Open Access Article
Open Access ArticleMaterials and methods for droplet microfluidic device fabrication
Katherine S.
Elvira
 a,
Fabrice
Gielen
a,
Fabrice
Gielen
 b,
Scott S. H.
Tsai
b,
Scott S. H.
Tsai
 cde and
Adrian M.
Nightingale
cde and
Adrian M.
Nightingale
 *fg
*fg
aDepartment of Chemistry, Faculty of Science, University of Victoria, BC, Canada
bLiving Systems Institute, College of Engineering, Physics and Mathematics, University of Exeter, Exeter, EX4 4QD, UK
cDepartment of Mechanical and Industrial Engineering, Ryerson University, ON, Canada
dInstitute for Biomedical Engineering, Science, and Technology (iBEST)—a partnership between Ryerson University and St. Michael's Hospital, ON, Canada
eKeenan Research Centre for Biomedical Science, St. Michael's Hospital, ON, Canada
fMechanical Engineering, Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, SO17 1BJ, UK
gCentre of Excellence for Continuous Digital Chemical Engineering Science, Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: a.nightingale@southampton.ac.uk
First published on 24th January 2022
Abstract
Since the first reports two decades ago, droplet-based systems have emerged as a compelling tool for microbiological and (bio)chemical science, with droplet flow providing multiple advantages over standard single-phase microfluidics such as removal of Taylor dispersion, enhanced mixing, isolation of droplet contents from surfaces, and the ability to contain and address individual cells or biomolecules. Typically, a droplet microfluidic device is designed to produce droplets with well-defined sizes and compositions that flow through the device without interacting with channel walls. Successful droplet flow is fundamentally dependent on the microfluidic device – not only its geometry but moreover how the channel surfaces interact with the fluids. Here we summarise the materials and fabrication techniques required to make microfluidic devices that deliver controlled uniform droplet flow, looking not just at physical fabrication methods, but moreover how to select and modify surfaces to yield the required surface/fluid interactions. We describe the various materials, surface modification techniques, and channel geometry approaches that can be used, and give examples of the decision process when determining which material or method to use by describing the design process for five different devices with applications ranging from field-deployable chemical analysers to water-in-water droplet creation. Finally we consider how droplet microfluidic device fabrication is changing and will change in the future, and what challenges remain to be addressed in the field.
1. Introduction
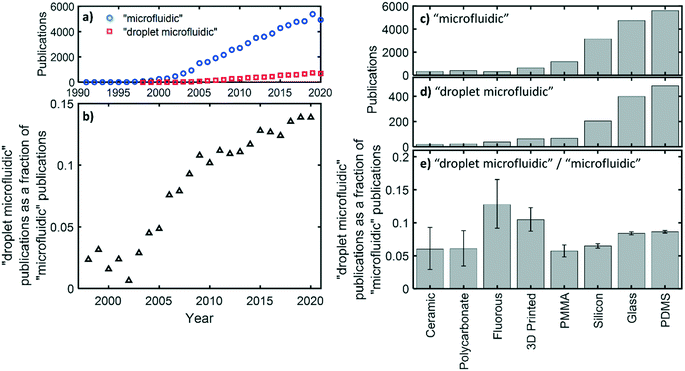
Droplet microfluidic devices are used for the generation, manipulation, and analysis of discrete liquid droplets within a secondary immiscible liquid phase flowing through channels with dimensions preferentially below 500 μm.1,2 Compared to standard single-phase flow, flowing a liquid as a sequence of sub-μL droplets has several practical advantages, such as the removal of Taylor dispersion,3 the encapsulation of viscous or fouling species away from channel walls,4,5 and the segregation of single cells or molecules so that they can be assayed or analysed individually in high throughput.6 Because of these advantages droplet microfluidics is becoming increasingly important within the microfluidic field as a whole, as shown in the bibliographic record: while the total number of both microfluidic and droplet microfluidic publications have steadily increased over time, the proportion of microfluidic publications concerning droplets has significantly increased, with droplet microfluidics currently making up ∼15% of all microfluidic papers, up from ∼5% fifteen years ago (shown in more detail later). This reflects the increasing interest in droplet microfluidics and its importance within the microfluidics community.Droplet flow is typically generated by bringing two immiscible liquids together at a microfluidic junction. Where the two flows meet, the balance of interfacial tension and shear forces (determined by flow rates, channel geometry, fluid composition and viscosity) causes the fluids to break up7 with the resulting droplet size and generation frequency determined by the fluid mechanics of the system.8 Which fluid becomes the “disperse” phase (droplets) and which the “continuous” or “carrier” phase (encapsulating the droplets) is chiefly determined by the relative affinity of each fluid for the channel wall; for example a hydrophobic fluid will preferentially wet a hydrophobic surface. Hence an oil/water fluid pair flowing within hydrophobic channels will flow as a succession of water droplets carried within the continuous oil phase. This is, however, dependent on the channels being uniformly hydrophobic over both space and time. If the surface changes over the length of the channel, or over time, then droplets will stick to the walls, causing a range of problems such as inter-droplet transfer of contents, increase in droplet polydispersity, and analyte adsorption to the channel walls.9,10 Consequently the surface properties of the channels, which determine how the fluids interact with the channel walls, are paramount to ensuring reliable droplet flow – not only during generation, but also through all subsequent operations such as merging, separation, storage, and analysis.
This review summarises how microfluidic devices can be fabricated to control those interactions and hence deliver reliable stable droplet flow. There are several comprehensive reviews that describe materials and fabrication techniques for microfluidic devices in general,11–13 focusing on the range of available materials, their properties, and how they can be physically micropatterned. They pay little attention, however, to the surface chemistry, fluid wetting and other considerations that are fundamental to the successful operation of a droplet microfluidic device. This review aims to address this gap in the literature by providing readers with a holistic guide to material choice and fabrication techniques for droplet microfluidic devices. Our focus will specifically be on channel-based microfluidic devices for flowing droplets rather than digital microfluidic (traditionally electrowetting-on-dielectric) devices, or indeed devices for generating free droplets in gaseous environment (e.g. inkjet printing). Readers interested in these areas are directed to one of the many authoritative reviews.14–17
This review will be especially useful to those new to the field but may also be of use to established researchers considering materials they have not used before. It will cover what materials can be used to make droplet microfluidic devices, describe the range of ways that the surface/fluid interactions can be controlled by surface functionalisation or spatial control of fluids, and then provide concrete examples of the thought process used when choosing a material and fabrication method by discussing five examples from our own research groups. We end the review by highlighting areas where we consider innovations in materials and fabrication methods will significantly impact droplet microfluidics in the future.
2. Device materials and physical fabrication
To begin we will summarise what materials can be used to make microfluidic devices in general, and what techniques can be used to physically fabricate them (including patterning and bonding) before paying more attention in the next section to surface/fluid interactions and methods to chemically modify the device, a common part of the fabrication process for droplet microfluidic devices. Various fabrication methods are available18–20 (summarised in Table 1), with a general trade off between ease/cost of manufacture and the minimum attainable feature sizes. A range of different materials can be used for microfluidic devices, each with different properties and possible physical fabrication methods, as summarised in Table 2. These are described in detail in several good reviews11–13 hence here we will provide a brief summary of the most common material options within the three main classes of materials: inorganic materials (chiefly silicon or glass, but also including ceramics), elastomers, and thermoplastics.13| Minimum feature size (μm) | Fabrication time | Manual interaction | Equipment costs | Running costs | Materials | Additional notes | |
|---|---|---|---|---|---|---|---|
| a Feature size dependent on feature resolution on mould. b Does not include the time, cost, and effort for mould manufacture. c Feature size given for common commercially available systems (e.g. fused deposition modelling, stereolithographic addition printers). Much higher resolutions are possible using more advanced systems (e.g. two-photon polymerisation34–36 can give resolutions in the order of 100 nm). | |||||||
| Photolithography24 | <1 | High | High | High | Medium | Photoresists, photocurable polymers | Cleanroom required |
| Micromachining25 | 50 | Medium | Medium | High | Medium | Inorganic, plastics | Typically produces rough surfaces |
| High aspect-ratio channels possible | |||||||
| Moulding/casting26–28 | Variablea | Lowb | Lowb | Lowb | Lowb | Elastomers, thermoplastics | |
| Laser ablation29,30 | 1 | Low | Low | High | Low | Inorganic, plastics | |
| 3D printing31,32 | 100c | Medium | Low | Low | Low | Thermoplastics | |
| Chemical etching33 | <1 | High | High | Low | Medium | Inorganics | Requires use of hazardous chemicals |
| Rigidity | Chemical compatibility | Thermal stability | Gas permeability | Surface hydrophilicity | Physical patterning | Bonding methods | |
|---|---|---|---|---|---|---|---|
| Inorganic materials (e.g. glass, silicon) | Rigid | High | High | Typically poor | Hydrophilic | Laser ablation, micromachining, chemical etching | Thermal bonding, adhesives |
| Elastomers (e.g. PDMS) | Soft | Moderate | Moderate to good | Good | Typically hydrophobic | Casting, 3D printing | Adhesives, covalent bonding, conformal bonding |
| Thermoplastics (e.g. PMMA, PTFE) | Moderate to rigid | Variable | Variable | Variable | Typically hydrophobic | Micromachining, moulding, laser ablation, 3D printing | Thermal bonding, adhesives |
Inorganic materials have the advantage of broad solvent compatibility, mechanical rigidity and, for glass, exceptional optical clarity at ultraviolet/visible wavelengths. They are expensive and difficult to fabricate, however, with the manufacturing process difficult to scale up. Monolithic microfluidic devices (i.e. those made exclusively from a single material with no observable joins once fabricated) made from glass or silicon are typically patterned by a combination of photolithography and wet-etching techniques followed by hot pressing above the glass transition temperature. While this is an expensive and manually intensive fabrication method, glass devices can be washed and reused, which is highly useful if device geometries are already established. As a cheaper alternative, off-the-shelf components can also be used; for example glass capillaries are often used as microfluidic devices with their tips tapered to small diameters using capillary pullers.21
Elastomers, such as the ubiquitous poly(dimethylsiloxane) (PDMS), are a low cost and easy-to-manufacture alternative to silicon and glass. These are typically patterned by moulding to masters created using other fabrication methods.22 While the techniques used to make the masters (most usually photolithography) can be time-consuming, the masters can be used repeatedly to mould many devices, with excellent reproducibility and sufficient scalability for academic requirements. Sealed channels are typically formed by covalent bonding of the patterned elastomer substrate to a glass surface via surface activation by a plasma. PDMS devices can also be reversibly sealed to another piece of PDMS, glass, or other substrates by simple contact between the surfaces, creating hybrid devices with hybrid surface properties,23 though this necessitates the use of low fluid pressures and hence low flow rates.
Thermoplastics include polymethylmethacrylate (PMMA), polycarbonate (PC), polystyrene (PS), polyvinylchloride (PVC), and cyclic olefin co-polymer (COC) as well as most common fluoropolymers.37–39 They have the major advantage that they can be large-scale manufactured using injection moulding or hot embossing, however smaller scale manufacture is more difficult, relying on micromachining (i.e. micromilling and other mechanical fabrication methods) which involves costly machinery and tooling and moreover has much lower feature resolution (hundreds of microns) compared to most lithography methods. Bonding is typically achieved by either thermal bonding of the substrates or by using adhesive tapes. Various thermoplastics and elastomers can also be 3D printed, but typically at lower resolutions. While high end two photon polymerisation printers can give resolutions in the order of 100 nm,34–36 most commercially available printing methods (fused deposition modelling, stereolithographic addition) produce channels 100 μm or larger.31
When considering how material choice impacts on droplet microfluidic devices in particular it is useful to examine what materials have been historically used. As previously mentioned, droplet microfluidics publications make up an increasing proportion of the microfluidics publications in general (Fig. 1a and b). If we look at the trends seen for several common device materials (Fig. 1c–e), we see that PDMS is associated with the greatest number of publications for both microfluidics in general (Fig. 1c) and droplet microfluidics in particular (Fig. 1d), consistent with its ease of use for small volume manufacturing and suitability for academic research. Glass and silicon also score highly, in part because they have been used from the very beginning of the field of microfluidics. While material popularity shows the same overall trend for droplet microfluidics (Fig. 1d) and microfluidics in general (Fig. 1c), if we look at the droplet microfluidics results as a proportion of the corresponding microfluidics publications (Fig. 1e), there are a few materials that appear to be disproportionately favoured for droplet microfluidics. Most materials comprise 6–9% of the droplet publications, but there are outliers with fluoropolymer materials (13%) and, to a lesser extent, 3D printed materials (11%) being particularly favoured for droplet microfluidic devices. Fluoropolymers are known for their superhydrophobic surface properties which, as later discussed, means that the hydrophobic continuous phases typically used in droplet flow will easily wet the surfaces without need of any surface modification procedures. 3D printed materials also score slightly higher than other materials but this may not be due to any inherent advantage that makes them better suited to droplet microfluidics, but rather due to trends in research focus; the recent use of 3D printing for microfluidics (since 2012 – fourteen years later than the first PDMS and fluoropolymer reports for example) has coincided with the increasing emphasis on droplet microfluidics publications (Fig. 1b), meaning we would expect a higher baseline compared to longstanding materials with similar suitability for droplet flow.
While this bibliographic analysis should be treated as indicative, it shows how a wide range of materials have been used for droplet microfluidic devices, and that there is no “right” material for droplet-based devices with ease of fabrication, access to facilities, cost, as well as the application requirements themselves, playing significant roles in material choice. Nonetheless, the relatively disproportionate prevalence of fluoropolymers, illustrates how droplet flow places additional considerations on surface/fluid interactions and hence device material choices. In the next section we look in more detail at these interactions and how they can be controlled.
3. Ensuring channel surfaces are preferentially wetted by the continuous phase
The interactions between fluids and the channel surface are key to determining which fluid becomes the dispersed phase and which the continuous. With the small channel sizes in microfluidic devices, and the accompanying high surface area to volume ratios, the channel/fluid interface dominates fluid behaviour. There are several ways to control the surface/fluid interactions, either by choosing a material with the correct surface properties, modifying a surface (either permanently or temporarily), or by careful spatial control at the point of droplet generation. Here we will examine each in turn.3a. Native material surfaces
The simplest way to control which fluid becomes the continuous phase is to make sure the device is fabricated from a material with similar chemical properties to the desired continuous phase, which will lead to that fluid preferentially wetting the channel surface. “Wetting” refers to the preference of a material to be in contact with one fluid rather than another. For instance, in a competition between an aqueous fluid and a hydrocarbon oil, a hydrophilic surface will be preferentially wetted by the aqueous fluid. A key parameter that describes this effect and can be used to predict good droplet formation is the advancing (maximal) contact angle – the angle between the fluid/fluid interface and the wall. For the example of a simple water/oil flow, if the contact angle for water exceeds a critical value (92° in the example shown in Fig. 2) water-in-oil droplets will be generated. Below that value oil-in-water droplets will be generated.40 It is important to note that droplet generation dynamics (droplet size, generation frequency) are independent of wetting assuming the contact angle is above/below the critical angle40 and also that for long term operation it is essential that the contact angle is maintained over time and space. If the angle crosses the critical value at a specific time and position in the channel, the disperse phase will then wet the channel walls leading to droplet pinning, cross contamination and other failure modes.10 Stable channel surfaces, reliably preferentially wetted by the continuous phase, are therefore an essential consideration when designing a droplet microfluidic device. It is preferable to make the device from a material with the required surface characteristics but this is not always possible, hence surface modification is often required as an additional fabrication step. We now describe in more detail the native surface chemistry of different device materials and the implications for fluid wetting.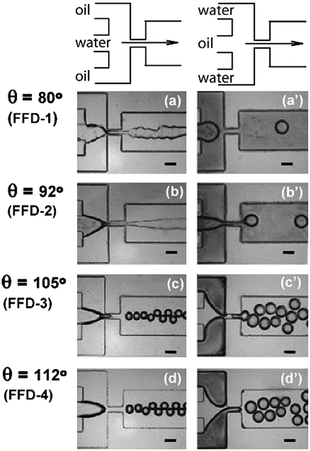 | ||
| Fig. 2 Generation of droplets by a flow-focusing device (FFD), with varying incoming fluid orientations and device surface characteristics. Fluids enter with either a water-in-oil (a–d, left) or oil-in-water (a′–d′,right) setup, and surfaces varying from relatively hydrophilic (a and a′) to relatively hydrophobic (d and d′) as shown by the increasing contact angle, θ. Water-in-oil droplets can be formed when the contact angle exceeds 92°. Phase inversion is visible in c′ and d′ when the oil phase wets the channels even though it is intended to be used as a dispersed phase. The scale bar is 100 μm in all cases.40 Reprinted (adapted) with permission from Li et al.40 Copyright 2007 American Chemical Society. | ||
Glass is naturally hydrophilic making it typically suitable for generating oil-in-water droplets, however its natural wettability by water can vary depending on several parameters including cleaning and drying protocols, and atmospheric conditions.41 Surface modifications for glass that are compatible with both water-in-oil and oil-in-water droplet generation are well established, as described below. The most commonly used elastomer, PDMS, features a contact angle for water of 112–120° when pristine,42 signifying a hydrophobic surface suitable for generating water-in-oil droplets without modification. Contact angles vary significantly however, depending on the preparation method and surface treatment, contact time with water, and velocity of the advancing contact line.43 As a result, pristine PDMS is commonly surface-treated to maintain surface properties and hence promote device longevity.
Most thermoplastics used to fabricate microfluidic devices are hydrophobic in nature, although the contact angles of water on their surface ranges from 80° to over 100°.44 Native PMMA, for example, has been used to create devices for stable monodisperse water-in-oil droplets with mineral oil as continuous phase and Span 80/Abil Em90 as surfactants.45 Surface modification is often needed for robust operation however,45 or for the generation of oil-in-water droplets. Fluoropolymers are special thermoplastics containing a large proportion of fluorine atoms and characteristically exhibiting highly useful properties such as high chemical resistance, good solvent compatibility compared to other thermoplastics, and low absorption of small molecules. It is their superhydrophobic surfaces that are of most interest for droplet microfluidics. Water contact angle for native smooth polytetrafluoroethylene (PTFE) is ∼125° and therefore does not usually need to be functionalized for the generation of water-in-oil droplets. Common fluoropolymers such as PTFE,46 perfluoroalkoxy alkane (PFA),47,48 and fluorinated ethylene-propylene (FEP),48,49 have been used to make droplet devices. They are typically difficult to fabricate as they have high glass transition temperatures and their softness makes them poorly suited to direct machining. Hence terpolymers of tetrafluoroethylene, hexafluoropropylene and vinylidene fluoride (THV) have recently attracted attention as they offer similar properties but are easier to fabricate as the lower melting points (<200 °C) are highly suitable for melt-processing.50,51 Speciality fluoroelastomers52,53 are also available but at higher cost than standard fluoropolymers.
3b. Surface modification of channel surfaces
If the material cannot be chosen to match the required continuous phase, surfaces can be altered after the devices have been physically formed to obtain a desired surface chemistry. Chemical surface modification of glass and PDMS microfluidic devices has been routinely performed since the early days of the field.54,55 Compared to simply choosing a material with appropriate surface chemistry, surface modification not only allows researchers to almost arbitrarily specify the nature of the surface, but also means a device fabricated from a single material can have separate sections with different surface types. This can be exploited, for example, to make devices for generating complex droplets-within-droplets.56 Surface modification does, however, come at the expense of additional fabrication steps which increase fabrication time, cost, and introduces additional potential failure modes. Here we describe some of the most common techniques for surface treatment, from the simplest to the most complex.Plasma treatment is used to activate PDMS surfaces for device bonding but, as it creates Si–OH groups on the surface of PDMS, can also be used as a method to render the surface hydrophilic. The hydrophilic surface is transient, however, and plasma treating can form cracks on the surface57 that can exacerbate unwanted molecular diffusion into the PDMS.58 Hence, plasma treatment is typically used as a method of enhancing capillary action to fill microfluidic channels with aqueous fluids,59 or as the first step for further surface modification. Similar treatments include corona discharge and UV light.60
Silanisation is a common method to modify PDMS, glass or silicon surfaces.61,62 Silanisation is usually performed in two steps, firstly the activation of the surface by oxygen plasma treatment to yield a hydroxy-rich surface, and then immediate introduction of a silane molecule which spontaneously covalently bonds to the device surface. The choice of silane determines the resulting surface characteristics, for example 1H,1H,2H,2H-perfluorooctyltrichlorosilane (PFOS) for hydrophobic surface modification and 3-aminopropyltriethoxysilane (APTES) for hydrophilic surface modification.63 Both silanes can be used in the same microfluidic device to create both hydrophobic and hydrophilic regions which can be used, for example, for forming multiple emulsions.56 If hydrophobic surfaces are required, a similar effect can be achieved at lower cost by flowing fluorosilane-based automotive screen rain repellent treatments through the channels.64,65 While silanisation is the most common method of surface treatment, it should not be considered a permanent change in surface properties (especially for PDMS), but rather one with a finite life span,42 and we note there is a lack of fundamental research on the longevity of chemical surface treatments and behaviour under real-use conditions.
Polymer coatings can also be used to modify the surfaces of microfluidic devices. The most common example is the use of fluoropolymers66,67 to make PDMS channels superhydrophobic. In this case, the fluoropolymer forms a layer on the surface of the PDMS, though, again, the longevity of the coating is affected by the nature of the underlying material. Nanostructuring is a more complicated method of surface modification. Nature provides numerous examples of surface properties being modified by surface structure, such as the superhydrophobic surfaces of the leaves of certain plants which allow water droplets to easily roll off, cleaning the leaves in the process (the so-called “lotus effect”).68 The superhydrophobicity of these leaves directly results from the nanostructured surface which reduces the contact area between the droplet and the leaf surface. Microfluidic researchers have used bioinspired nanostructuring approaches to make both hydrophobic and hydrophilic surfaces with recent reviews summarising the different applications and fabrication methods.69,70 While this approach has not been widely applied to droplet flow, likely due to the extra fabrication steps involved, one group in particular has used it to render PMMA microchips superhydrophobic,71 with this method chosen as PMMA is difficult to functionalise using other techniques. In this case, channel surfaces were modified by depositing silica nanoparticles (generating a nanotextured hydrophilic surface) which were subsequently rendered hydrophobic using n-dodecyltrichlorosilane to yield the final superhydrophobic surface. This technique has been utilised in several different devices for droplet-based microbial toxicity assays.71–73
3c. Use of surfactants
As an alternative to permanent functionalisation of the channel surface, channel surfaces can be non-covalently altered by utilising a continuous phase containing a surfactant. Surfactants (also referred to as emulsifiers or stabilisers) are amphiphilic molecules that are primarily used to stabilise the fluid/fluid interface, however they can also interact with channel surfaces74 and as such be used as a temporary form of surface modification. The ability of surfactants to radically change the surface chemistry of the channels has been shown in previous studies where both water-in-oil or oil-in-water droplets could be formed in the same device by simply changing the surfactant, without any further modification of the channel surfaces.75There are several commercial surfactants available that are made specifically for droplet microfluidics such as QX100 by Bio Rad, PicoSurf by Sphere Fluidics, and the more recently available FluoSurf by Emulseo. However it is also possible to use common detergents used in biological research such as sodium dodecyl sulfate (SDS), Span80 or polyethylene glycol (PEG).74 When using a surfactant, one must decide whether to introduce it via the disperse or continuous phase. If the surfactant is dosed in the disperse phase, it is contained away from the channel walls, however if dosed in the continuous phase, surfactant molecules are free to migrate to the channel/fluid interface.75,76 In this case an equilibrium exists between the surfactant molecules in solution in the continuous phase, those that self-assemble at the droplet surface, and those that reversibly adhere to the channel walls. To ensure that the surface of the channels is coated with the surfactant, in practice devices are often first “primed”, whereby the continuous phase is flowed through the device for several minutes before the disperse phase is introduced.
Prior work by Elvira and co-workers shows how, when using surfactants as a temporary surface modification, stable droplet formation is dependent on a certain proportion of the surfactants being present on the channel wall.10 They showed both through modelling and experimental work how addition of droplets to an continuous phase disrupts this equilibrium, with each additional droplet effectively being a “surfactant sink” that draws surfactant away from the walls of the device. This can in certain circumstances lead to droplet failure modes such as dripping, where the droplet does not form cleanly at a T-junction due to wetting of the junction walls. For a guide in choosing surfactants for each aqueous/oil phase combination and the droplet failure modes that may occur in PDMS devices, a flow chart is provided in the ESI of their 2015 paper.10
3d. Geometries to control wall interactions during droplet generation
As well as the interfacial tensions at the surface/fluid interface, the spatial relation between the fluids and the surface can also have an effect on obtaining reliable droplet flow. Droplets are generated at a junction where the dispersed and continuous fluid phases meet and the dispersed phase is broken up into discrete droplets. The shape of the microfluidic geometry dictates the spatial arrangement by which the two phases meet, which in turn, influences the mode of droplet generation as well as whether and what surface treatments are necessary. Here we briefly describe the most commonly used microfluidic designs for making droplets and how careful design, used in conjunction with the surface modifications described previously, can ensure that only the continuous phase wets the channel walls.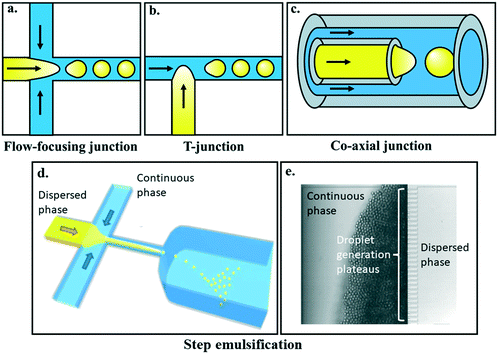 | ||
| Fig. 3 Commonly used geometries for microfluidic droplet generation include a) flow-focusing, b) T-junction, and c) co-axial geometries. Each of these microfluidic designs enable the dispersed and continuous phases to meet at a junction and generate droplets of the dispersed phase downstream of the junction. Images provided by Kaitlyn Ramsay. d) Step emulsification and its subset, e) edge-based droplet generation (EDGE) devices enable controlled monodisperse droplet generation, and the potential for massive scale-up. Images reproduced from Z. Li et al.77 with permission from the Royal Society of Chemistry and S. ten Klooster et al.78 under a CC BY 4.0 licence. | ||
In both flow-focusing and T-junction setups, whether droplets form and via what mechanism depends on the ratio of the dispersed and continuous phase volumetric flow rates, as well as the dimensionless capillary number, which is the ratio of continuous phase viscosity and velocity to the liquid–liquid interfacial tension between the two phases. Droplet generation regimes transition between the well-studied squeezing, dripping, and jetting regimes, with changes to the capillary number.8,80 The popularity of these geometries is likely due to their ease-of-manufacture, featuring planar designs with uniform channel heights, and are typically made from PDMS following classical soft lithography protocols.22 Consequently flow-focusing and T-junction geometries have been used in a wide range of microfluidic applications and the fluid mechanics behind their droplet generation regimes have been well studied and are well understood.8
One consequence of using planar geometries is that both the dispersed and continuous phase fluids are in contact with the “ceiling” and “floor” of the channels when the fluids first meet. This presents a challenge to droplet generation. As the disperse phase is already in contact with the channel walls, there is a strict requirement that the continuous phase must preferentially wet the channel walls. This is the primary reason why, in devices that generate water-in-oil droplets using flow-focusing or T-junctions, the microchannels must be made using hydrophobic materials or treated with hydrophobic coatings, as described earlier.
Where co-axial geometries are used functionalisation is often not required,21,86 however this is not true in all cases.85 Even in cases where functionalisation has been necessary however, spatial separation of the dispersed phase from the channel walls means that surface chemistry requirements are less stringent, making the devices more robust and expanding the possible fluid/material options.87
4. Examples of design rationale in five different applications
With so many possible routes to control surface/fluid interactions and deliver successful droplet devices, how does a researcher choose the best option when first deciding to make a droplet microfluidic device? In practice, this is done on a case-by-case basis driven by individual experimental requirements, available resources within the laboratory, and fabrication complexity – if there are multiple routes to a similarly performing device, the route that has fewer fabrication steps, and hence fewer potential failure points, should be chosen. To provide concrete practical examples of how these choices are made in practice, here we describe five separate examples of device fabrication. In each case we focus on the experimental requirements of the microfluidic device and how that led to the material and fabrication choice. For further descriptions of droplet microfluidics applications, interested readers are directed to several more application-focussed reviews.90–924a. Single-cell encapsulation for growing clonal stem cell colonies
Single cell assays are a historically important application of droplet microfluidics, allowing omics and phenotypic studies across thousands or more cells at a time.93 Culturing individual cells long-term and understanding the fate of single cells is crucial to developmental biology. The Gielen Lab, in collaboration with others, has developed a microfluidic method that enables optical interrogation of single mouse embryonic stem cells cultured over days, enabling a better understanding of cellular heterogeneity and differentiation processes.94 Although cells can survive and stay functional for days within water-in-oil emulsions, an increasingly popular method is to encapsulate single cells into hydrogels acting as 3D scaffolds in which cells can proliferate and form cellular aggregates.95 This approach enables complete removal of the oil phase following polymerization of the gel. Two distinct devices were used in this work: one for single-cell encapsulation into hydrogel (agarose) and a second one for hydrogel bead trapping. Key considerations were the need for high cell survival rates during encapsulation and incubation within microfluidic devices, and high optical transparency for transmitted light and fluorescence imaging. Hence both devices were fabricated in PDMS and covalently bonded onto thin borosilicate glass coverslips. PDMS was chosen because of its compatibility with cell culture conditions (especially good gas exchange and optical clarity), easy local access to facilities to fabricate master moulds, and overall low cost and fast turnaround times. The thin coverslip substrate allows for high-resolution imaging using inverted epifluorescence microscopes. The microfluidic chip was rendered superhydrophobic by treating with 1% (v/v) (PFOS) dissolved in HFE-7500 fluorocarbon oil directly after plasma bonding, making all surfaces fluorophilic. Excess PFOS molecules were thoroughly washed away with pure HFE oil before use to ensure high cell viability when transiting through the device and during the incubation phase in gels. Glass and PDMS both coated with the fluorosilane molecules provided for robust droplet generation required to form highly monodisperse gels. Overall, long-term cell viability relied on keeping surfaces sterile, careful selection of the gel polymerization conditions and cell handling protocols. Other biocompatible materials such as thermoplastics could have alternatively been used for the droplet generation device but would have required more expensive and longer fabrication.964b. Robust field-deployable droplet microfluidics using PTFE capillary tubing
Measurement of chemical levels in rivers, lakes and oceans is important, both in the short term for monitoring pollutant levels, and more generally for learning more about the basic biogeochemical processes that govern life on earth. Recently Nightingale and co-workers reported a droplet-based sensor for in situ monitoring of nitrate and nitrite levels in rivers (Fig. 4a) and its field testing in a tidal river (Fig. 4b) over three weeks.97 The system works by continuously taking water samples, performing a colorimetric assay in droplets and recording the result using onboard optics and electronics. The use of droplet flow is important for removing Taylor dispersion and hence increasing temporal resolution (seconds vs. minutes) and decreasing the consumption rate of assay reagents when compared to the existing state of the art single phase systems.98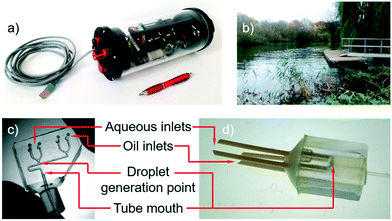 | ||
| Fig. 4 a) Droplet based nitrite sensor which was deployed for 3 weeks in the River Itchen in Southampton (b).97 c) PDMS chip for generating droplets and introducing them into PTFE tubing100 similar to that used in early sensor prototypes. d) 3D-printed device for droplet generation at the mouth of a PTFE tube as used in the final sensor. Images reproduced from A. M. Nightingale et al.,97 copyright 2019 American Chemical Society, and A. M. Nightingale et al.100 under a CC BY 4.0 licence. | ||
One of the foremost requirements for a field-deployable droplet flow system is robustness – users need to be sure that despite changes in ambient conditions (most notably temperature) droplet generation is reproducible and non-drifting (i.e. constant generation rate, droplet volume and droplet composition), and that there will be no droplet pinning or other unwanted surface interactions that will compromise droplet integrity and hence measurement quality. To ensure reproducible droplet generation dynamics irrespective of ambient changes, an anti-phase pulsatile pumping method was chosen, with droplet size and frequency hard-coded into the pump design,99 however, maintaining the droplet integrity was directly dependent on correct material choice.
In development, the team initially used PDMS T-junctions to generate the droplets which were then subsequently fed into PTFE capillary tubing (Fig. 4c) for droplet incubation and optical analysis.101 The use of a PDMS chip meant that droplet generation could be controlled by changing geometries if required and the PTFE tubing offered a simpler means to retain the droplets during incubation. The PDMS droplet generation junctions were formed from 3D printed moulds made using a Objet500 Connex3 polyjet printer. PDMS was chosen for its transparency and easy manufacture, with 3D printing used to generate the moulds as it allowed channels of the required size (∼300 μm in the smallest dimension) to be generated much quicker and easier compared to traditional cleanroom methods. A fluorocarbon continuous phase (Fluorinert FC-40) was used to encapsulate the aqueous droplets to ensure maximum interfacial tension and hence droplet integrity. While PTFE tubing is naturally wetted by the oil and hence supports good water-in-oil droplet flow, the PDMS needed to be functionalised to render it superhydrophobic. This was achieved using a commercially available fluoroalkylsilane normally marketed for automotive screens (Aquapel, PPG Industries) however in practical testing the surface coating had a finite lifespan of days to weeks (exact time dependent on batch-to-batch variation) with surface deterioration leading to droplet pinning and polydisperse droplet sizes. Rather than working to improve the surface functionalisation of the PDMS chip, the team decided to remove the problem completely by generating droplets directly at the PTFE tubing entrance and thus removing the need for a PDMS device. An alternative would have been to make the chip out of a fluoropolymer, however this route was much simpler. To generate the droplets at the tubing mouth a 3D printed manifold was used to converge the oil and aqueous streams at the tubing mouth so that the droplets formed as the fluids entered the tubing (Fig. 4d). As the droplet flow did not contact any material except PTFE, which has a naturally superhydrophobic surface which will not deteriorate over time, there was minimal risk of droplets pinning or breaking up. In practice this was found to be the case with continuous droplet flow in a river over three weeks.
It is worth noting that while tubing-based systems49 such as this are advantageous for their simplicity and robustness and were the right choice here, they have some notable disadvantages compared to microfluidic chips – most notably that channels cannot be arbitrarily designed for specific applications. Hence the group have more recently looked towards exploring routes to bespoke fabricated fluoropolymer devices for cases where more complicated channel architectures are required.51
4c. Microfluidic geometry for water-in-water droplet generation without surface modification
Droplet microfluidics typically involves a water/oil fluid pair, however, there is an emerging class of droplet microfluidics that generates water-surrounded-by-water (water-in-water) droplets, which have advantages in terms of biocompatibility102 and a powerful selective partitioning ability to separate biological particles such as cells, proteins, and viruses.103 Water-in-water droplets are generated using a set of fluids called aqueous two phase systems (ATPS) of which the most studied uses dextran-rich (DEX) and PEG phases. While there is sufficient surface tension between the two aqueous phases to render them immiscible, the differences in the hydrophilicity of each phase are only slight. This means droplet breakup often needs external stimulus104,105 and while DEX-in-PEG droplets have been commonly reported it is particularly difficult to tune channel surfaces to generate PEG-in-DEX droplets.104–107It is here that microfluidic geometry design is very important. The Tsai Group recently showed how flowing the PEG phase as the dispersed phase in a typical planar flow-focusing microchannel results in a long PEG thread that attaches to the “ceiling” and “floor” of the microchannel, but flowing the same PEG phase into a needle that is inserted into a rectangular microchannel, such that the dispersed phase enters the channel without contact with the main channel “ceiling” and “floor”, enables robust PEG phase water-in-water droplet formation (Fig. 5).83
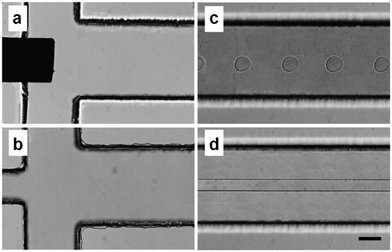 | ||
| Fig. 5 Microscopy images of microchannels a) with and b) without an inserted needle. c) The PEG-in-DEX water-in-water droplets are formed when the dispersed phase enters via the needle. d) Without the needle, the dispersed PEG phase enters the channel in contact with the “ceiling” and “floor” of the channel, and forms a long thread that does not break into monodisperse droplets. Scale bar represents 100 μm. Reprinted from M. Jeyhani et al.,83 copyright 2019, with permission from Elsevier. | ||
Water-in-water droplet microfluidics is still an emerging topic in the microfluidics field, with only a few dozen papers in the literature, and this hybrid needle-PDMS approach reported in 2019. While there are currently no general design rules for the required distance between the needle and the “floor” or “ceiling” of the microchannel, the main principle is clear: successful droplet generation is enabled by the spatial organisation of the fluids as they enter the cross junction. The design enables the dispersed phase, which can be either the PEG or DEX phases, to be sufficiently separated from the “ceiling” and “floor” of the downstream microchannel, such that any interfacial interaction forces between the dispersed phase and the channel surface can be overcome by spatial separation. Flowing the PEG phase through a needle creates a coaxial-like flow, whereby the dispersed PEG phase is surrounded by the continuous DEX phase as soon as the PEG phase enters the microchannel. In the context of fluid pairs with similar wettability, where channel surface modifications have minimal impact, this design is essential to ensuring reliable droplet breakup. A similar approach, whereby a microneedle and glass capillaries are embedded into a PDMS microfluidic channel, can be also used to create ATPS water-in-water-in-water double emulsions.82
4d. Democratising microfluidic technologies using 3D printing and off-the-shelf tubing
Microfluidic technologies are commonly promoted as tools to enable new scientific discoveries, however their use is mostly confined to academic laboratories with specialist microfluidic expertise. For microfluidic systems to make the most scientific impact, they need to be used widely, however the infrastructure (cleanroom), instrumentation (high-speed cameras, pumps, microscopes), and knowhow (photolithography, soft-lithography, device design) typically required create a barrier to uptake of microfluidic technologies as a commonplace tool. While the development of new microfluidic techniques and devices is probably always going to be confined to specialist research laboratories,108 there are many examples in the literature where overly complicated designs are used for simple on-chip operations. Devices tend to be custom-made for each new application and it is rare indeed that a single microfluidic platform is reused even within the same research group. The balance of innovation and utility needs to be equilibrated such that simple microfluidic devices are easily accessible for use in non-specialised laboratories. As described above, 3D printing can be used to make the microfluidic devices themselves. However, 3D printing can also be used to fabricate moulds for casting elastomeric devices, which is much simpler, cheaper and easier than traditional photolithographic mould fabrication.The Elvira Group has recently developed a plug-and-play microcapillary platform for the creation of multicompartmental double emulsions that simply requires an inexpensive consumer-grade bench-top 3D printer for mould fabrication and syringe pumps for operation.109 This is the type of microfluidic device that can be mailed to collaborators so that they can make droplets in their own laboratory. There were several design parameters they considered when developing this microfluidic platform. Firstly, they wanted to limit the fabrication techniques required to those readily available. Hence, they used a 3D printer that can be purchased for under 200 USD to make the mould, rather than relying on access to a cleanroom. Secondly, they wanted to remove the need for surface treatment while not limiting the types of droplets that could be made. Hence, they used off-the-shelf PTFE tubing (for hydrophobic surfaces) and glass capillaries (for hydrophilic surfaces). And lastly, they wanted to ensure that no microfluidic expertise was required to fabricate this device. Hence, the tubing and capillaries are simply inserted into “junction boxes” made from 3D printed moulds using a flexible polymer that also prevents leakage (Fig. 6a–f). The 3D printed mould was made from the standard resin supplied by the printer manufacturer to keep costs low and ensure that printing was straightforward. The junction boxes themselves were cast from polyurethane resin because this flexible material creates a seal around the tubing and capillaries inserted into the junction boxes, removing the need for gaskets or other sealants.
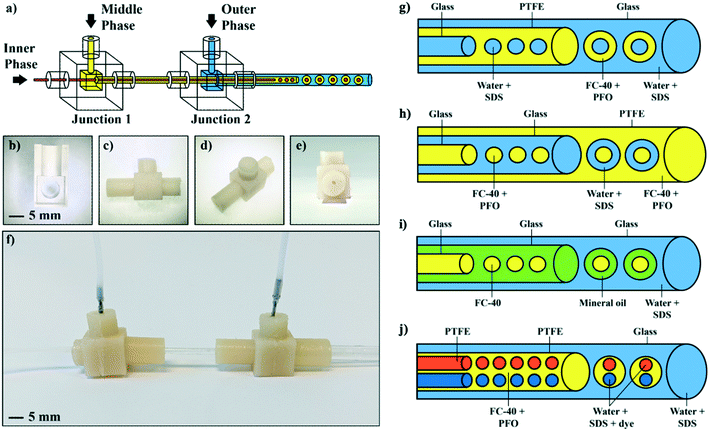 | ||
| Fig. 6 A microcapillary platform for the formation of multicompartmental double emulsions. a) Schematic showing the overall design of the junction boxes that hold the capillaries in the correct configuration for droplet formation. b) 3D printed mold to cast the junction boxes and c–e) images of the flexible junction boxes used to hold the capillaries in place and seal them. f) Image of the assembled platform. g) Formation of water-in-oil-in-water multicompartmental double emulsions using a glass capillary to make the inner aqueous droplets (water stabilised with SDS), PTFE tubing to encapsulate them in oil (FC-40), and a glass capillary to form the double emulsions in a surrounding aqueous phase (water stabilised with SDS). h) Formation of oil-in-water-in-oil multicompartmental double emulsions using a glass capillary to make the inner oil droplets (FC-40 stabilised with PFO), a glass capillary to encapsulate them in an aqueous phase (water stabilised with SDS), and a glass capillary to form the double emulsions in oil (FC-40 stabilised with PFO). i) Formation of oil-in-oil-in-water multicompartmental double emulsions using a glass capillary to make the inner oil droplets (FC-40), a second glass capillary to encapsulate them in another oil (mineral oil), and a third glass capillary to form the double emulsions in a surrounding aqueous phase (water stabilised with SDS). j) Formation of binary water-in-oil-in-water multicompartmental double emulsions using two pieces of PTFE tubing to make the inner aqueous droplets (water stabilised with SDS), a second PTFE tubing to encapsulate them in oil (FC-40 stabilised with PFO), and a glass capillary to form the double emulsions in a surrounding aqueous phase (water stabilised with SDS). Reproduced from S. Farley et al.109 with permission from the Royal Society of Chemistry. | ||
To demonstrate the versatility of their platform, they showed water-in-oil-in-water, oil-in-water-in-oil and oil-in-oil-in-water multicompartmental double emulsions with between 1 and 10 inner droplets. The junction boxes are designed to hold glass capillaries and PTFE tubing in place and hence there is no need to manually align or glue the capillaries as with other microcapillary platforms.81,110 In all cases, inexpensive off-the-shelf surfactants such as SDS to stabilise the water phases, and 1H,1H,2H,2H-perfluoro-1-octanol (PFO) to stabilise the oil phases are used to create the multiple emulsions. They also show the formation of binary water-in-oil-in-water multicompartmental double emulsions with predetermined combinations of two different types of inner droplets (Fig. 6g–j). This means that with this microcapillary platform complex multicompartmental droplet emulsions can be built using readily available components that do not require expertise to assemble and operate.
4e. Interfacing microwells with nanolitre droplets for library screening applications
A key advantage often cited for droplet microfluidics is the possibility to perform reactions in a massively parallel format. Traditional droplet formation such as flow-focusing devices allow the generation of very large numbers of droplets at high rates, however, such large numbers of droplets are less useful for experiments in which small libraries (e.g. drug compounds), typically stored in microtiter plates, are to be screened individually. In these instances, droplet-on-demand platforms have been developed to provide a low-throughput alternative whereby droplets can be sampled from multiple wells in sequence. The Gielen Lab is developing similar interfaces that permit rapid screening of small compound libraries (i.e. kept in 96 or 384 well plates), in an individual or combinatorial manner, combining the on-demand access of different samples with the droplet-based advantages of low reagent consumption and statistical averaging from multiple droplets.Gielen and co-workers previously developed an unsupervised platform to screen enzyme substrates and inhibitors kept in microwells (∼20 μL) that yielded high-quality dose–response curves from up to 24 individual compounds.111 Their strategy was to compartmentalise enzymes, substrates and inhibitors in droplets kept in sequence, relying on spatial encoding for droplet identification. In practice this was achieved using a two-stage process comprised of a tubing-based platform to generate the droplets and a chip to process the droplets. Droplets were produced by aspiration (Fig. 7A) using a tubing inlet that moved alternately between oil and sample while connected to a negative pressure source. This is a convenient way to achieve controlled, stable production albeit at low throughputs (<10 Hz)46 and results in the generation of a confined drop every cycle.46 There were several requirements for the tubing material: firstly the continuous phase (FC-40) had to preferentially wet the tubing to avoid any contamination between aqueous samples. Secondly, as a UV-vis absorbance-based method was used to analyse the droplets, the tubing needed to be optically transparent. Thirdly it had to be mechanically resilient enough to allow being squeezed and pulled through a hook-shaped stainless steel guide tube that held the PTFE tube and moved it vertically. Consequently, they settled on a microbore PTFE tubing which had the required superhydrophobic surface, had walls thin enough to be effectively transparent, and was soft enough to be threaded through the stainless steel guide.
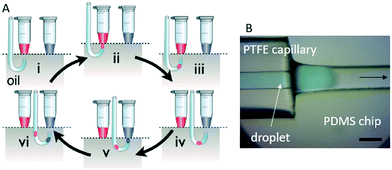 | ||
| Fig. 7 A) Capillary-based droplet generation by aspiration. During all steps of operation, the PTFE tubing is aspirating liquid at a constant rate. (i) The tip of the tubing is aligned with a given sample. (ii) The tip is lifted so that it sits in the aqueous phase of sample 1 (red). (iii) The tip returns to the oil phase. The change from aqueous to oil phase creates a microcompartment containing a controlled quantity of sample 1 (red). (iv) The tip is aligned below a second sample. (v) The tip is lifted analogously to step (i), but now sample 2 (blue) is taken up. (vi) The tip comes back to the carrier fluid. As a result of this process, a sequence of microdroplets with defined contents (sample 1, red; sample 2, blue) emerges in the tubing in a pre-planned order. Reproduced from F. Gielen et al.46 under a CC BY 4.0 licence. B) Interfacing with PDMS devices. A custom-made side channel allows capillary insertion and transitioning to a microchannel. The scalebar represents 200 μm. | ||
While production of arbitrary sequences of droplets is not easily done on-chip, chips are much better suited to complex, sequential droplet operations which require complex channel architectures. To enable one-to-one droplet fusion and serial droplet dilution, the droplet-containing tubing was therefore connected to specially designed PDMS microfluidic chips (Fig. 7B). The chips were fabricated using stereolithography, bonded to thin PDMS layers via oxygen plasma and then the channels were surface modified using a fluorosilane dissolved in fluorinated oil. PDMS was used as the chip material as it had the required deformability that allowed easy insertion and sealing of PTFE tubing. The chips were designed with a side-port in which the tubing could be inserted until contact with the end of a pre-designed channel. The side connection is essential to preserve the spatial arrangement of droplets and provides a convenient way to monitor transfer between tubing and the device (Fig. 7B). The PDMS–capillary interface was made permanent using silicone sealants which solidified to create a mechanically solid seal. Thanks to this connection, they could demonstrate added functionality such as droplet dilution and fusion, expanding the capabilities and analytical throughput of the platform.
5. Future perspectives
We end by highlighting several areas where changes in material usage and development of new techniques are anticipated to lead to changes in the way researchers fabricate droplet microfluidic devices in the future.5a. 3D printed microfluidic devices
As noted above, 3D printed microfluidic devices feature highly in recent microfluidic publications. While resolution limits mean 3D printing is unlikely to become the go-to fabrication method for most researchers (at least not in the short to medium term), it is likely to continue to be a highly popular fabrication method. The maturity of printing technologies has led to decreasing costs and widespread adoption. This increasing popular uptake has a reciprocal effect in further developing the technology and the wider commercial industry behind it. Accordingly, it is likely that 3D printing will continue to be a popular fabrication method, driven by the ease and low cost of manufacture which, as highlighted earlier, has the potential to democratise microfluidics by allowing a wider pool of researchers to fabricate microfluidic devices.For 3D printed fabrication to have maximum utility for droplet microfluidics, we would hope that in future cost improvements are also accompanied by technical improvements that allow more material choices with good feature sizes. Of the two most popular and accessible methods, fused deposition modelling (FDM) and stereolithography (SL), FDM offers a broad range of commercially available materials, including fluoropolymers, but most standard FDM printers struggle to reliably produce channels below 500 μm. SL conversely offers channel sizes down to ∼100 μm,112 but suffers from a much narrower range of potential materials. As reliably defined channel sizes and channel surface chemistries are both paramount to droplet microfluidics, the use of 3D printing is likely to continue to increase, but will become truly valuable when low feature sizes and a wide range of materials can be combined within an affordable printer.
5b. Restriction of PFAS (per/poly-fluoroalkyl substances)
Droplet microfluidics makes routine use of fluorinated substances, be it in fluorocarbon carrier fluids, fluoroalkylsilane-derived surface coatings, surfactants, and/or fluoropolymer device materials. The environmental persistence of PFAS has become increasingly apparent over recent years. Consequently there has been a legislative push to restrict their use113,114 with legislation already addressing PFAS in fire extinguishing foams, and food contact paper and cardboard, for example.115,116 While legal moves to restrict PFAS will focus on applications with the greatest usage and highest environmental impact, it seems unlikely that microfluidics will be immediately affected by legislation. However the long-term direction of travel is clear and should be a consideration for those wishing to commercialise microfluidic technology. It also raises the question whether the microfluidic community should be devoting more effort to investigating alternative materials that provide similar performance with less environmental impact.5c. Standard microfluidic modules
Microfluidic devices should ideally be tools that any laboratory could use without needing to have access to specialist fabrication techniques or knowledge, so that more scientists can make use of the technique. This would be aided if standard microfluidic modules for set operations (such as droplet generation, incubation, dosing, optical analysis etc.) were easily available and could be combined as required for a given application. Standardisation would promote availability as it would aid mass production117 however for this to happen the microfluidic devices would also need to be made from materials with the required material and surface properties for the targeted application and suitable for simple large scale production.A recent example of an approach to address standardisation is the work of Owens and Hart,118 who used micromilling to pattern store-bought LEGO bricks (made by standard injection moulding) to create LEGO-like blocks that contained microchannels. Each type of block could achieve different functions, such as fluid mixing and droplet generation, and could be reconfigurably fitted together for different sequential fluid operations. Such an approach to standardisation is innovative with injection moulding as a fabrication technique having the advantage that it can be used to pattern the microfluidic channels, works with a wide variety of polymers (such as PS and acrylonitrile butadiene styrene), is suitable for mass production, and results in smooth surfaces and small tolerances. One potential disadvantage is that these materials, like other thermoplastics, are generally incompatible with organic solvents but this could be rectified by coating with a resistant material like parylene-C, as the authors demonstrated.
3D printing (as described in the previous section) offers a different potential approach to achieving standardisation, whereby set designs can be shared easily, 3D printed and combined as required. Other approaches to standardisation are also being proposed by researchers, however more innovations from the microfluidics community will be needed to truly achieve useful standardisation.
5d. Surfactant innovation
In one of the example applications above we touched on how droplet microfluidics would benefit by being available to a wider range of researchers. One such area where this is an issue is in the surfactants which are typically used. Currently, the most reliable surfactants are commercially produced, but they are expensive, and suppliers do not provide detailed information on what exactly is in the bottle. This limits how easily researchers in resource-limited settings can use them and provides a barrier to the development of new droplet-based assays. A significant advance in this field would be the development of a range of inexpensive surfactants designed for specific applications, from cell culture to chemical synthesis.Surfactants also have potential in terms of providing extra functionality in a droplet-based system, if surfactants could be used as active surfaces to enhance the application rather than just to stabilise the droplets. For example, surfactants could be synthesised to include catalysts or reporter molecules for reactions taking place within the droplet, or to immobilise cells on the droplet surface.
5e. Hybrid material devices
Incorporation of functional materials within the microfluidic device allows fabrication of hybrid devices that can perform complex functions. For example, indium tin oxide coated glass is frequently used for patterning planar electrodes inducing dielectrophoretic forces,119 while piezoelectric substrates120 (e.g. LiNbO3) are used for generating surface acoustic waves. As other functional materials are developed there is significant scope to create new and innovative devices. Light-sensitive polymers appear especially promising as they can display reversible hydrophobicity/hydrophilicity121 so that one could imagine on-demand patterning of chip areas with precisely controlled surface energy to unlock novel applications such as the creation of multiple emulsions (e.g. more than three) in a single device, generation of hydrophilic spots for creating detachable sessile droplets, or configurable droplet extraction to liquid phase without the need for electrodes. Likewise, the recent trend in liquid-metal based microfluidics using low-melting point metals122 is likely to apply to the droplet field to create electro-fluidic devices. These allow the creation of devices made entirely with flexible materials but also can be used to design components such as pumps, heaters, or valves, adding a range of low power functions to create fully embedded systems.6. Conclusion
With various different potential native surfaces, surface modification techniques, and channel geometry options, there are a range of strategies to deliver microfluidic devices that provide reliable droplet flow. While there are often several potential different fabrication routes to a device that fulfils the required performance criteria, it is important to think holistically; ultimately the fabrication route chosen should also take account of the complexity and reproducibility of the fabrication process. Indeed, a consistent theme of the example devices given above is that devices should only be as complex as they need to be, with fewer and simpler fabrication steps reducing failure modes, time, and cost. The range of possible fabrication options will continue to increase over time. New techniques, such as the growth of 3D printing offer new routes to successful devices and mean that microfluidic devices are becoming, and will hopefully continue to become, more accessible to a wider range of researchers. As a consequence, we expect the popularity of droplet microfluidics to be sustained into the future and newcomers to the field to catalyse droplet-based research in new and unexpected directions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
KSE's position is funded by the Canada Research Chairs program and the Michael Smith Foundation for Health Research in partnership with the Pacific Alzheimer Research Foundation. FG has received funding from the Biotechnology and Biological Sciences Research Council (grant BB/T011777/1) and the European Union's Horizon 2020 research and innovation programme (grant agreement No. 101000560). SSHT is thankful for support from the Government of Canada's Natural Sciences and Engineering Research Council (NSERC), Discovery Grants program (RGPIN-2019-04618). AMN is supported by the Natural Environment Research Council via an Industrial Innovation Fellowship (NE/R013578/1) and the Signals in the Soil program (NE/T010584/1).References
- A. J. deMello, Nature, 2006, 442, 394–402 CrossRef CAS PubMed.
- K. S. Elvira, X. Casadevalli i Solvas, R. C. R. Wootton and A. J. deMello, Nat. Chem., 2013, 5, 905–915 CrossRef CAS PubMed.
- H. Song and R. F. Ismagilov, J. Am. Chem. Soc., 2003, 125, 14613–14619 CrossRef CAS PubMed.
- J. H. Bannock, S. H. Krishnadasan, A. M. Nightingale, C. P. Yau, K. Khaw, D. Burkitt, J. J. M. Halls, M. Heeney and J. C. de Mello, Adv. Funct. Mater., 2013, 23, 2123–2129 CrossRef CAS.
- I. Shestopalov, J. D. Tice and R. F. Ismagilov, Lab Chip, 2004, 4, 316–321 RSC.
- J. J. Agresti, E. Antipov, A. R. Abate, K. Ahn, A. C. Rowat, J.-C. Baret, M. Marquez, A. M. Klibanov, A. D. Griffiths and D. A. Weitz, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4004–4009 CrossRef CAS PubMed.
- C. N. Baroud, F. Gallaire and R. Dangla, Lab Chip, 2010, 10, 2032–2045 RSC.
- G. F. Christopher and S. L. Anna, J. Phys. D: Appl. Phys., 2007, 40, R319–R336 CrossRef CAS.
- S. W. Hu, X. Q. Ren, M. Bachman, C. E. Sims, G. P. Li and N. Allbritton, Anal. Chem., 2002, 74, 4117–4123 CrossRef CAS PubMed.
- A. P. Debon, R. C. R. Wootton and K. S. Elvira, Biomicrofluidics, 2015, 9, 024119 CrossRef PubMed.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550–2583 CrossRef CAS PubMed.
- J. B. Nielsen, R. L. Hanson, H. M. Almughamsi, C. Pang, T. R. Fish and A. T. Woolley, Anal. Chem., 2020, 92, 150–168 CrossRef CAS PubMed.
- K. Ren, J. Zhou and H. Wu, Acc. Chem. Res., 2013, 46, 2396–2406 CrossRef CAS PubMed.
- S. Bammesberger, A. Ernst, N. Losleben, L. Tanguy, R. Zengerle and P. Koltay, Drug Discovery Today, 2013, 18, 435–446 CrossRef CAS PubMed.
- P. Ben-Tzvi and W. Rone, Microsyst. Technol., 2010, 16, 333–356 CrossRef.
- K. Choi, A. H. C. Ng, R. Fobel and A. R. Wheeler, Annu. Rev. Anal. Chem., 2012, 5, 413–440 CrossRef CAS PubMed.
- E. Samiei, M. Tabrizian and M. Hoorfar, Lab Chip, 2016, 16, 2376–2396 RSC.
- A. Waldbaur, H. Rapp, K. Länge and B. E. Rapp, Anal. Methods, 2011, 3, 2681–2716 RSC.
- A.-G. Niculescu, C. Chircov, A. C. Bîrcă and A. M. Grumezescu, Int. J. Mol. Sci., 2021, 22, 2011 CrossRef CAS PubMed.
- S. M. Scott and Z. Ali, Micromachines, 2021, 12, 319 CrossRef PubMed.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef CAS PubMed.
- Y. Xia and G. M. Whitesides, Annu. Rev. Mater. Sci., 1998, 28, 153–184 CrossRef CAS.
- V. Sunkara, D.-K. Park, H. Hwang, R. Chantiwas, S. A. Soper and Y.-K. Cho, Lab Chip, 2011, 11, 962–965 RSC.
- H. Becker and C. Gärtner, Anal. Bioanal. Chem., 2008, 390, 89–111 CrossRef CAS PubMed.
- D. J. Guckenberger, T. E. de Groot, A. M. D. Wan, D. J. Beebe and E. W. K. Young, Lab Chip, 2015, 15, 2364–2378 RSC.
- J. C. McDonald, D. C. Duffy, J. R. Anderson, D. T. Chiu, H. K. Wu, O. J. A. Schueller and G. M. Whitesides, Electrophoresis, 2000, 21, 27–40 CrossRef CAS PubMed.
- S. S. Deshmukh and A. Goswami, Mater. Manuf. Processes, 2021, 36, 501–543 CrossRef CAS.
- U. M. Attia, S. Marson and J. R. Alcock, Microfluid. Nanofluid., 2009, 7, 1 CrossRef CAS.
- K. Sugioka, J. Xu, D. Wu, Y. Hanada, Z. Wang, Y. Cheng and K. Midorikawa, Lab Chip, 2014, 14, 3447–3458 RSC.
- C. G. Khan Malek, Anal. Bioanal. Chem., 2006, 385, 1351–1361 CrossRef CAS PubMed.
- S. Waheed, J. M. Cabot, N. P. Macdonald, T. Lewis, R. M. Guijt, B. Paull and M. C. Breadmore, Lab Chip, 2016, 16, 1993–2013 RSC.
- F. Li, N. P. Macdonald, R. M. Guijt and M. C. Breadmore, Lab Chip, 2019, 19, 35–49 RSC.
- J. Hwang, Y. H. Cho, M. S. Park and B. H. Kim, Int. J. Precis. Eng. Manuf., 2019, 20, 479–495 CrossRef.
- A. J. G. Otuka, N. B. Tomazio, K. T. Paula and C. R. Mendonça, Polymer, 2021, 13, 1994 CAS.
- T. W. Lim, Y. Son, Y. J. Jeong, D.-Y. Yang, H.-J. Kong, K.-S. Lee and D.-P. Kim, Lab Chip, 2011, 11, 100–103 RSC.
- L. Amato, Y. Gu, N. Bellini, S. M. Eaton, G. Cerullo and R. Osellame, Lab Chip, 2012, 12, 1135–1142 RSC.
- I. Bilican and M. Tahsin Guler, Appl. Surf. Sci., 2020, 534, 147642 CrossRef CAS.
- S. Su, G. Jing, M. Zhang, B. Liu, X. Zhu, B. Wang, M. Fu, L. Zhu, J. Cheng and Y. Guo, Sens. Actuators, B, 2019, 282, 60–68 CrossRef CAS.
- S. A. Aghvami, A. Opathalage, Z. K. Zhang, M. Ludwig, M. Heymann, M. Norton, N. Wilkins and S. Fraden, Sens. Actuators, B, 2017, 247, 940–949 CrossRef CAS.
- W. Li, Z. Nie, H. Zhang, C. Paquet, M. Seo, P. Garstecki and E. Kumacheva, Langmuir, 2007, 23, 8010–8014 CrossRef CAS PubMed.
- I. R. Durán and G. Laroche, Prog. Mater. Sci., 2019, 99, 106–186 CrossRef.
- D. Bodas and C. Khan-Malek, Sens. Actuators, B, 2007, 123, 368–373 CrossRef CAS.
- W. S. Y. Wong, L. Hauer, A. Naga, A. Kaltbeitzel, P. Baumli, R. Berger, M. D'Acunzi, D. Vollmer and H.-J. Butt, Langmuir, 2020, 36, 7236–7245 CrossRef CAS PubMed.
- C. Zilio, L. Sola, F. Damin, L. Faggioni and M. Chiari, Biomed. Microdevices, 2014, 16, 107–114 CrossRef CAS PubMed.
- V. Sahore, S. R. Doonan and R. C. Bailey, Anal. Methods, 2018, 10, 4264–4274 RSC.
- F. Gielen, L. van Vliet, B. T. Koprowski, S. R. A. Devenish, M. Fischlechner, J. B. Edel, X. Niu, A. J. deMello and F. Hollfelder, Anal. Chem., 2013, 85, 4761–4769 CrossRef CAS PubMed.
- A. C. Sun, D. J. Steyer, A. R. Allen, E. M. Payne, R. T. Kennedy and C. R. J. Stephenson, Nat. Commun., 2020, 11, 6202 CrossRef CAS PubMed.
- K. Ren, W. Dai, J. Zhou, J. Su and H. Wu, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8162–8166 CrossRef CAS PubMed.
- M. Horka, S. Sun, A. Ruszczak, P. Garstecki and T. Mayr, Anal. Chem., 2016, 88, 12006–12012 CrossRef CAS PubMed.
- N. Aboud, D. Ferraro, M. Taverna, S. Descroix, C. Smadja and N. T. Tran, Analyst, 2016, 141, 5776–5783 RSC.
- A. M. Nightingale, S.-u. Hassan, K. Makris, W. T. Bhuiyan, T. J. Harvey and X. Niu, RSC Adv., 2020, 10, 30975–30981 RSC.
- A. H. McMillan, J. Mora-Macías, J. Teyssandier, R. Thür, E. Roy, I. Ochoa, S. De Feyter, I. F. J. Vankelecom, M. B. J. Roeffaers and S. C. Lesher-Pérez, Nano Sel., 2021, 2, 1385–1402 CrossRef CAS.
- I. Morita, Y. Ando and Y. J. Heo, J. Adv. Mech. Des. Syst. Manuf., 2017, 11, JAMDSM0031 CrossRef.
- I. Wong and C.-M. Ho, Microfluid. Nanofluid., 2009, 7, 291 CrossRef CAS PubMed.
- H. Makamba, J. H. Kim, K. Lim, N. Park and J. H. Hahn, Electrophoresis, 2003, 24, 3607–3619 CrossRef CAS PubMed.
- T. Trantidou, Y. Elani, E. Parsons and O. Ces, Microsyst. Nanoeng., 2017, 3, 16091 CrossRef CAS PubMed.
- M. J. Owen and P. J. Smith, J. Adhes. Sci. Technol., 1994, 8, 1063–1075 CrossRef CAS.
- M. Lenz, B. Sebastian and P. S. Dittrich, Small, 2019, 15, 1901547 CrossRef PubMed.
- F. Jahangiri, T. Hakala and V. Jokinen, Microfluid. Nanofluid., 2019, 24, 2 CrossRef.
- S. K. Nemani, R. K. Annavarapu, B. Mohammadian, A. Raiyan, J. Heil, M. A. Haque, A. Abdelaal and H. Sojoudi, Adv. Mater. Interfaces, 2018, 5, 1801247 CrossRef.
- U. Srinivasan, M. R. Houston, R. T. Howe and R. Maboudian, J. Microelectromech. Syst., 1998, 7, 252–260 CrossRef CAS.
- Gelest Inc, Silane Coupling Agents - Connecting Across Boundaries, https://www.gelest.com/wp-content/uploads/Silane_Coupling_Agents.pdf, 2014.
- J. H. L. Beal, A. Bubendorfer, T. Kemmitt, I. Hoek and W. M. Arnold, Biomicrofluidics, 2012, 6, 036503 CrossRef PubMed.
- A. B. Theberge, G. Whyte and W. T. S. Huck, Anal. Chem., 2010, 82, 3449–3453 CrossRef CAS PubMed.
- A. R. Abate, D. Lee, T. Do, C. Holtze and D. A. Weitz, Lab Chip, 2008, 8, 516–518 RSC.
- C. T. Riche, C. Zhang, M. Gupta and N. Malmstadt, Lab Chip, 2014, 14, 1834–1841 RSC.
- T. Yang, J. Choo, S. Stavrakis and A. de Mello, Chem. – Eur. J., 2018, 24, 12078–12083 CrossRef CAS PubMed.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1–8 CrossRef CAS.
- S. Wang, X. Yang, F. Wu, L. Min, X. Chen and X. Hou, Small, 2020, 16, 1905318 CrossRef CAS PubMed.
- Y. Zuo, L. Zheng, C. Zhao and H. Liu, Small, 2020, 16, 1903849 CrossRef CAS PubMed.
- R. Ortiz, J. L. Chen, D. C. Stuckey and T. W. J. Steele, ACS Appl. Mater. Interfaces, 2017, 9, 13801–13811 CrossRef CAS PubMed.
- R. Ortiz, J. L. Chen, D. C. Stuckey and T. W. J. Steele, Micro Nano Eng., 2019, 2, 92–103 CrossRef.
- R. Ortiz, D. C. Stuckey and T. W. J. Steele, Micro Nano Eng., 2019, 3, 82–91 CrossRef.
- J.-C. Baret, Lab Chip, 2012, 12, 422–433 RSC.
- J. H. Xu, S. W. Li, J. Tan, Y. J. Wang and G. S. Luo, Langmuir, 2006, 22, 7943–7946 CrossRef CAS PubMed.
- B. Riechers, F. Maes, E. Akoury, B. Semin, P. Gruner and J.-C. Baret, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11465–11470 CrossRef CAS PubMed.
- Z. Li, A. M. Leshansky, L. M. Pismen and P. Tabeling, Lab Chip, 2015, 15, 1023–1031 RSC.
- S. ten Klooster, S. Sahin and K. Schroën, Sci. Rep., 2019, 9, 7820 CrossRef CAS PubMed.
- M. Seo, C. Paquet, Z. Nie, S. Xu and E. Kumacheva, Soft Matter, 2007, 3, 986–992 RSC.
- J. K. Nunes, S. S. H. Tsai, J. Wan and H. A. Stone, J. Phys. D: Appl. Phys., 2013, 46, 114002 CrossRef PubMed.
- R. K. Shah, H. C. Shum, A. C. Rowat, D. Lee, J. J. Agresti, A. S. Utada, L.-Y. Chu, J.-W. Kim, A. Fernandez-Nieves, C. J. Martinez and D. A. Weitz, Mater. Today, 2008, 11, 18–27 CrossRef CAS.
- M. Jeyhani, R. Thevakumaran, N. Abbasi, D. K. Hwang and S. S. H. Tsai, Small, 2020, 16, 1906565 CrossRef CAS PubMed.
- M. Jeyhani, V. Gnyawali, N. Abbasi, D. K. Hwang and S. S. H. Tsai, J. Colloid Interface Sci., 2019, 553, 382–389 CrossRef CAS PubMed.
- M. Navi, N. Abbasi, M. Jeyhani, V. Gnyawali and S. S. H. Tsai, Lab Chip, 2018, 18, 3361–3370 RSC.
- M. B. Romanowsky, A. R. Abate, A. Rotem, C. Holtze and D. A. Weitz, Lab Chip, 2012, 12, 802–807 RSC.
- Z. Nie, S. Xu, M. Seo, P. C. Lewis and E. Kumacheva, J. Am. Chem. Soc., 2005, 127, 8058–8063 CrossRef CAS PubMed.
- L. Y. Chu, A. S. Utada, R. K. Shah, J. W. Kim and D. A. Weitz, Angew. Chem., Int. Ed., 2007, 46, 8970–8974 CrossRef CAS PubMed.
- K. van Dijke, G. Veldhuis, K. Schroën and R. Boom, Lab Chip, 2009, 9, 2824–2830 RSC.
- Z. Shi, X. Lai, C. Sun, X. Zhang, L. Zhang, Z. Pu, R. Wang, H. Yu and D. Li, Chem. Commun., 2020, 56, 9056–9066 RSC.
- L. Shang, Y. Cheng and Y. Zhao, Chem. Rev., 2017, 117, 7964–8040 CrossRef CAS PubMed.
- S. Sohrabi, N. Kassir and M. Keshavarz Moraveji, RSC Adv., 2020, 10, 27560–27574 RSC.
- T. S. Kaminski and P. Garstecki, Chem. Soc. Rev., 2017, 46, 6210–6226 RSC.
- K. Matuła, F. Rivello and W. T. S. Huck, Adv. Biosyst., 2020, 4, 1900188 CrossRef PubMed.
- H. Kleine-Brüggeney, L. D. van Vliet, C. Mulas, F. Gielen, C. C. Agley, J. C. R. Silva, A. Smith, K. Chalut and F. Hollfelder, Small, 2019, 15, 1804576 CrossRef PubMed.
- S. Allazetta and M. P. Lutolf, Curr. Opin. Biotechnol., 2015, 35, 86–93 CrossRef CAS PubMed.
- E. W. K. Young and D. J. Beebe, Chem. Soc. Rev., 2010, 39, 1036–1048 RSC.
- A. M. Nightingale, S.-u. Hassan, B. M. Warren, K. Makris, G. W. H. Evans, E. Papadopoulou, S. Coleman and X. Niu, Environ. Sci. Technol., 2019, 53, 9677–9685 CrossRef CAS PubMed.
- A. M. Nightingale, A. D. Beaton and M. C. Mowlem, Sens. Actuators, B, 2015, 221, 1398–1405 CrossRef CAS.
- A. M. Nightingale, G. W. H. Evans, P. X. Xu, B. J. Kim, H. Sammer-ul and X. Z. Niu, Lab Chip, 2017, 17, 1149–1157 RSC.
- A. M. Nightingale, C. L. Leong, R. A. Burnish, S.-u. Hassan, Y. Zhang, G. F. Clough, M. G. Boutelle, D. Voegeli and X. Niu, Nat. Commun., 2019, 10, 2741 CrossRef PubMed.
- A. M. Nightingale, S.-u. Hassan, G. W. H. Evans, S. M. Coleman and X. Niu, Lab Chip, 2018, 18, 1903–1913 RSC.
- Y. Chao and H. C. Shum, Chem. Soc. Rev., 2020, 49, 114–142 RSC.
- Y. S. Huh, S. J. Jeon, E. Z. Lee, H. S. Park and W. H. Hong, Korean J. Chem. Eng., 2011, 28, 633–642 CrossRef CAS.
- J. A. De Lora, F. A. Fencl, A. D. Y. Macias Gonzalez, A. Bandegi, R. Foudazi, G. P. Lopez, A. P. Shreve and N. J. Carroll, ACS Appl. Bio Mater., 2019, 2, 4097–4105 CrossRef CAS PubMed.
- I. Ziemecka, V. van Steijn, G. J. M. Koper, M. Rosso, A. M. Brizard, J. H. van Esch and M. T. Kreutzer, Lab Chip, 2011, 11, 620–624 RSC.
- B.-U. Moon, N. Abbasi, S. G. Jones, D. K. Hwang and S. S. H. Tsai, Anal. Chem., 2016, 88, 3982–3989 CrossRef CAS PubMed.
- H. C. Shum, J. Varnell and D. A. Weitz, Biomicrofluidics, 2012, 6, 012808 CrossRef PubMed.
- D. Sinton and S. O. Kelley, Lab Chip, 2021, 21, 2330–2332 RSC.
- S. Farley, K. Ramsay and K. S. Elvira, Lab Chip, 2021, 21, 2781–2790 RSC.
- Y. Iwasa, K. Yamanoi, Y. Kaneyasu and T. Norimatsu, Fusion Sci. Technol., 2018, 73, 258–264 CrossRef.
- F. Gielen, T. Buryska, L. Van Vliet, M. Butz, J. Damborsky, Z. Prokop and F. Hollfelder, Anal. Chem., 2015, 87, 624–632 CrossRef CAS PubMed.
- N. P. Macdonald, J. M. Cabot, P. Smejkal, R. M. Guijt, B. Paull and M. C. Breadmore, Anal. Chem., 2017, 89, 3858–3866 CrossRef CAS PubMed.
- C. F. Kwiatkowski, D. Q. Andrews, L. S. Birnbaum, T. A. Bruton, J. C. DeWitt, D. R. U. Knappe, M. V. Maffini, M. F. Miller, K. E. Pelch, A. Reade, A. Soehl, X. Trier, M. Venier, C. C. Wagner, Z. Wang and A. Blum, Environ. Sci. Technol. Lett., 2020, 7, 532–543 CrossRef CAS PubMed.
- I. T. Cousins, G. Goldenman, D. Herzke, R. Lohmann, M. Miller, C. A. Ng, S. Patton, M. Scheringer, X. Trier, L. Vierke, Z. Wang and J. C. DeWitt, Environ. Sci.: Processes Impacts, 2019, 21, 1803–1815 RSC.
- Bekendtgørelse om fødevarekontaktmaterialer og om straffebestemmelser for overtrædelse af relaterede EU-retsakter, https://www.retsinformation.dk/eli/lta/2020/681, 2020.
- State of Maine - An Act To Protect the Environment and Public Health by Further Reducing Toxic Chemicals in Packaging, https://www.maine.gov/dep/safechem/packaging/LD1433-PL277.pdf, 2019.
- D. R. Reyes, H. van Heeren, S. Guha, L. Herbertson, A. P. Tzannis, J. Ducrée, H. Bissig and H. Becker, Lab Chip, 2021, 21, 9–21 RSC.
- C. E. Owens and A. J. Hart, Lab Chip, 2018, 18, 890–901 RSC.
- X. Niu, F. Gielen, A. J. deMello and J. B. Edel, Anal. Chem., 2009, 81, 7321–7325 CrossRef CAS PubMed.
- T. Franke, A. R. Abate, D. A. Weitz and A. Wixforth, Lab Chip, 2009, 9, 2625–2627 RSC.
- E. Rossegger, D. Nees, S. Turisser, S. Radl, T. Griesser and S. Schlögl, Polym. Chem., 2020, 11, 3125–3135 RSC.
- L. Zhu, B. Wang, S. Handschuh-Wang and X. Zhou, Small, 2020, 16, 1903841 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |