Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Actuated 3D microgels for single cell mechanobiology
Berna
Özkale
ab,
Junzhe
Lou
ab,
Ece
Özelçi
c,
Alberto
Elosegui-Artola
ab,
Christina M.
Tringides
ab,
Angelo S.
Mao
ab,
Mahmut Selman
Sakar
*c and
David J.
Mooney
*ab
aHarvard John A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA. E-mail: mooneyd@seas.harvard.edu
bWyss Institute for Biologically Inspired Engineering, Cambridge, MA 02138, USA
cInstitute of Mechanical Engineering and Institute of Bioengineering, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015, Lausanne, Switzerland. E-mail: selman.sakar@epfl.ch
First published on 17th August 2022
Abstract
Correction for ‘Actuated 3D microgels for single cell mechanobiology’ by Berna Özkale et al., Lab Chip, 2022, 22, 1962–1970, https://doi.org/10.1039/D2LC00203E.
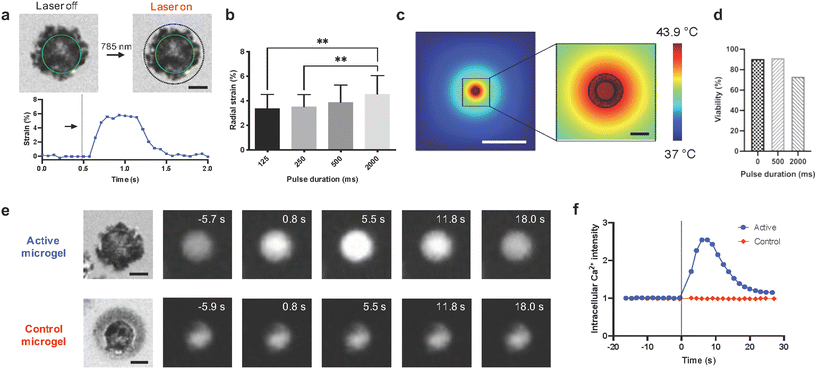
The arrows in Fig. 3e were shown incorrectly in the original article. The corrected Fig. 3 is shown below. In addition the x-axis in Fig. 5d was not labelled correctly. The corrected Fig. 5 is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2022 |