 660, and 808 nm, which lays a good foundation of Te nanomaterials to be used for PDT in NIR region to achieve deeper penetration depths.
As an attractive candidate, Te nanomaterials can not only produce PTT through the generation and relaxation of electron–hole pairs but also trigger the generation of ROS through the reaction between excitons and water/oxygen to realize PTT/PDT synergetic therapy.130–132 It has been reported that Te nanomaterials have great potential in cancer synergetic therapy.55,92,121 Guo et al.121 loaded the chemotherapeutic drug cantharidin and Te nanoparticles into the 4T1 cell membrane with a homologous targeting ability to develop cancer-cell-camouflaged nano therapeutic agent m-CTD@Te (Fig. 9a). As shown in Fig. 9b, compared with bare Te, the Te nanomaterials coated with cell membrane showed higher cell uptake, which had decisive significance for the realization of targeted anticancer therapy; after 808 nm laser treatment, the generation of ROS was verified, as seen in Fig. 9c. The infrared thermal imaging photographs in Fig. 9d clearly show the higher photothermal effect of the tumor area than the normal area, confirming the targeting ability of m-CTD@Te in vivo. At the same time, the significant difference in tumor volume (Fig. 9e) also proved that m-CTD@Te realized high-efficiency PDT/PTT anticancer effects through the single light source.
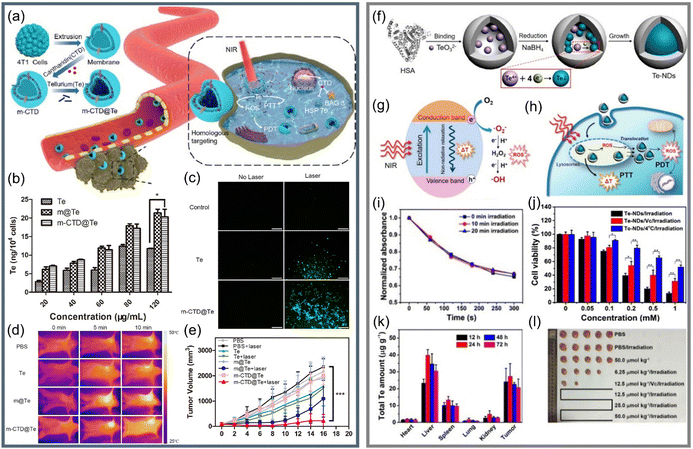 | ||
| Fig. 9 (a) Schematic diagram of preparation of m-CTD@Te and synergetic anticancer applications.121 (b) Content of Te in 4T1 cells after treatment with different groups by Te, m@Te, and m-CTD@Te.121 (c) Exploring of the ability of Te and m-CTD@Te to generate ROS through DCFH-DA.121 (d) Real-time thermography of mice after intravenous injection of Te, m@Te, m-CTD@Te, and PBS.121 (e) Change curve of tumor volume during 16 days of treatment (Laser wavelength = 808 nm, 1.0 W cm−2).121 Copyright 2020, Elsevier. (f) Schematic diagram of bifunctional Te-NDs synthesis with HSA as a nano reactor.55 (g) and (h) show the photothermal effect and ROS generation of Te-NDs to realize PTT/PDT synergistic treatment, respectively.55 (i) Relationship between the standard absorption of DPBF and Te-NDs irradiation time for 0 min, 10 min, and 20 min.55 (j) A chart comparing the cell survival rate of the three groups.55 (k) The total Te content of several main tissues after treated by Te-NDs for different periods of time.55 (l) Pictures of mice tumors after treated for 30 days (Laser wavelength = 785 nm, 1.5 W cm−2).55 Copyright 2017, American Chemical Society. | ||
Yang et al.55 used human serum albumin (HSA) as a nano reactor to synthesize bifunctional Te nanodots (Te-NDs) to realize PTT/PDT synergetic therapy under the irradiation of a single 785 nm light, as shown in Fig. 9f–h. Apart from the known photothermal effect, DPBF was used to capture ROS, and the Te-NDs still maintained the ability to produce ROS even after 10 and 20 min of irradiation (Fig. 9i). In order to verify the synergetic effect, they used a scavenger of ROS, vitamin C, to inhibit the photodynamic effect, and the environment at 4 °C inhibited the photothermal effect; the comparison of cell survival rates of the three groups in Fig. 9j proves the synergetic PTT/PDT effect. Due to the EPR effect and the tumor accumulation capacity of albumin,133,134 the Te-NDs would mainly distribute in tumor and liver areas (Fig. 9k), and the significant tumor ablation in Fig. 9l proves phototherapeutic ability of Te nanomaterials.
Under extensive laser irradiation, because Ru can catalyze peroxide to produce oxygen to improve PDT efficiency, spotted Ru–Te hollow nanorods were able to achieve high-efficiency synergetic PTT/PDT for anoxic pancreatic cancer,63 which provided a reference for the fusion of Te nanomaterials and other materials to realize multifunctional anticancer modes. Li et al.135 prepared Te nanorods using peptides as nano reactors to achieve efficient PTT/sonodynamic therapy. The presence of peptides and HSA ensured that the Te nanomaterials had high dispersion and stability, avoiding complex modification. Huang et al.136 explored a combination of triangular nanocrystals in radiotherapy and immunotherapy, which provided a new idea for phototherapy combined with immunotherapy via Te nanomaterials.
3.3 Breakthrough of Te nanomaterials in cancer treatment
As a chiral-chain-structure semiconductor material, Te has the outstanding advantage of a high light absorption and can realize PTT and PDT simultaneously under the excitation of a single light source, which greatly inspires and promotes the realization of efficient and synergistic cancer treatment.Unfortunately, the biggest threat of Te nanomaterials to organisms is that we have a limited understanding of their metabolism, transformation and possible toxicity in vivo.137 In addition, Te has poor compatibility in organisms and may have potential toxicity to the human body. How to improve the biocompatibility and reduce the biological toxicity of Te nanomaterials are questions that must be solved urgently. Here, based on previous studies, we propose the surface modification and size control of Te nanomaterials to affect their metabolic behavior in organisms with the hope to achieve a breakthrough of Te nanomaterials in cancer treatment through more research.
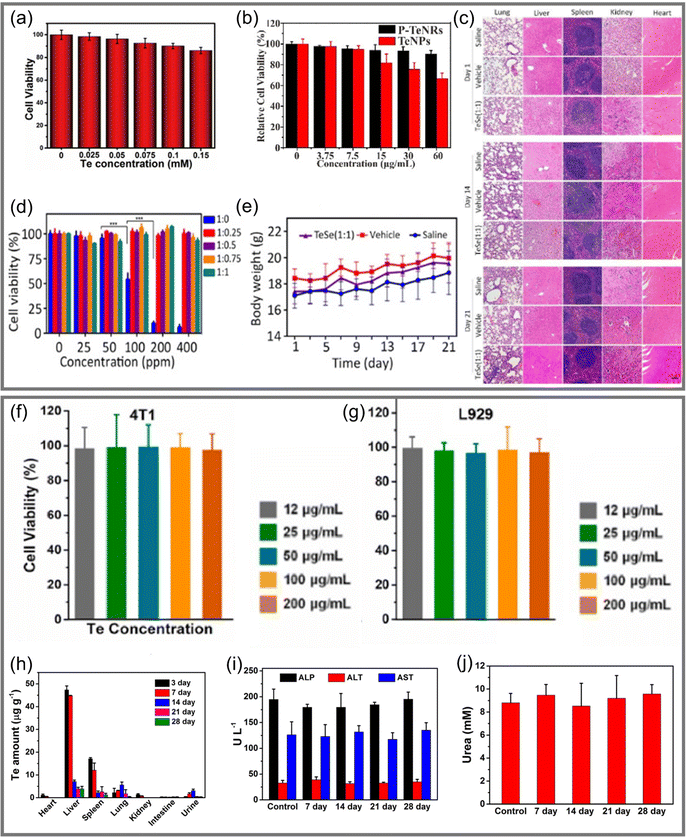 | ||
Fig. 10 (a) Relative viability of HeLa cells after incubated by Te nanosheets@GSH for 24 hours.23 Copyright 2018, Royal Society of Chemistry. (b) Relative viability of 4T1 cells after incubated with Te nanomaterials.135 Copyright 2021, Elsevier. (c) H&E slice image of different tissues treated by several groups at days 1, 14, and 21.83 (Scale bar = 100 μm.) (d) Survival rate of SMMC-7721 cancer cells treated with nanomaterials under different Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratios.83 (e) Body weight changes of mice treated in different groups from day 1 to day 21, with 5 mice in each group.83 Copyright 2020, American Association for the Advancement of Science. (f) and (g) are the relative viability of 4T1 cells and L929 after incubated with ultrathin Te nanosheets for 24 hours, respectively.97 Copyright 2021, Elsevier. (h) Distribution of Te in various mouse tissues that were treated with 50.0 μmol kg−1 Te over 28 days.55 (i) Levels of ALP, ALT, and AST in blood of mice in a control group and mice injected with 50.0 μmol kg−1 Te at different times.55 (j) Level of urea in blood of mice in a control group and mice injected with 50.0 μmol kg−1 Te at different times.55 Copyright 2017, American Chemical Society. Se ratios.83 (e) Body weight changes of mice treated in different groups from day 1 to day 21, with 5 mice in each group.83 Copyright 2020, American Association for the Advancement of Science. (f) and (g) are the relative viability of 4T1 cells and L929 after incubated with ultrathin Te nanosheets for 24 hours, respectively.97 Copyright 2021, Elsevier. (h) Distribution of Te in various mouse tissues that were treated with 50.0 μmol kg−1 Te over 28 days.55 (i) Levels of ALP, ALT, and AST in blood of mice in a control group and mice injected with 50.0 μmol kg−1 Te at different times.55 (j) Level of urea in blood of mice in a control group and mice injected with 50.0 μmol kg−1 Te at different times.55 Copyright 2017, American Chemical Society. | ||
Chen et al.83 developed a TeSe nano-heterojunction structure (Fig. 5e) comprising a Se coating covering a Te nanosheet. Mixing with SMMC-7721 cells, it was observed that the cell survival rate under pure Te decreased sharply at a concentration of 100 ppm, while the Te nanomaterials encapsulated by Se were non-toxic at a concentration of 400 ppm, as shown in Fig. 10d. The consistency of tissue structures in organ-slice images in Fig. 10c and no significant changes in body weight, as shown in Fig. 10e, demonstrated that Te nanomaterials coated with Se had better biocompatibility. A similar work with Ru–Te hollow nanorods also proved the feasibility of this program.63
Therefore, it is safe to conclude that reasonable modification of Te nanomaterials is a breakthrough idea to reduce their toxicity, improve their biocompatibility, and ensure their therapeutic effect, which is significant for their application in biomedical field. In addition, ligands with a targeting effect (such as towards 4T1 cell membranes121) can be employed to improve the specific recognition ability of materials, so as to improve their targeting ability in tumor therapy.
Ultrathin Te nanosheets prepared by Pan et al.97 could still maintain a cellular survival rate of 90% after incubation with cancer cells (4T1) and normal cells (L929 fibroblast cells) for 24 hours, as shown in Fig. 10f and g, indicating that they had very low biological toxicity. Te NDs synthesized by Yang et al.55 had an ultra-small size (<10 nm), which was conducive to renal excretion.20,142 In order to explore the excretion and clearance behavior of Te-NDs in vivo, the elimination ability was evaluated by monitoring the distribution of Te in heart, liver, spleen, lung, and kidney over 28 days, as shown in Fig. 10h. It was clearly observed that after the third day, the content of Te in each tissue decreased dramatically. In addition, the markers of liver and kidney function (ALP/ALT/AST and urea) had no significant difference compared with the original levels after being injected for 28 days, as shown in Fig. 10i and j. It was confirmed that Te-NDs had no obvious toxicity to normal tissues, and that their ultra-small size possibly led them to be excreted from normal tissues through the kidney. This work provided a breakthrough idea for the study of long-term retention and toxicity of Te nanomaterials in vivo and significantly enlightens the study of inorganic phototherapeutic nanomaterials, which has great significance for their clinical application.
4. Summary and prospective
This review summarizes the synthesis methods, properties, and mechanism of Te nanomaterials, as well as their applications in cancer phototherapy, and it puts forward some breakthroughs in the direction of cancer phototherapy with regards to possible biocompatibility and potential biological toxicity. We hope to fully utilize the advantageously high light absorption and phototherapeutic potential of Te nanomaterials, so as to promote their application in biomedicine.At present, studies have shown the application of Te nanomaterials in the biological field, as well as their fascinating characteristics, such as high carrier mobility, strong light absorption, and PTT/PDT synergetic therapy excited by a single light source, making them outstanding in tumor phototherapy. Especially, tellurene can control/accelerate the release of drugs from the delivery platform due to the NIR photothermal effect, owing to the unique ultra-high surface area to volume ratio and ultra-thin 2D nanosheet structure. These prominent merits of Te nanomaterials make them very likely to realize the synergistic treatment of tumor radio/chemo/phototherapy, indicating their great potential as effective anticancer agents.
However, there has been little research on Te nanomaterials in the biomedical field, which indicates many gaps and great challenges; the harsh conditions required by PVD, such as the high vacuum environment, and the difficulty in size-control of LPE are factors that limit their development. Therefore, much exploration is still needed to discover simple and efficient methods to synthesize Te nanomaterials with uniform size and large scale. In addition, as we all know, the controversial problem of some nano biomaterials is the biocompatibility of the as-prepared materials. The production of Te nanomaterials usually involves the use of toxic reactants and organic solvents, which limits their application in the clinical field. In the process of material preparation, a solvent with good biocompatibility and high hydrophilicity can be used as the reaction base, which can greatly improve the biocompatibility of the final product. Moreover, surface modification and size control to improve biocompatibility, produce a small immune response, and reduce or even eliminate biological toxicity are the major breakthrough points of Te nanomaterials in cancer treatment.
In addition, the excellent performance of Te nanomaterials in phototherapy such as fast response to NIR laser and good photothermal performance makes it possible for more diseases. For example, in cardiovascular and cerebrovascular diseases, the warm photothermal effect through controlling the dose of Te nanomaterials can improve the water solubility of blood lipids and reduce blood viscosity, meanwhile, it can also soften blood vessels to accelerate blood circulation. What's more, the layered structure of tellurene has high drug loading capacity, and has promising approach in targeted drug delivery after reasonable modification. We expect that Te nanomaterials can maximize the therapeutic effect and minimize side effects. Although there have been some preliminary theoretical proofs and experimental studies on the application of Te nanomaterials in the biological field, the interaction between Te and organisms, metabolism in organisms, and short-term and long-term biological toxicity still call for in-depth exploration. Therefore, more effort must be exerted to realize the development and breakthrough of Te nanomaterials in the biomedical field. We maintain an optimistic point of view that there will be more opportunities for Te nanomaterials in the near future.
Abbreviations
| EPR | Enhanced permeation and retention |
| 2D | Two-dimensional |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| Te | Tellurium |
| NIR | Near-infrared |
| 1D | One-dimensional |
| PVD | Physical vapor deposition |
| MBE | Molecular beam epitaxy |
| PLD | Pulsed laser deposition |
| vdWE | van der Waals epitaxy |
| LPE | Liquid-phase exfoliation |
| NMP | N-Methylpyrrolidinone |
| PVP | Polyvinylpyrrolidone |
| ROS | Reactive oxygen species |
| UV | Ultraviolet light |
| DOX | Doxorubicin hydrochloride |
| PEG | Polyethylene glycol |
| 1O2 | Singlet oxygen |
| GSH | Glutathione |
| DCFH-DA | Dichlorofluorescin-diacetate |
| DPBF | 1,3-Diphenylisobenzofuran |
| HSA | Human serum albumin |
| Te-NDs | Te nanodots |
| BSA | Bovine serum albumin |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (Grant No. 52101287 and U1806219). The Special Funding also supports this work in the Project of the Qilu Young Scholar Program of Shandong University.References
- W. P. Fan, B. Yung, P. Huang and X. Y. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed.
- The Lancet, Lancet, 2018, 392, 985 Search PubMed.
- Z. Cheng, M. Y. Li, R. Dey and Y. H. Chen, J. Hematol. Oncol., 2021, 14, 27 CrossRef PubMed.
- X. Yi, K. Yang, C. Liang, X. Y. Zhong, P. Ning, G. S. Song, D. L. Wang, C. C. Ge, C. Y. Chen, Z. F. Chai and Z. Liu, Adv. Funct. Mater., 2015, 25, 4689–4699 CrossRef CAS.
- C. Early Breast Canc Trialists, Lancet, 2011, 378, 1707–1716 CrossRef.
- Z. J. Zhang, J. Wang and C. H. Chen, Adv. Mater., 2013, 25, 3869–3880 CrossRef CAS PubMed.
- P. Y. Chen, Y. C. Ma, Z. Zheng, C. F. Wu, Y. C. Wang and G. L. Liang, Nat. Commun., 2019, 10, 1192 CrossRef PubMed.
- Q. Wang, Y. N. Dai, J. Z. Xu, J. Cai, X. R. Niu, L. Zhang, R. F. Chen, Q. M. Shen, W. Huang and Q. L. Fan, Adv. Funct. Mater., 2019, 29, 12 Search PubMed.
- J. Nam, S. Son, L. J. Ochyl, R. Kuai, A. Schwendeman and J. J. Moon, Nat. Commun., 2018, 9, 13 CrossRef PubMed.
- S. L. Gai, G. X. Yang, P. P. Yang, F. He, J. Lin, D. Y. Jin and B. G. Xing, Nano Today, 2018, 19, 146–187 CrossRef CAS.
- D. M. Sun, X. Y. Zhuang, S. Q. Zhang, Z. B. Deng, W. Grizzle, D. Miller and H. G. Zhang, Adv. Drug Delivery Rev., 2013, 65, 342–347 CrossRef CAS PubMed.
- W. W. Qin, G. Huang, Z. G. Chen and Y. Q. Zhang, Front. Pharmacol., 2017, 8, 15 Search PubMed.
- A. Y. Lin, J. K. Young, A. V. Nixon and R. A. Drezek, Small, 2014, 10, 3246–3251 CrossRef CAS PubMed.
- N. Karousis, I. Suarez-Martinez, C. P. Ewels and N. Tagmatarchis, Chem. Rev., 2016, 116, 4850–4883 CrossRef CAS PubMed.
- H. P. Cui, K. Zheng, L. Q. Tao, J. B. Yu, X. Y. Zhu, X. D. Li and X. P. Chen, IEEE Electron Device Lett., 2019, 40, 1522–1525 CAS.
- X. Y. Yang, G. Y. Liu, Y. H. Shi, W. Huang, J. J. Shao and X. C. Dong, Nanotechnology, 2018, 29, 15 Search PubMed.
- W. Tao, N. Kong, X. Y. Ji, Y. P. Zhang, A. Sharma, J. Ouyang, B. W. Qi, J. Q. Wang, N. Xie, C. Kang, H. Zhang, O. C. Farokhzad and J. S. Kim, Chem. Soc. Rev., 2019, 48, 2891–2912 RSC.
- H. Lin, X. G. Wang, L. D. Yu, Y. Chen and J. L. Shi, Nano Lett., 2017, 17, 384–391 CrossRef CAS PubMed.
- Y. B. Li, W. Lu, Q. A. Huang, M. A. Huang, C. Li and W. Chen, Nanomedicine, 2010, 5, 1161–1171 CrossRef CAS PubMed.
- H. Kang, S. Mintri, A. V. Menon, H. Y. Lee, H. S. Choi and J. Kim, Nanoscale, 2015, 7, 18848–18862 RSC.
- W. Tao, X. Y. Ji, X. B. Zhu, L. Li, J. Q. Wang, Y. Zhang, P. E. Saw, W. L. Li, N. Kong, M. A. Islam, T. Gan, X. W. Zeng, H. Zhang, M. Mahmoudi, G. J. Tearney and O. C. Farokhzad, Adv. Mater., 2018, 30, 11 Search PubMed.
- W. Tao, X. Y. Ji, X. D. Xu, M. A. Islam, Z. J. Li, S. Chen, P. E. Saw, H. Zhang, Z. Bharwani, Z. L. Guo, J. J. Shi and O. C. Farokhzad, Angew. Chem., Int. Ed., 2017, 56, 11896–11900 CrossRef CAS PubMed.
- Y. Lin, Y. Wu, R. Wang, G. Tao, P. F. Luo, X. Lin, G. M. Huang, J. Li and H. H. Yang, Chem. Commun., 2018, 54, 8579–8582 RSC.
- S. J. Gao, Y. X. Wang, R. X. Wang and W. Z. Wu, Semicond. Sci. Technol., 2017, 32, 9 Search PubMed.
- Y. Wang, C. C. Xiao, M. G. Chen, C. Q. Hu, J. D. Zou, C. Wu, J. Z. Jiang, S. Y. A. Yang, Y. H. Lu and W. Ji, Mater. Horiz., 2018, 5, 521–528 RSC.
- S. Deckoff-Jones, Y. X. Wang, H. T. Lin, W. Z. Wu and J. J. Hu, ACS Photonics, 2019, 6, 1632–1638 CrossRef CAS.
- W. Z. Wu, G. Qiu, Y. X. Wang, R. X. Wang and P. D. Ye, Chem. Soc. Rev., 2018, 47, 7203–7212 RSC.
- J. S. Qiao, Y. H. Pan, F. Yang, C. Wang, Y. Chai and W. Ji, Sci. Bull., 2018, 63, 159–168 CrossRef CAS.
- S. Berweger, G. Qiu, Y. X. Wang, B. Pollard, K. L. Genter, R. Tyrrell-Ead, T. M. Wallis, W. Z. Wu, P. D. Ye and P. Kabos, Nano Lett., 2019, 19, 1289–1294 CrossRef CAS PubMed.
- G. Qiu, Y. X. Wang, Y. F. Nie, Y. P. Zheng, K. Cho, W. Z. Wu and P. D. D. Ye, Nano Lett., 2018, 18, 5760–5767 CrossRef CAS PubMed.
- S. Sharma, N. Singh and U. Schwingenschlogl, ACS Appl. Energy Mater., 2018, 1, 1950–1954 CrossRef CAS.
- K. K. Wen, L. F. Wu, X. X. Wu, Y. Lu, T. Duan, H. Ma, A. D. Peng, Q. Q. Shi and H. Huang, Angew. Chem., Int. Ed., 2020, 59, 12756–12761 CrossRef CAS PubMed.
- B. Mayers and Y. N. Xia, Adv. Mater., 2002, 14, 279–282 CrossRef CAS.
- B. Mayers and Y. N. Xia, J. Mater. Chem., 2002, 12, 1875–1881 RSC.
- Y. X. Wang, G. Qiu, R. X. Wang, S. Y. Huang, Q. X. Wang, Y. Y. Liu, Y. C. Du, W. A. Goddard, M. J. Kim, X. F. Xu, P. D. Ye and W. Z. Wu, Nat. Electron., 2018, 1, 228–236 CrossRef.
- Z. B. Gao, G. Liu and J. Ren, ACS Appl. Mater. Interfaces, 2018, 10, 40702–40709 CrossRef CAS PubMed.
- B. Z. Wu, X. H. Liu, J. R. Yin and H. Lee, Mater. Res. Express, 2017, 4, 8 Search PubMed.
- C. Wang, X. Y. Zhou, J. S. Qiao, L. W. Zhou, X. H. Kong, Y. H. Pan, Z. H. Cheng, Y. Chai and W. Ji, Nanoscale, 2018, 10, 22263–22269 RSC.
- X. H. Wang, D. W. Wang, A. J. Yang, N. Koratkar, J. F. Chu, P. L. Lv and M. Z. Rong, Phys. Chem. Chem. Phys., 2018, 20, 4058–4066 RSC.
- J. H. Yan, X. Y. Zhang, Y. Y. Pan, J. Z. Li, B. W. Shi, S. Q. Liu, J. Yang, Z. G. Song, H. Zhang, M. Ye, R. G. Quhe, Y. Y. Wang, J. B. Yang, F. Pan and J. Lu, J. Mater. Chem. C, 2018, 6, 6153–6163 RSC.
- R. J. Turner, R. Borghese and D. Zannoni, Biotechnol. Adv., 2012, 30, 954–963 CrossRef CAS PubMed.
- M. M. Luo, T. J. Fan, Y. Zhou, H. Zhang and L. Mei, Adv. Funct. Mater., 2019, 29, 19 Search PubMed.
- H. J. Guo, K. Zheng, H. P. Cui, J. B. Yu, L. Q. Tao, X. D. Li, C. R. Liao, L. Xie and X. P. Chen, Appl. Surf. Sci., 2020, 532, 7 CrossRef.
- Z. B. Gao, F. Tao and J. Ren, Nanoscale, 2018, 10, 12997–13003 RSC.
- J. L. Chen, Y. W. Dai, Y. Q. Ma, X. Q. Dai, W. K. Ho and M. H. Xie, Nanoscale, 2017, 9, 15945–15948 RSC.
- Z. He, Y. Yang, J. W. Liu and S. H. Yu, Chem. Soc. Rev., 2017, 46, 2732–2753 RSC.
- M. Amani, C. L. Tan, G. Zhang, C. S. Zhao, J. Bullock, X. H. Song, H. Kim, V. R. Shrestha, Y. Gao, K. B. Crozier, M. Scott and A. Javey, ACS Nano, 2018, 12, 7253–7263 CrossRef CAS PubMed.
- Y. N. Xia, W. Y. Li, C. M. Cobley, J. Y. Chen, X. H. Xia, Q. Zhang, M. X. Yang, E. C. Cho and P. K. Brown, Acc. Chem. Res., 2011, 44, 914–924 CrossRef CAS PubMed.
- L. Zhang, Z. X. Xie and J. L. Gong, Chem. Soc. Rev., 2016, 45, 3916–3934 RSC.
- Z. Shi, R. Cao, K. Khan, K. Tareen Ayesha, X. Liu, W. Liang, Y. Zhang, C. Ma, Z. Guo, X. Luo and H. Zhang, Nano-Micro Lett., 2020, 12, 99 CrossRef CAS PubMed.
- J. M. Pietryga, Y. S. Park, J. H. Lim, A. F. Fidler, W. K. Bae, S. Brovelli and V. I. Klimov, Chem. Rev., 2016, 116, 10513–10622 CrossRef CAS PubMed.
- Y. H. Liu, H. Huang, W. J. Cao, B. D. Mao, Y. Liu and Z. H. Kang, Mater. Chem. Front., 2020, 4, 1586–1613 RSC.
- M. Tuerhong, Y. Xu and X. B. Yin, Chin. J. Anal. Chem., 2017, 45, 139–149 Search PubMed.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- T. Yang, H. T. Ke, Q. L. Wang, Y. A. Tang, Y. B. Deng, H. Yang, X. L. Yang, P. Yang, D. S. Ling, C. Y. Chen, Y. L. Zhao, H. Wu and H. B. Chen, ACS Nano, 2017, 11, 10012–10024 CrossRef CAS PubMed.
- W. D. He, A. Krejci, J. H. Lin, M. E. Osmulski and J. H. Dickerson, Nanoscale, 2011, 3, 1523–1525 RSC.
- J. K. Qin, P. Y. Liao, M. W. Si, S. Y. Gao, G. Qiu, J. Jian, Q. X. Wang, S. Q. Zhang, S. Y. Huang, A. Charnas, Y. X. Wang, M. J. Kim, W. Z. Wu, X. F. Xu, H. Y. Wang, L. Yang, Y. K. Yap and P. D. D. Ye, Nat. Electron., 2020, 3, 141–147 CrossRef CAS.
- O. Erol, I. Uyan, M. Hatip, C. Yilmaz, A. B. Tekinay and M. O. Guler, Nanomedicine, 2018, 14, 2433–2454 CrossRef CAS PubMed.
- M. F. Pantano and I. Kuljanishvili, Nano Express, 2020, 1, 25 CrossRef.
- B. Y. Geng, Y. Lin, X. S. Peng, G. W. Meng and L. D. Zhang, Nanotechnology, 2003, 14, 983–986 CrossRef CAS.
- M. Safdar, X. Y. Zhan, M. T. Niu, M. Mirza, Q. Zhao, Z. X. Wang, J. P. Zhang, L. F. Sun and J. He, Nanotechnology, 2013, 24, 8 CrossRef PubMed.
- P. Bhol, S. Swain, S. Jena, K. Bhatte, C. S. Rout, M. Saxena, A. H. Jadhav and A. K. Samal, ACS Appl. Nano Mater., 2021, 4, 9008–9021 CrossRef CAS.
- S. Kang, Y. G. Gil, D. H. Min and H. Jang, ACS Nano, 2020, 14, 4383–4394 CrossRef CAS PubMed.
- Q. S. Wang, M. Safdar, K. Xu, M. Mirza, Z. X. Wang and J. He, ACS Nano, 2014, 8, 7497–7505 CrossRef CAS PubMed.
- C. D. Zhang, A. Johnson, C. L. Hsu, L. J. Li and C. K. Shih, Nano Lett., 2014, 14, 2443–2447 CrossRef CAS PubMed.
- A. Apte, E. Bianco, A. Krishnamoorthy, S. Yazdi, R. Rao, N. Glavin, H. Kumazoe, V. Varshney, A. Roy, F. Shimojo, E. Ringe, R. K. Kalia, A. Nakano, C. S. Tiwari, P. Vashishta, V. Kochat and P. M. Ajayan, 2D Mater., 2019, 6, 9 Search PubMed.
- Z. J. Xie, C. Y. Xing, W. C. Huang, T. J. Fan, Z. J. Li, J. L. Zhao, Y. J. Xiang, Z. N. Guo, J. Q. Li, Z. G. Yang, B. Q. Dong, J. L. Qu, D. Y. Fan and H. Zhang, Adv. Funct. Mater., 2018, 28, 11 Search PubMed.
- F. Cai, X. Huang, Q. Yang and D. Nagy, Trans. ASME: J. Eng. Gas Turbines Power, 2012, 134, 8 CrossRef.
- F. Cernuschi, L. Lorenzoni, S. Capelli, C. Guardamagna, M. Karger, R. Vassen, K. von Niessen, N. Markocsan, J. Menuey and C. Giolli, Wear, 2011, 271, 2909–2918 CrossRef CAS.
- J. H. Yuan, Q. Zhan, Q. Lei, S. Y. Ding and H. Li, Appl. Surf. Sci., 2012, 258, 6672–6678 CrossRef CAS.
- D. H. Boone, Mater. Sci. Technol., 1986, 2, 220–224 CrossRef CAS.
- M. Kracht, J. Schormann and M. Eickhoff, J. Cryst. Growth, 2016, 436, 87–91 CrossRef CAS.
- A. Koma, Thin Solid Films, 1992, 216, 72–76 CrossRef CAS.
- J. P. Ji, X. F. Song, J. Z. Liu, Z. Yan, C. X. Huo, S. L. Zhang, M. Su, L. Liao, W. H. Wang, Z. H. Ni, Y. F. Hao and H. B. Zeng, Nat. Commun., 2016, 7, 9 Search PubMed.
- X. C. Huang, J. Q. Guan, Z. J. Lin, B. Liu, S. Y. Xing, W. H. Wang and J. D. Guo, Nano Lett., 2017, 17, 4619–4623 CrossRef CAS PubMed.
- X. G. Gu, Y. Zhao, K. Sun, C. L. Z. Vieira, Z. J. Jia, C. Cui, Z. J. Wang, A. Walsh and S. D. Huang, Ultrason. Sonochem., 2019, 58, 12 CrossRef PubMed.
- C. Gibaja, D. Rodriguez-San-Miguel, P. Ares, J. Gomez-Herrero, M. Varela, R. Gillen, J. Maultzsch, F. Hauke, A. Hirsch, G. Abellan and F. Zamora, Angew. Chem., Int. Ed., 2016, 55, 14343–14347 Search PubMed.
- G. W. Liu, J. J. Yuan, T. G. Wu, F. Zhang, F. Xing, W. F. Zhang, H. N. Zhang and S. G. Fu, IEEE J. Sel. Top. Quantum Electron., 2021, 27, 6 Search PubMed.
- K. Manna, H. N. Huang, W. T. Li, Y. H. Ho and W. H. Chiang, Chem. Mater., 2016, 28, 7586–7593 CrossRef CAS.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- H. Qi, B. Liang and U. Kaiser, SmartMat, 2021, 2, 131–138 CrossRef.
- Y. C. Du, G. Qiu, Y. X. Wang, M. W. Si, X. F. Xu, W. Z. Wu and P. D. D. Ye, Nano Lett., 2017, 17, 3965–3973 CrossRef CAS PubMed.
- S. Y. Chen, C. Y. Xing, D. Z. Huang, C. H. Zhou, B. Ding, Z. H. Guo, Z. C. Peng, D. Wang, X. Zhu, S. Z. Liu, Z. Cai, J. Y. Wu, J. Q. Zhao, Z. Z. Wu, Y. H. Zhang, C. Y. Wei, Q. T. Yan, H. Z. Wang, D. Y. Fan, L. P. Liu, H. Zhang and Y. H. Cao, Sci. Adv., 2020, 6, 11 CrossRef PubMed.
- M. Qiu, D. Wang, H. Huang, T. Yin, W. L. Bao, B. Zhang, Z. J. Xie, N. Xie, Z. Z. Wu, C. C. Ge, Q. Wang, M. Gu, H. L. Kutscher, L. P. Liu, S. Y. Bao, P. N. Prasad and H. Zhang, Adv. Mater., 2021, 33, 2102562 CrossRef CAS PubMed.
- M. Qiu, Y. H. Duo, W. Y. Liang, Y. L. Yang, B. Zhang, Z. J. Xie, X. L. Yang, G. Q. Wang, N. Xie, G. H. Nie, O. A. Alhartomy, A. A. Alghamdi, S. Wageh, Y. H. Cao and H. Zhang, Adv. Funct. Mater., 2021, 31, 17 Search PubMed.
- A. Walther and A. H. E. Muller, Chem. Rev., 2013, 113, 5194–5261 CrossRef CAS PubMed.
- T. D. Schladt, K. Schneider, H. Schild and W. Tremel, Dalton Trans., 2011, 40, 6315–6343 RSC.
- Y. P. Guo, H. S. Wang, X. Feng, Y. B. Zhao, C. Y. Liang, L. Yang, M. J. Li, Y. G. Zhang and W. Gao, Nanotechnology, 2021, 32, 11 Search PubMed.
- H. J. Zhu, P. H. Cheng, P. Chen and K. Y. Pu, Biomater. Sci., 2018, 6, 746–765 RSC.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- Z. Q. Meng, Y. Chao, X. F. Zhou, C. Liang, J. J. Liu, R. Zhang, L. Cheng, K. Yang, W. Pan, M. F. Zhu and Z. Liu, ACS Nano, 2018, 12, 9412–9422 CrossRef CAS PubMed.
- J. Wen, K. Yang, X. C. Ding, H. J. Li, Y. Q. Xu, F. Y. Liu and S. G. Sun, Inorg. Chem., 2019, 58, 2987–2996 CrossRef CAS PubMed.
- Q. Y. He, Y. Liu, C. L. Tan, W. Zhai, G. H. Nam and H. Zhang, ACS Nano, 2019, 13, 12294–12300 CrossRef CAS PubMed.
- G. Kresse, J. Furthmuller and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 13181–13185 CrossRef CAS PubMed.
- R. Borghese, M. Brucale, G. Fortunato, M. Lanzi, A. Mezzi, F. Valle, M. Cavallini and D. Zannoni, J. Hazard. Mater., 2017, 324, 31–38 CrossRef CAS PubMed.
- X. Y. Ji, N. Kong, J. Q. Wang, W. L. Li, Y. L. Xiao, S. T. Gan, Y. Zhang, Y. J. Li, X. R. Song, Q. Q. Xiong, S. J. Shi, Z. J. Li, W. Tao, H. Zhang, L. Mei and J. J. Shi, Adv. Mater., 2018, 30, 11 Search PubMed.
- W. Pan, C. Liu, Y. Li, Y. Yang, W. Li, C. Feng and L. Li, Bioact. Mater., 2022, 13, 96–104 CrossRef CAS PubMed.
- C. Dai, H. Lin, G. Xu, Z. Liu, R. Wu and Y. Chen, Chem. Mater., 2017, 29, 8637–8652 CrossRef CAS.
- B. Xia, B. Wang, J. S. Shi, Y. Zhang, Q. Zhang, Z. Y. Chen and J. C. Li, Acta Biomater., 2017, 51, 197–208 CrossRef CAS PubMed.
- J. Li, L. Xie, W. Sang, W. Li, G. Wang, H. Tian, Z. Zhang, J. Liu, Q. Fan and Y. Dai, ChemPhysMater, 2022, 1, 51–55 CrossRef.
- W. C. Huang, C. Y. Xing, Y. Z. Wang, Z. J. Li, L. M. Wu, D. T. Ma, X. Y. Dai, Y. J. Xiang, J. Q. Li, D. Y. Fan and H. Zhang, Nanoscale, 2018, 10, 2404–2412 RSC.
- Z. H. Wang, L. L. Wang, J. R. Huang, H. Wang, L. Pan and X. W. Wei, J. Mater. Chem., 2010, 20, 2457–2463 RSC.
- J. Wang, Y. Y. Li, L. Deng, N. N. Wei, Y. K. Weng, S. Dong, D. P. Qi, J. Qiu, X. D. Chen and T. Wu, Adv. Mater., 2017, 29, 6 Search PubMed.
- Q. W. Tian, M. H. Tang, Y. G. Sun, R. J. Zou, Z. G. Chen, M. F. Zhu, S. P. Yang, J. L. Wang, J. H. Wang and J. Q. Hu, Adv. Mater., 2011, 23, 3542–3547 CrossRef CAS PubMed.
- X. D. Yang, Y. B. Yang, L. N. Fu, M. C. Zou, Z. H. Li, A. Y. Cao and Q. Yuan, Adv. Funct. Mater., 2018, 28, 9 Search PubMed.
- D. X. Xu, Z. D. Li, L. S. Li and J. Wang, Adv. Funct. Mater., 2020, 30, 21 Search PubMed.
- Y. Wang, W. Feng, M. Chang, J. Yang, Y. Guo, L. Ding, L. Yu, H. Huang, Y. Chen and J. Shi, Adv. Funct. Mater., 2021, 31, 2005093 CrossRef CAS.
- C.-W. Lo, D. Zhu and H. Jiang, Soft Matter, 2011, 7, 5604–5609 RSC.
- Z. L. Zhu, X. L. Cai, S. H. Yi, J. L. Chen, Y. W. Dai, C. Y. Niu, Z. X. Guo, M. H. Xie, F. Liu, J. H. Cho, Y. Jia and Z. Y. Zhang, Phys. Rev. Lett., 2017, 119, 5 Search PubMed.
- L. D. Xian, A. P. Paz, E. Bianco, P. M. Ajayan and A. Rubio, 2D Mater., 2017, 4, 7 Search PubMed.
- W. Huang, Y. Y. Huang, Y. Y. You, T. Q. Nie and T. F. Chen, Adv. Funct. Mater., 2017, 27, 15 Search PubMed.
- L. Q. Duan, T. Liu and T. F. Chen, Biomater. Sci., 2021, 9, 1767–1778 RSC.
- N. Yu, J. N. Li, Z. J. Wang, S. Y. Yang, Z. X. Liu, Y. S. Wang, M. F. Zhu, D. B. Wang and Z. G. Chen, Adv. Healthcare Mater., 2018, 7, 12 Search PubMed.
- L. F. Wang, Y. Li, L. Zhao, Z. J. Qi, J. Y. Gou, S. Zhang and J. Z. Zhang, Nanoscale, 2020, 12, 19516–19535 RSC.
- K. Plaetzer, B. Krammer, J. Berlanda, F. Berr and T. Kiesslich, Lasers Med. Sci., 2009, 24, 259–268 CrossRef CAS PubMed.
- A. Gazzi, L. Fusco, A. Khan, D. Bedognetti, B. Zavan, F. Vitale, A. Yilmazer and L. G. Delogu, Front. Bioeng. Biotechnol., 2019, 7, 15 CrossRef PubMed.
- Y. Ma, Y. X. Ma, M. Q. Gao, Z. H. Han, W. Jiang, Y. Q. Gu and Y. Liu, Adv. Sci., 2021, 8, 10 Search PubMed.
- X. Li, S. Lee and J. Yoon, Chem. Soc. Rev., 2018, 47, 1174–1188 RSC.
- F. Q. Fan, L. Wang, F. Li, Y. Fu and H. P. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 17004–17010 CrossRef CAS.
- L. T. Xu, K. Zhou, H. L. Ma, A. Q. Lv, D. D. Pei, G. P. Li, Y. F. Zhang, Z. F. An, A. Li and G. He, ACS Appl. Mater. Interfaces, 2020, 12, 18385–18394 CrossRef CAS PubMed.
- Z. M. Guo, Y. Liu, X. Cheng, D. Wang, S. B. Guo, M. L. Jia, K. Ma, C. H. Cui, L. Wang and H. Zhou, Acta Biomater., 2020, 110, 208–220 CrossRef CAS PubMed.
- M. Guo, H. J. Mao, Y. L. Li, A. J. Zhu, H. He, H. Yang, Y. Y. Wang, X. Tian, C. C. Ge, Q. L. Peng, X. Y. Wang, X. L. Yang, X. Y. Chen, G. Liu and H. B. Chen, Biomaterials, 2014, 35, 4656–4666 CrossRef CAS PubMed.
- M. Qiu, D. Wang, H. Huang, T. Yin, W. L. Bao, B. Zhang, Z. J. Xie, N. Xie, Z. Z. Wu, C. C. Ge, Q. Wang, M. Gu, H. L. Kutscher, L. P. Liu, S. Y. Bao, P. N. Prasad and H. Zhang, Adv. Mater., 2021, 33, 2012562 Search PubMed.
- M. Qiu, Y. Duo, W. Liang, Y. Yang, B. Zhang, Z. Xie, X. Yang, G. Wang, N. Xie, G. Nie, O. A. Alhartomy, A. A. ALGhamdi, S. Wageh, Y. Cao and H. Zhang, Adv. Funct. Mater., 2021, 31, 17 Search PubMed.
- Y. F. Li, W. Lv, L. Wang, Y. M. Zhang, L. P. Yang, T. Y. Wang, L. Y. Zhu, Y. F. Wang and W. P. Wang, Nano Res., 2021, 14, 2630–2636 CrossRef CAS.
- C. Alvarez-Lorenzo, L. Bromberg and A. Concheiro, Photochem. Photobiol., 2009, 85, 848–860 CrossRef CAS PubMed.
- J. Y. Kim, W. I. Choi, M. Kim and G. Tae, J. Controlled Release, 2013, 171, 113–121 CrossRef CAS PubMed.
- W. Shao, C. Yang, F. Li, J. Wu, N. Wang, Q. Ding, J. Gao and D. Ling, Nano-Micro Lett., 2020, 12, 147 CrossRef CAS PubMed.
- B. Tian, C. Wang, S. Zhang, L. Feng and Z. Liu, ACS Nano, 2011, 5, 7000–7009 CrossRef CAS PubMed.
- J. C. Ge, M. H. Lan, B. J. Zhou, W. M. Liu, L. Guo, H. Wang, Q. Y. Jia, G. L. Niu, X. Huang, H. Y. Zhou, X. M. Meng, P. F. Wang, C. S. Lee, W. J. Zhang and X. D. Han, Nat. Commun., 2014, 5, 8 Search PubMed.
- Y. P. Li, T. Y. Lin, Y. Luo, Q. Q. Liu, W. W. Xiao, W. C. Guo, D. Lac, H. Y. Zhang, C. H. Feng, S. Wachsmann-Hogiu, J. H. Walton, S. R. Cherry, D. J. Rowland, D. Kukis, C. X. Pan and K. S. Lam, Nat. Commun., 2014, 5, 15 CrossRef.
- C. Mongin, S. Garakyaraghi, N. Razgoniaeva, M. Zamkov and F. N. Castellano, Science, 2016, 351, 369–372 CrossRef CAS PubMed.
- A. M. Merlot, D. S. Kalinowski and D. R. Richardson, Front. Physiol., 2014, 5, 7 Search PubMed.
- F. Kratz, J. Controlled Release, 2008, 132, 171–183 CrossRef CAS PubMed.
- C. Q. Li, D. H. Zhao, X. L. Hou, B. Zhang, L. B. Song, R. M. Jin, Y. D. Zhao and B. Liu, Chem. Eng. J., 2021, 417, 10 Search PubMed.
- W. Huang, L. Z. He, J. Ouyang, Q. Chen, C. Liu, W. Tao and T. F. Chen, Matter, 2020, 3, 1725–1753 CrossRef.
- D. Chimene, D. L. Alge and A. K. Gaharwar, Adv. Mater., 2015, 27, 7261–7284 CrossRef CAS PubMed.
- Z. X. Tu, G. Guday, M. Adeli and R. Haag, Adv. Mater., 2018, 30, 27 Search PubMed.
- W. Huang, H. L. Wu, X. L. Li and T. F. Chen, Chem. – Asian J., 2016, 11, 2301–2311 CrossRef CAS PubMed.
- J. Wang and B. B. Zhang, Curr. Med. Chem., 2018, 25, 2938–2953 CrossRef CAS PubMed.
- Y. R. Zhou, Y. L. Bai, H. B. Liu, X. H. Jiang, T. Tong, L. R. Fang, D. Wang, Q. Y. Ke, J. G. Liang and S. B. Xiao, ACS Appl. Mater. Interfaces, 2018, 10, 25241–25251 CrossRef CAS PubMed.
- S. H. Tang, M. Chen and N. F. Zheng, Small, 2014, 10, 3139–3144 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |