A survey of 3D printing technology applied to paper microfluidics
Elain
Fu†
 * and
Lael
Wentland†
* and
Lael
Wentland†

School of Chemical, Biological, and Environmental Engineering, Oregon State University, Corvallis, OR 97331, USA. E-mail: elain.fu@oregonstate.edu
First published on 13th December 2021
Abstract
Paper microfluidics is a rapidly growing subfield of microfluidics in which paper-like porous materials are used to create analytical devices that are well-suited for use in field applications. 3D printing technology has the potential to positively affect paper microfluidic device development by enabling tools and methods for the creation of devices with well-defined and tunable fluidic networks of porous matrices for high performance signal generation. This critical review focuses on the progress that has been made in using 3D printing technologies to advance the development of paper microfluidic devices. We describe printing work in three general categories: (i) solid support structures for paper microfluidic device components; (ii) channel barrier definition in existing porous materials; and (iii) porous channels for capillary flow, and discuss their value in advancing paper microfluidic device development. Finally, we discuss major areas of focus for highest impact on the next generation of paper microfluidics devices.
Introduction
Paper microfluidics, the subfield focused on the use of porous materials to create field-usable devices, has been recognized as an excellent platform for time-critical health and environmental testing outside of a laboratory setting. The porous nature of the materials is a key feature that provides flow. Specifically, capillarity provides transport of liquid samples, and the reagents within, without external pumps and in an automated fashion. By removing the need for trained technicians or expensive pumping equipment, the devices are suitable for point-of-use applications. Critical progress has been made, since the 2007 demonstration by the Whitesides group of multianalyte detection in paper,1 on adding functionality to porous materials-based devices by developing fabrication methods to enable more complex 3D channel geometries,2 and flow control tools3,4 for multi-step processing.5,6 However, a main challenge of developing effective point-of-use devices for a specific application is achieving the required robust, analytical performance, while balancing the other application needs with respect to operator ease of use, cost, and manufacturability. 3D printing technology has the potential to positively affect paper microfluidic device development by enabling tools and methods for multi-layer fluidic channel definition, new porous materials and non-porous supporting components, and methods for the seamless integration of multiple device components, thereby extending device capabilities and manufacturability.3D printing is an additive manufacturing technology that builds an object from a digital model by joining materials layer by layer to create a three-dimensional structure. This technology has already been established as a tool in the fabrication of classic microfluidic devices, i.e., the 3D printing of solid structures with small open fluidic channels. A review by Nielsen et al. is suggested for an overview of recent developments regarding 3D printed classic microfluidic devices from 2012–2019,7 and a review by Naderi et al. from 2019 provides an analysis of the advantages and limitations of different 3D printing technologies used in conventional microfluidic fabrication.8 Common themes in the demonstrations of 3D printing for conventional microfluidics so far include the relative speed of 3D printing for prototyping devices compared to conventional methods (e.g., lithography), and the restricted applicability of 3D printing to devices intended for larger volume processing (for a useful discussion of 3D printing resolution and cost, see ref. 8). Regarding the latter, as the technology progresses, it is expected that higher resolution printers will become more accessible and enable smaller width features for applications that require it.
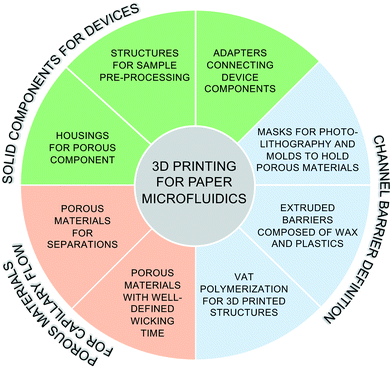
This work aims to survey the recent literature on 3D printing technology applied to support the development of paper microfluidic devices. Past work can be divided into three categories (Fig. 1): (I) 3D printing of solid components for paper microfluidic devices, including housings for the porous components, structures for sample pre-processing or detection, and adapters connecting device components; (II) 3D printing to define channel barriers in existing porous materials, including masks and molds to support barrier definition, and the creation of hydrophobic barriers; and (III) 3D printing (and related printing technology) of porous structures directly. Before exploring this work, we briefly describe the three categories of 3D printing technologies relevant to this review.
Review of 3D printing categories relevant to this discussion
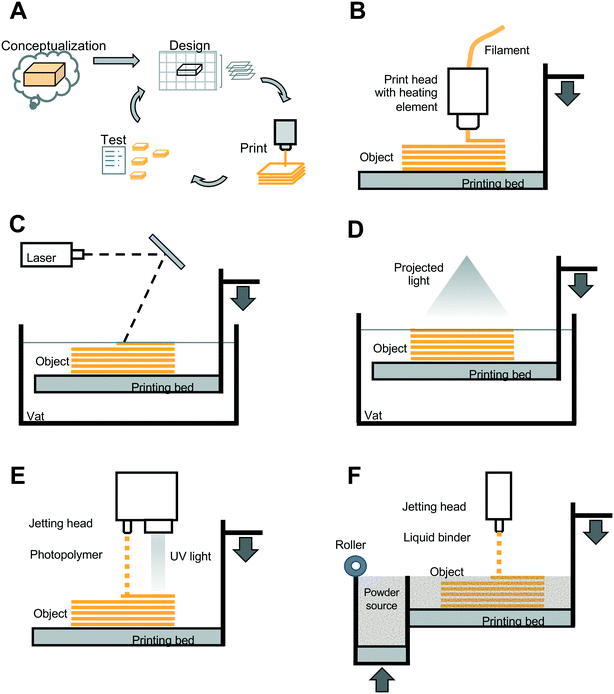
3D printing technology is well known for its effective prototyping cycle in which one can move rapidly from conceptualization, through design, printing, testing, and then redesign iterations as needed (Fig. 2A). The 3D printing categories that have been applied to paper microfluidic devices discussed in this review include (i) material extrusion, (ii) vat photopolymerization, and (iii) jetting-based methods (note that there are several other categories of 3D printing technologies and basic overviews of these can be found in multiple online resources9–11). In material extrusion (also referred to as fused deposition modeling (FDM) or fused filament fabrication (FFF)), thermoplastic material in the form of a long fiber or “filament” is heated to form a liquid, and is then dispensed from a nozzle, or “extruded” in a layer-by-layer manner to form a solid object upon cooling of the plastic (Fig. 2B). In contrast, in vat photopolymerization, a container of photocurable resin, i.e., a polymer in liquid form that cures or solidifies when exposed to an energy source, is selectively exposed to a light source in a layer-by-layer manner. Two forms of vat photopolymerization 3D printing include stereolithography (SLA), in which the focused light source, often a laser, essentially traces out the selective pattern to be cured within a given layer (Fig. 2C), and digital light projection (DLP), in which the light source is optically-manipulated to form the desired pattern for a given layer in a single step (Fig. 2D). In jetting-based methods, a third category of 3D printing technology, droplets of liquid are jetted from a printhead. Two types of jetting-based 3D printing include materials jetting and binder jetting. For materials jetting (Fig. 2E), a photocurable polymer is dispensed in droplets, and then subsequently exposed to UV light for curing in a layer-by-layer process. For binder jetting (Fig. 2F), a binder is dispensed selectively onto a layer of powder, and acts to fuse the powder in that layer. Powder is then smoothed over the fused layer, and binder is applied in the desired pattern for the next layer, and this process iterated until the print of the object is completed.Each 3D printing method has strengths and was developed for specific applications. Material extrusion is currently considered the most accessible form of 3D printing, with a lower cost and larger range of commercially-available materials. However, it cannot achieve the lower minimum feature sizes characteristic of SLA/DLP or jetting-based methods.12 Printer resolution, characterized by the minimum feature size that is printable, is a combination of the resolution in the XY direction (within a layer) and the minimum Z-axis layer height, and these parameters are dependent on the specific material, machine, and object shape used in the printing process. The material extrusion method has been noted by Chan et al. to have been used in the majority of academic papers designing point-of-use diagnostics.13 This may be due to the aforementioned accessibility of lower cost material extrusion-based printing systems that can provide a rapid turnaround for design iterations, and in particular, for device designs that may not require high resolution features. In the work reviewed below, in addition to materials extrusion, vat polymerization methods, jetting-based methods, and related customizations of those technologies have been used to advance paper microfluidic device development.
| Function | 3D printing method and material | Description |
|---|---|---|
| Housings for porous components | FDM extrusion 3D printing (Replicator 2X from Makerbot) using transparent and black ABS polymer15 | Cartridge for paper-based device with transparent region for viewing cholesterol detection zone15 |
| Stereolithography 3D printing (Freeform Pico from Asiga) using PlasCLEAR photopolymer25 | Housing to contain the paper component in a syringe-based assay for malaria protein detection25 | |
| Polyjet printing (Objet30 Scholar from Stratasys) using DuraWhitePlus26 | Housings for a porous material-based nucleic acid amplification device for the detection of methicillin-resistant Staphylococcus aureus bacteria26 | |
| Stereolithography 3D printing (Form2 from Formlabs) using white methacrylate polymer27 | Cartridge for a paper-based electrochemical sensor with an opening above the electrodes to serve as sample reservoir and a hinge for easy reuse for cholinesterase activity quantification27 | |
| FDM extrusion 3D printing using ABS polymer28 | Housing for a paper component that attached to a mouth guard for oral saliva collection, and detection of glucose and nitrite28 | |
| Structures for sample pre-processing | Polyjet printing (Objet30 Scholar from Stratasys) using DuraWhitePlus material26 | Lysis valve component for sample pre-processing in a device to detect methicillin-resistant Staphylococcus aureus bacteria26 |
| Stereolithography 3D printing using clear methacrylate-based polymer29 | Chamber for nucleic acid amplification upstream of paper-based component for SARS-CoV-2 detection29 | |
| Adapters connecting device components | FDM extrusion 3D printing (Replicator 2X from Makerbot) using ABS polymer15 | Smartphone camera adapter that interfaced with the 3D-printed housing of the paper-based component (see above)15 |
| 3D printing (instrument and material not specified)16 | Imaging box with integrated paper-based glucose test holder for colorimetric readout using a smartphone camera16 | |
| FDM extrusion 3D printing (Replicator 2X from MakerBot), material not specified17 | Adapter module (including a 3D printed component to hold the porous substrate) for smartphone camera-based fluorescence detection of avian influenza from a lateral flow assay17 | |
| FDM extrusion 3D printing (Einstart S from Shining 3D) using white and black PLA polymer18 | Adapter module (including a 3D printed component to hold the porous substrate) for smartphone data acquisition demonstrated in the context of a nanozyme-based lateral flow assay for the detection of bacteria18 | |
| FDM extrusion 3D printing (Makerbot) using black PLA polymer19,20 | Adapter for smartphone camera-based luminescence detection of human chorionic gonadotropin19 and prostate specific antigen20 | |
| FDM extrusion 3D printing (Ultimaker 3), material not specified21 | Adapter module (including a 3D printed component to hold the porous substrate) for smartphone camera-based detection of human cardiac troponin I using colorimetric labels21 | |
| FDM extrusion 3D printing (Ultimaker 3) with black and red PLA filament23 | Adapter module (including a 3D printed component to hold the porous substrate) for smartphone camera-based detection of thrombin and interleukin-6 using quantum dots as labels23 | |
| FDM extrusion 3D printing (TECHB V30) using PLA polymer22 | Smartphone camera imaging box with associated 3D printed component to hold the paper-based colorimetric hemoglobin test22 | |
| FDM extrusion 3D printing (LulzBot TAZ6), material not specified24 | Adapter for smartphone camera-based detection of bacteria using commercial lateral flow tests coupled with machine learning classifiers24 |
Roda et al. used material extrusion to 3D print a minicartridge (Fig. 3A) and a smartphone adapter for their devices to quantify total bile acids and total cholesterol using biochemiluminescence-based signals.15 Specifically, their disposable minicartridge was designed to house porous membrane assay components and a sample well, and was implemented in a combination of transparent (for the detection window) and black acrylonitrile butadiene styrene (ABS) resin. Complementary to this, their smart phone adapter, that encased the phone camera and an additional magnifying lens, was designed to interface with the minicartridge in a lock and key fashion, and was composed of black ABS resin in order to accurately and reproducibly quantify assay signal in various environmental lighting conditions. The capability of their 3D printing system (Replicator 2X from Makerbot, Boston, MA, USA) to rapidly (e.g., printing times of 10 or 30 minutes for the two components) and cost-effectively generate multiple iterations of prototypes for characterization was cited as key in their assay development process. More recently, Choobbari et al. used 3D printing to create a “lighting box” to contain their colorimetric paper-based glucose assay.16 The design of the box enabled optimization (and consistency) of the distance between the assay detection region and the smartphone camera that was placed on a window on top of the otherwise optically opaque box. Further, as above, the 3D printed housing provided uniform lighting for image acquisition, regardless of the environmental lighting conditions, which is critically important for obtaining robust assay results in field settings.16
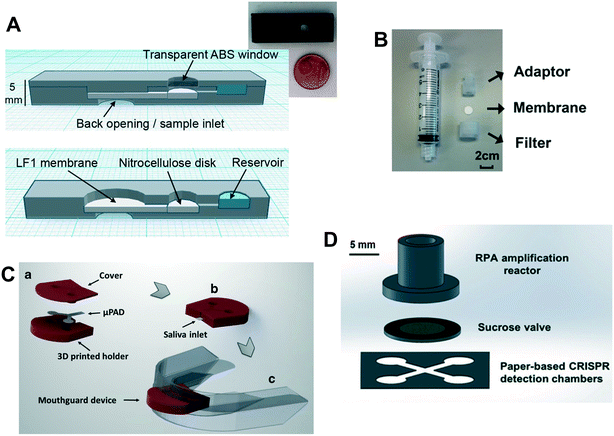 | ||
| Fig. 3 (A) Schematic and photograph of the minicartridge, composed of 3D printed transparent and black ABS using material extrusion, that houses the porous component of the cholesterol assay from Roda et al. Reprinted with permission from ref. 15. Copyright 2014 American Chemical Society. (B) Photograph of the unassembled components of the 3D printed syringe attachment from Dirkzwager et al. for their malaria diagnostics device. Reprinted with permission from ref. 25. Copyright 2016 American Chemical Society. (C) Schematic of the 3D printed holder for the porous component in an intraoral device from de Castro et al. Reprinted with permission from ref. 28. Copyright 2019 Springer-Verlag GmbH Germany. (D) Schematic of the 3D printed nucleic acid amplification chamber in a paper-based device from Yin et al. Reprinted with permission from ref. 29. Copyright 2021 the Royal Society of Chemistry. | ||
Many other groups have used material extrusion 3D printing to create components for robust image data acquisition from their lateral flow assays using smartphone-based optical detection. For example, Yeo et al. created a custom adapter module to interface a smartphone camera with their lateral flow assays for fluorescence detection of various avian influenza strains.17 Similarly, Cheng et al. fabricated a smartphone camera adapter module for quantification of signal from their nanozyme-based lateral flow assay for the detection of bacteria.18 Additional demonstrations of 3D printed adapter modules supported the smartphone camera-based luminescence detection of human chorionic gonadotropin19 and prostate specific antigen,20 the colorimetric detection of human cardiac troponin I21 and hemoglobin,22 UV excitation of quantum dot-labels for the detection of thrombin and interleukin-6,23 and colorimetric detection with machine learning classifiers for bacterial detection.24
Multiple groups have used 3D printing to create housings for their paper-based substrates. Dirkzwager et al.25 reported on the utility of 3D printing for rapid prototyping in the context of a paper- and syringe-based assay for colorimetric malaria detection (Fig. 3B). Specifically, they used a stereolithography-based 3D printer (Freeform Pico from Asiga) to create a housing composed of PlasCLEAR photopolymer to contain the paper component. The authors emphasized the importance of being able to quickly iterate on initial designs in order to achieve the high-fidelity fluidic seal that enabled stringent rinsing of the detection region in the final version of their device. Lafleur et al. used polyjet printing (Objet30 Scholar from Stratasys) with DuraWhitePlus material to create multiple housing components to hold the porous substrates in their nucleic acid amplification device for methicillin-resistant Staphylococcus aureus detection.26 In complementary work, Scordo et al. described the use of stereolithography 3D printing (Form2 from Formlabs) using white methacrylate polymer to create a cartridge for a paper-based electrochemical sensor.27 The housing contained an opening above the electrodes to serve as sample reservoir and a hinge for easy reuse for cholinesterase activity quantification.27 And most recently, 3D printing has been used to create a housing for the paper component in the context of a wearable. For their intraoral monitor, de Castro et al. created a FDM-printed structure using the polymer ABS that enclosed most of their paper component, but left one end uncovered for wicking in the saliva sample.28 The 3D printed structure interfaced with a wearable mouthguard for easy and secure placement/replacement of the disposable paper component (Fig. 3C). The authors demonstrated colorimetric glucose and nitrite detection using their device.
3D printing technology has also been used to fabricate upstream components of more complex paper-based assays. Lafleur et al. reported the fabrication of a custom valve for upstream lysis using 3D printing (in addition to multiple housings for porous substrates, see above) for their nucleic acid amplification and detection device with on-board sample processing.26 More recently, Yin et al. created a reaction chamber for nucleic acid amplification in a methacrylate-based polymer using SLA (Formlabs).29 The reaction chamber was integrated with a downstream sucrose valve and paper-based colorimetric detection (Fig. 3D). Further, on-device detection of SARS-CoV-2 from nasopharyngeal samples was demonstrated.
As shown by the work discussed in this section, 3D printing technology has been effectively used to advance paper microfluidic device development through the design and creation of solid polymer device components. For paper microfluidic assays, the housing for the porous component serves several critical functions. Specifically, the housing can provide physical support and protection for the often-fragile porous component during manipulation by the operator, as well as serve as a barrier between the assay and the operator/environment (e.g., minimizing dust or other environmental contaminants, minimizing evaporation, and encasing potentially harmful reagents). Further, 3D printing has been used to produce high-quality housings during the development phase that are robust (e.g., compared to support layers of plastic and adhesive that have been commonly used‡), and can be rapidly customized as the design of the porous component is optimized for a particular application. 3D printing has also been effectively used to create other components that are critical to overall device performance, such as camera housings and adapters for the acquisition of image data, which needs to be acquired reproducibly at a specific magnification and under uniform lighting conditions. In addition, 3D printing has been used to create upstream 3D printed components for sample pre-processing in more complex paper-based devices. Thus, the main strength of 3D printing technology for work in this category is the ability to rapidly create custom components and iterate this process to support the effective development of novel paper microfluidic devices. As 3D printing technology continues to advance, 3D printers will continue to become lower cost and more accessible to research and development labs. Note that although 3D printing technology can serve a critical role in the development stage of novel devices when the number of printed pieces required is modest, transition to another higher-volume fabrication method may be needed once the device has advanced to the commercialization stage and requires large-scale production.
| Function | 3D printing method and material | Description |
|---|---|---|
| Masks for photolithography and molds | Polyjet 3D printing (Objet30 Pro from Stratsys) of a photolithography mask using an acrylic monomer-based mixture; exposed chromatography (No. 51B) paper hydrophobized with n-hexane to 253 nm source31 |
Demonstrated colorimetric iron assay in river/tap water31
Min. barrier width: 106 ± 14 μm (CV of 13%) Min. channel width: 563 ± 15 μm (CV of 3%) |
|
Comparison with direct wax printing
Via hot plate heating on Whatman chromatography paper:32 Min. barrier width: 850 ± 50 μm (CV of 6%) Min. channel width: 561 ± 45 μm (CV of 8%) Via laminator heating on Whatman No. 1 filter paper:33 Min. barrier width: 467 ± 33 μm (CV of 7%) Min. channel width: 228 ± 30 μm (CV of 13%) |
||
| Material extrusion printing (D-Force 400) of polylactic acid that contained the desired open channel design and was subsequently filled with rehydrated cellulose34 |
Demonstrated colorimetric nitrite assay34
Min. barrier width: 493 ± 22 μm (CV of 4%) Min. channel width: 118 ± 17 μm (CV of 14%) |
|
| Material extrusion printing (D-Force 400) of polylactic acid to create modular variations of the work in ref. 34 with more complex functions36 | Demonstrated modular components that interfaced like Legos to perform a stent degradation study and cell culture36 | |
| Extruded barriers of wax and plastics | Material extrusion printing (Prusa Mendel RepRap) of wax on paper to form hydrophobic barriers41 | Detailed description of the open-source commercially available 3D printing system41 |
| Material extrusion printing (Prusa i3 printing framework) of paraffin wax on filter paper42 |
Demonstrated colorimetric nitrite and glucose assays using buffer solutions42
Min. barrier width: 468 ± 72 μm (CV of 15%) Min. channel width: 445 ± 38 μm (CV of 8%) |
|
| Material extrusion printing (Creator Pro from Flash Forge) of polycaprolactone on Whatman chromatography paper43–45 | Demonstrated viscosity measurements43–45 including of protein solutions and saliva | |
| Material extrusion printing (Ultimaker 2+ 3D printer) of polypropylene barriers in Whatman filter paper46 | Demonstrated devices with mechanical valves (requires operator to actuate)46 | |
| Vat polymerization for 3D printed structures | Stereolithography printing (custom system similar to Form1) with 400 nm LED of hydrophobic resin barriers in Whatman No. 1 filter paper47 |
Demonstrated colorimetric nitrite assay (required printing on both sides of the paper)47
Min. barrier width: 349 ± 18 μm (CV of 5%) Min. channel width: 332 ± 17 μm (CV of 5%) |
| Digital light projection printing (MINIQ III) with 405 nm LED of hydrophobic resin barriers in paper48 | Demonstrated colorimetric glucose and albumin assays in artificial urine48 | |
| Digital light projection printing (DP 110) with UV source of resin barriers in cellulose filter paper49–55 | Demonstrated a 3D network of channels for the detection of glucose, cholesterol and triglyceride from serum50 | |
| Integration of a plasma separation membrane with their 3D printing method49 | ||
| Demonstrated the detection of eight markers of health from serum51 | ||
| Demonstrated enclosed channels (for minimization of evaporation) using their 3D printing method52 |
In the first sub-category of 3D printed components to aid in porous channel definition, Asano et al. used a polyjet 3D printer (Objet30 Pro Stratasys) to create a photolithography mask from VeroWhitePlus RGD835 resin for defining fluidic channels.31 The polymer mask was used to selectively expose hydrophobized chromatography paper (treated with octadecyltrichlorosilane (OTS)-n-hexane solution) to UV light at 253 nm in order to define the hydrophilic channels. Compared to the conventional method of creating a mask in a cleanroom, the advantages of mask fabrication using 3D printing are a shorter fabrication time and a lower cost. The authors achieved a minimum barrier width and a minimum channel width of 106 ± 14 μm and 563 ± 15 μm, respectively, using their 3D printed mask for patterning chromatography paper (No. 51B). These widths are smaller than that reported for the commonly used paper microfluidic fabrication method of direct wax printing with hot plate heating (850 ± 50 μm and 561 ± 45 μm, respectively) on Whatman chromatography paper,32 but smaller in barrier width and greater in channel width than that reported for direct wax printing with laminator heating (467 ± 33 μm and 228 ± 30 μm, respectively) on Whatman No. 1 filter paper.33 Reproducibility, as measured by the coefficient of variation (CV) in barrier and channel widths (CVs of 13% and 3% (ref. 31)) is reasonable, and similar in range to CVs for direct wax printing with hot plate heating (6% and 8%) and direct wax printing with laminator heating (7% and 13%).
Porous channel definition in paper microfluidic devices has also been assisted using 3D printed structures containing open channels into which porous cellulose could be reconstituted. Specifically, He et al. used material extrusion 3D printing (D-Force 400) to create a substrate composed of polylactic acid (PLA) that contained the desired open channel design.34 A layer of PDMS was then added to the substrate (and dried at 60 °C for 1 h) in order to fill and seal imperfections in its surface (that would otherwise affect device operation) and provide a hydrophobic coating. Note that in general, extrusion-based printing produces larger surface imperfections compared to other printing methods, and that these must be sealed in order to create robust (i.e., leak-free) open fluidic channels.35 Finally, a solution of cellulose powder in water was added to the substrate and the assembly was placed in an oven (60 °C for 30 min) in order to form the porous structure within the 3D printed substrate as a completed device. The authors noted that an advantage of their fabrication method is being able to reuse the 3D printed substrate. Although this could have value during development, it has limited utility in the context of diagnostic device fabrication, where mold reuse in the field would not be straightforward and perhaps not desired (e.g., due to contamination or other issues with creating reproducible structures). A potential advantage of this method is in defining narrower channels than are feasible with other common paper microfluidic fabrication methods. Specifically, the minimum channel width reported was 118 ± 17 μm, smaller than that reported by many of the current paper microfluidic fabrication methods including direct wax printing with laminator heating.33 The reported minimum barrier width using this method was 493 ± 22 μm and comparable to barrier widths achievable by direct wax printing with laminator heating.33 Reproducibility, as measured by the coefficient of variation (CV) in barrier and channel widths (CVs of 4% and 14% for barrier and channel width, respectively34) is reasonable, and comparable to CVs with laminator heating.
Nie et al. then expanded on the fabrication concept introduced by He et al. Building on the 3D printed substrate filled in with porous material, Nie et al. designed modular variations of these (including valving, mixing, concentration, and collection modules) that interfaced like Legos to perform more complex functions including multistage co-culture, drug screening, parallel testing, and timed mixing (Fig. 4).36 While the open channels need to be filled with dried cellulose by hand, the authors noted that the 3D printing fabrication is lower cost and more convenient than fabrication of PDMS-based microfluidic devices. The ability to connect pre-printed components in specific device configurations as needed is an intriguing concept for decentralizing the prototyping of paper microfluidic devices.
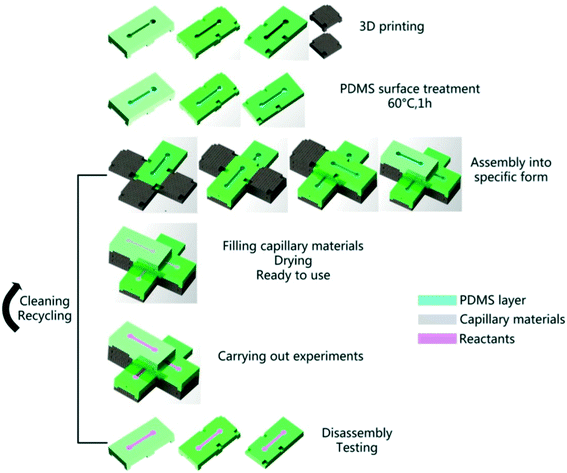 | ||
| Fig. 4 Schematic of 3D printing stackable components for configurable paper microfluidic devices. Printed PLA modules that are treated with PDMS were filled with rehydrated cellulose from Nie et al. The modules were then combined to create more complex microfluidic devices. Reprinted with permission from ref. 36. Copyright 2018 IOP Publishing Ltd. | ||
The second sub-category of work consists of the use of 3D printing technology to directly define hydrophobic barriers within existing porous materials. Two 3D printing methods, materials extrusion and vat polymerization, have been used in multiple demonstrations creating 2D and 3D networks in porous materials. While the capability of patterning 2D devices is adequate and appropriate for many paper microfluidic device applications, the capability to print 3D structures that include fluid reservoirs and channels in different layers, can increase device functionality for various field settings and applications. Examples of 3D printing methods of varying complexity that have been used to create both 2D and 3D networks, and the use of those porous networks in specific applications are described below.
For porous channel definition using material extrusion, wax has been one material of choice. As alluded to above, wax has been used extensively for fabrication in the paper microfluidics community. Specifically, wax printing, the transfer of heated wax to paper or other porous material in order to form hydrophobic barriers for fluidic channels, has been one of the most commonly used methods since the first demonstrations of a commercially-available wax printer for this purpose.32,37 Since then, others have expanded the use of direct wax printing, including coupling to laminator heating,33 indirect wax transfer printing,38,39 and wax screen printing.40 Thus, with the increasing accessibility of 3D printing in research groups, a natural extension is wax 3D printing. For example, Pearce et al. described their modification of a commercially-available 3D printer, the open-source Prusa Mendel RepRap, to enable wax extrusion for hydrophobic channel definition on porous substrates.41 The wax was heated above its melting temperature so it could be extruded onto paper, and the wax then formed a solid barrier within the fibers of the paper when cooled. In another example, Chiang et al. printed paraffin wax on filter paper with a custom 3D printer (open-source Prusa i3 printing framework) (Fig. 5A).42 As a proof of concept, they fabricated devices to detect nitrite and glucose in buffer using colorimetric methods. The smallest wax barrier width and channel width that successfully wicked fluid without leaking was reported as 468 ± 72 μm and 445 ± 38 μm, respectively. These are smaller than the minimum barrier and channel widths reported using direct wax printing with hot plate heating,32 though not as small as those reported for wax printing with laminator heating.33 Reproducibility, as measured by the coefficient of variation (CV) in barrier and channel widths (CVs of 15% and 8% (ref. 42)) is reasonable, and comparable to CVs with laminator heating. The authors note that an advantage of their method compared to the conventional wax printing method (using a commercial wax printer) is that 3D wax printing only requires a single step of heated wax deposition to form the barrier, while conventional wax printing requires additional heating after wax application for robust barrier formation. Further, 3D printing wax barriers only requires applying wax to one side of the porous substrate, while some of the conventional wax printing methods require wax deposition on both sides of the porous substrate (e.g., ref. 33). Disadvantages of 3D wax printing are the extra time and resources that are required to create the 3D printer capable of wax extrusion.
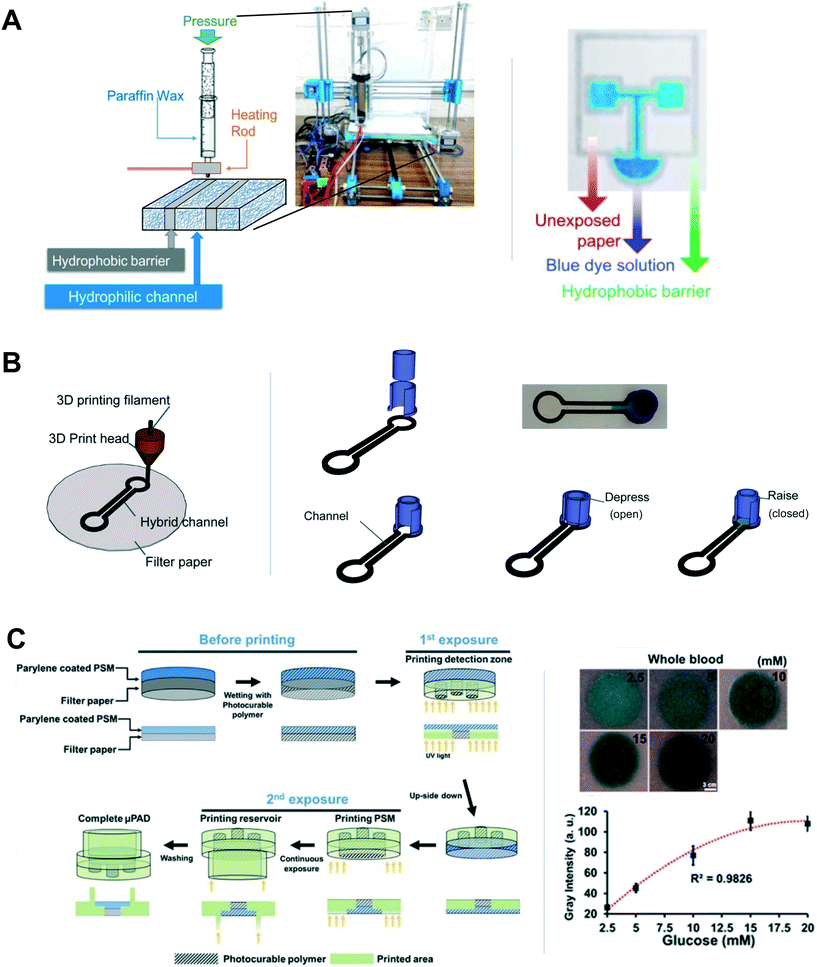 | ||
| Fig. 5 3D printing hydrophobic channels in existing porous materials. (A) Schematic and photograph of paraffin wax printing on paper from Chiang et al. (left), and an image of a printed device in which the wax barriers have contained the test fluid consisting of blue dye in water (right). Reproduced with permission from ref. 42. Copyright 2018 Elsevier B.V. (B) Extrusion-based 3D printing of polypropylene on paper to define hydrophobic barriers from Zargaryan et al. (left). Finger-activated fluid reservoir, also 3D printed, and channel are depicted (right). Adapted from ref. 46. (C) DLP-based 3D printing from Park et al. that incorporates two different porous materials within the device. Reprinted with permission from ref. 49. Copyright 2019 American Chemical Society. | ||
In addition to 3D printing hydrophobic barriers of wax, the 3D printing of plastic barriers has also been demonstrated. Specifically, commercially-available extrusion-based printers (Creator Pro, Flash Forge) have been used to 3D print polycaprolactone (PCL) barriers on chromatography paper.43–45 In the case of PCL, after printing the designed channels onto paper, the combination was heated in an oven at 150 °C for 30 minutes, so the PCL could fully seep into the pores of the paper and form the hydrophobic boundaries. The fabrication method was used to create devices for viscosity measurements using both electrochemical detection with integrated screen-printed electrodes (for milk samples44) and colorimetric detection (for human saliva45). One potential limitation of their method is that the hydrophobic barrier width and the hydrophilic channel width that were reported to be optimal (with respect to maximizing repeatability in flow time) was 2 mm and 6 mm, respectively,43 which is substantially larger than dimensions robustly achieved using other low-cost paper patterning methods like direct wax printing.
More recently, Zargaryan et al. developed ‘hybrid’ 3D printed devices by integrating 3D printed valves and reservoirs into paper substrates in which fluidic channels had been defined using hydrophobic barriers.46 Specifically, Zargaryan et al. used an extrusion-based 3D printer (Ultimaker 2+ 3D printer) to pattern polypropylene barriers in Whatman filter paper, and then baked the combination at 170 °C for 45 min in order to form robust barriers when cooled. The 3D printed reservoir or valve components were attached to the paper substrate via heat gun, glue, or double-sided tape (Fig. 5B). The addition of 3D printed components for reservoirs and valves provides advantages of improved repeatability and increased usability for untrained operators. Specifically, their finger-operated 1 mL fluid reservoirs provided a method of consistent fluid application, and the valves added control for fluid flow timing. Limitations of the work include (i) lower resolution features, i.e., the smallest barrier width that produced 100% barrier integrity (N = 3) via a fluid leak test was reported as 1 mm, and (ii) high sample volumes, 1 mL and greater. Regarding the applicability of other polymers, the authors noted that polylactic acid (PLA) and polycarbonate could be used, but that polypropylene was preferred because of its lower melting temperature which did not discolor the paper substrate.
Porous channel definition has also been achieved using commercially-available vat polymerization printers with digital light projection (DLP) or stereolithography (SLA). For these methods, the paper substrate was first submerged into the printer resin vat, the resin-filled substrate was selectively exposed to a light source for curing, and the substrate was rinsed to remove uncured resin from the porous substrate, leaving behind solid barriers that defined fluid flow. He et al. demonstrated an early application of stereolithography to create hydrophobic barriers for fluidic channels in filter paper.47 Printing on both sides of the paper was required because the UV light could not penetrate through the thickness of the paper to harden the resin and create the leak-proof barrier. The minimum hydrophobic barrier width and the minimum hydrophilic channel width reported with this method was 349 ± 18 μm and 332 ± 17 μm, respectively, compared to 470 μm and 220 μm, respectively, for direct wax printing with laminator heating.33 The coefficients of variation of the barrier and channel widths, at 5%, indicate a high level of reproducibility for this method. As a proof-of-concept, this fabrication method was used to create a paper microfluidic colorimetric nitrite test.
In a further advance, X. Fu et al. created multi-layer, bonded filter paper devices using a modified digital light projection (DLP) 3D printer (MINIQ III).48 After the hydrophobic boundaries in a given paper layer were printed, a new piece of paper was immersed in the resin bath and aligned with the previously printed paper layer, such that the two could be bonded and the new layer printed. The authors reported a minimum channel width of 350 μm, modestly larger than the channel widths of 220 μm achieved using direct wax printing with laminator heating.33 The authors noted that their lateral resolution was limited by the LCD pixel spacing and that a more advanced optical system could produce narrower channels if desired. This novel method of bonding multiple layers of paper channels was applied to create a colorimetric test for glucose and albumin from human urine. This work also demonstrated the integration of fluid valves that could be actuated via an electric field or airflow by taking advantage of the small gap which formed between layers of the filter paper. An additional advantage of this method is the elimination of a separate heating step after barrier application to the porous material. A disadvantage of the current implementation of this method is that it required manual placement of the paper layers during the printing process, but this could be automated in future work.
The Park lab has expanded the set of 3D printing-based fabrication tools for paper microfluidic devices. In their method, they used DLP (DP 110) to print a simple 3D network of channels within a single piece of cellulose filter paper.49–51 Briefly, a piece of resin-soaked paper was placed in the 3D printer resin bath and multiple layers were differentially exposed to the desired channel pattern for each layer. An advance in their designs was the integration of reservoirs via the 3D printing process, rather than the incorporation of components via sticky tape or adhesive after the printing process (which requires additional labor and resources). Additionally, their designs incorporated a 3D network of channels, rather than being limited to 2D. Regarding the latter, the authors noted that because their device detection region was accessed by fluid entering from a channel directed upward from below, rather than laterally into the region, their colorimetric reactions produced signals with greater uniformity than that reported in some 2D-μPADs. Using their method, they demonstrated integration of a second porous material, a plasma separation membrane (PSM), in-line with filter paper, within a 3D printed device (Fig. 5C left), and detection of glucose from blood (Fig. 5C right).49 As might be expected, due to the wide range in porous material properties, each material required a different set of parameter values for optimal printing. In addition, the PSM required a coating of parylene C in order to protect it from organic solvents in the 3D printing process (an option for other porous materials of interest that are not organic solvent resistant). In another study, they demonstrated the parallel detection of eight markers of health from serum using one of their 3D printed devices with individual channel width of 2 mm (minimum channel and barrier widths were not characterized) and a centrally-located sample reservoir.51 Most recently, the Park lab extended their 3D printing-based fabrication work to include enclosed channels which minimize sample evaporation and may be critical for the smallest volume applications.52
The work of this section demonstrated the use of 3D printing to define hydrophobic barriers in porous substrates. Not surprisingly, wax, having often been used to define hydrophobic barriers in paper microfluidic devices, has been a material of choice in extrusion-based 3D printing of hydrophobic barriers in porous materials. 3D printing protocols have been developed that enable reasonable channel and barrier resolution for many applications of paper microfluidics,§ thus providing an alternative method of applying wax to porous substrates. Extrusion-based 3D printing of polymers has also been used to create more complex devices in which porous channels defined with 3D printed hydrophobic barriers were combined with separately 3D printed polymer reservoirs or valves to produce devices with the potential for greater repeatability and usability. However, a disadvantage of this work is that it required post-printing integration of components using time- and resource-intensive methods based on tape or adhesives. Most notably in this category of work, vat polymerization 3D printing technology has been used to create 3D porous networks. The protocols that have been demonstrated vary in complexity, with some requiring additional alignment steps, and all requiring a post-printing rinse of the porous substrate to remove uncured resin. These recent demonstrations49–51 are an important advance that leverages the strength of additive manufacturing and eliminates the need for post-printing integration steps that could compromise repeatability of device performance. Further, the use of 3D printing technology to create 3D networks of channels in a single printing session may enable effective solutions to the challenge of seamlessly fabricating and integrating fluidic control tools within paper microfluidic devices.
| Category | Printing method and material | Description |
|---|---|---|
| Porous materials for separations | FDM extrusion printing (Prusa i3 rework) of porous silica gel matrix57 | Printed an ∼200 μm thin layer for the separation of multiple dyes57 |
| Coating of calcium carbonate with microfibrillated cellulose binder58 | Adjusted chromatographic resolution via the inkjet printing of surface charge modifiers onto their porous substrate and demonstrated the separation of a metabolite umbelliferone and NADH58 | |
| Porous materials for well-defined wicking time | Coating of calcium carbonate with microfibrillated cellulose binder58,59 | Generated coatings with a bimodal pore distribution and a range of wicking speeds for different formulations containing varying fractions of calcium carbonate59 |
| Created a porous matrix for a lateral flow assay to study cytochrome P450 metabolic activity58 | ||
| Extrusion (BioDot XYZ3060 with the PolyDrop™ touch-off dispenser) of thin porous layer of precipitated calcium carbonate with latex binder60 | Generated porous layers with a range of pore sizes (170 nm to 670 nm in diameter), and therefore flow rates, using four different types of PCC60 | |
| Direct write printing with a modified 3D printer (K8200 Vellerman) of silica gel particles in polyvinyl acetate61 | Generated porous channels with a wetting time of 35.3 s/2 cm (CV of 22%) and a minimum of 2.3 mm channel width (CV of 16%)61 | |
| Customized printing of electrospun nanofiber mats (polyimide doped with silicon powder) and hydrophobic wax boundaries62 | Created a porous matrix for an assay to quantify iron content in drinking water62 | |
| Binder jetting (modified Z650 3DSystems) of poly (methyl methacrylate) powder plus a liquid binder and dispensing of a binder with alkyl succinic anhydride for hydrophobic barriers63 | Reported a wicking time coefficient of variation of less than 2.5% for fluid in their hydrophilic porous channels and fabricated an IgE assay63 |
Porous layers with fluid wicking capabilities for paper microfluidic applications have been created using a 3D printer. For example, Fichou et al. modified a material extrusion-based 3D printer (Prusa i3 rework, Emotiontech, Toulouse, France) to create a porous silica gel matrix for thin layer chromatography.57 Specifically, they replaced the FDM extruder with a custom silica gel slurry applicator that included multiple 3D printed ABS components to hold and manipulate the syringe (Fig. 6A). The system was used to print an ∼200 μm thin layer for the separation of multiple dyes. Advantages of this system include the ability to print different layers in different patterns, the availability of the printer design and components (i.e., open source), and the overall low cost (less than 710 euros for the system components and less than 0.25 euro for a print). A potential disadvantage is the need for resources to perform the modifications. Further characterization would be needed to determine printing resolution and explore the range of possible porous material characteristics achievable with this method.
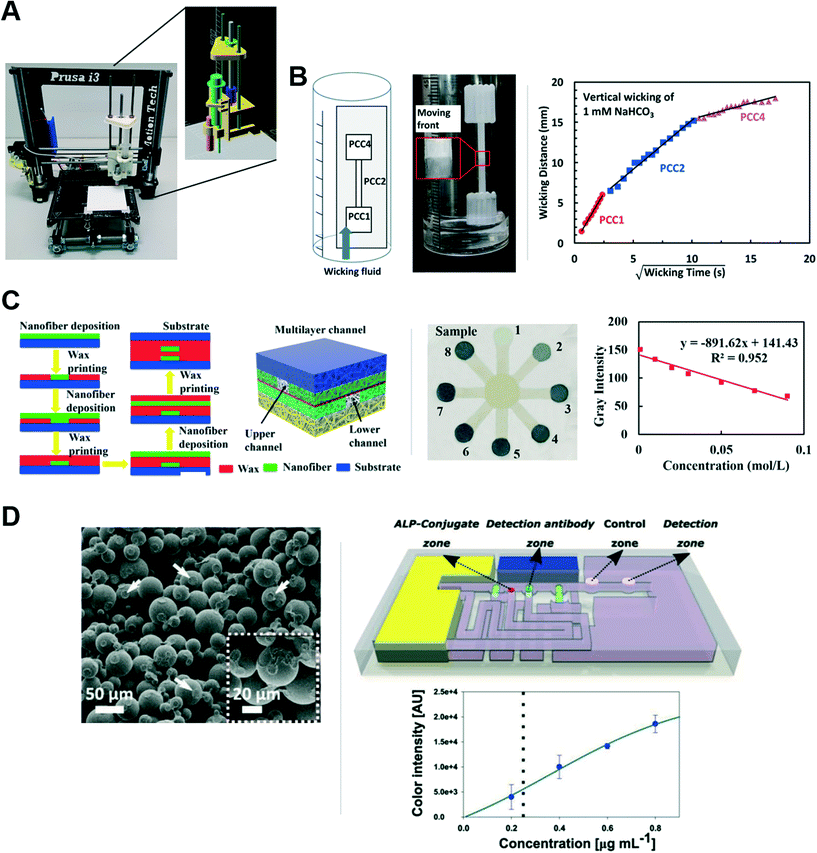 | ||
| Fig. 6 Advances in printing porous materials. (A) Photograph and schematic of the modified 3D printing system used to create a silica gel-based porous matrix for chromatography from Fichou et al. Reprinted with permission from ref. 57. Copyright 2017 American Chemical Society. (B) Schematic and photograph (left) of an extruded porous membrane containing regions with different wicking characteristics from Li et al., and their supporting data (right). Reprinted with permission from ref. 60. Copyright 2019 American Chemical Society. (C) Schematic of nanofiber matrix fabrication coupled to wax barrier printing from Chen et al. (left), and their implementation of an iron assay (middle and right). Reproduced from ref. 62. (D) SEM image of the porous matrix created by binder jetting PMMA powder and liquid binder from Achille et al. (left). A schematic of their printed device for IgE detection (right top), and their response curve for IgE in buffer (right bottom). Reprinted with permission from ref. 63. Copyright 2021 Wiley-VCH GmbH. | ||
Jutila et al. demonstrated the fabrication of a custom porous substrate from functionalized calcium carbonate with microfibrillated cellulose binder for chromatographic separations.58,59 Chromatographic resolution of their porous substrate could be varied via the inkjet printing of surface charge modifiers onto their porous substrate, and their system was used to successfully separate their metabolite of interest, umbelliferone, from NADPH.58 Further, they notably incorporated hydrophobic barriers into their porous substrate using inkjet-printing (Fujifilm, Dimatix) of either polystyrene, for partial barriers, or alkyl ketene dimer, for permanent barriers, in order to manipulate fluid flow within the substrate.58 They then used this system to create a lateral flow assay to study cytochrome P450 metabolic activity that incorporated both chromatographic separation and fluorescence detection.58 A strength of this work is the demonstration of key functionality that can be attained though successive additive inkjet printing steps. In related work, the authors performed characterization of their custom films, which showed a bimodal pore distribution and a range of wicking speeds for the different formulations containing varying fractions of calcium carbonate.59
Complementary to this, Li et al. demonstrated the extrusion of thin porous layers targeted for use in lateral flow assays.60 Specifically, they used a liquid dispensing system (BioDot XYZ3060 with the PolyDrop™ touch-off dispenser) for the extrusion of precipitated calcium carbonate (PCC) with latex binder. The PCC inks were limited to 15 wt% in order to maintain printing uniformity, and the porous layers (∼20 μm dry thickness) were prepared on a glass slide due to the requirement by the liquid dispensing system for a rigid substrate. Importantly, porous layers with a range of pore sizes (170 nm to 670 nm in diameter), and therefore flow rates, were created using four different types of PCC. The different PCC layers had comparable flow rates to filter paper, and lower than that in the nitrocellulose characterized. Further, the ability to print a continuous porous layer with three different wicking characteristics was demonstrated (Fig. 6B left shows the set up, and Fig. 6B right shows the supporting data), a significant potential advantage over the use of multiple components in conventional lateral flow assays. Finally, nonspecific binding of alkaline phosphatase (ALP) within the strip was demonstrated as an example of reagent immobilization and colorimetric reagent generation in PCC. Although promising in the context of an assay requiring ALP immobilization, both bioconjugation strategies for and nonspecific binding characteristics of PCC would need to be explored in more depth in order to determine the general utility of the PCC layers in lateral flow assay development. In the future, related printing systems could be used to build upon this work to create 3D networks of the porous material.
More recently, Evard et al. demonstrated direct write printing of a porous matrix using silica gel particles with polyvinyl acetate binder and glycerol, and printed with a modified 3D printer (K8200 Velleman).61 In order to remove the glycerol, the printed porous matrix required heating in a 75 °C oven for approximately five days. Characterization of the porous channels fabricated by 3D printing included a comparison with channels made by photolithography and screen printing of a similar porous mixture. The 3D printed channels had the fastest wicking time of the three methods of 35.3 s/2 cm (CV of 22%) and the largest pore sizes of 34–215 μm. A minimum channel width of 2.3 mm (CV of 16%) was achieved, but the authors noted that the use of more uniform and smaller particles could enable narrower channel widths.
Further enabling the creation of more complex porous networks, Chen et al. combined the layer-by-layer spraying of electrospun nanofiber mats with the deposition of hydrophobic wax boundaries using a customized printing system. After the fabrication of the desired number of layers, the assembly was heated at 120 °C to allow the wax in each layer to fully penetrate that layer. The resulting 3D networks were composed of multiple layers of porous channels of polyimide doped with silicon powder that could wick fluid within the wax barriers defined in that layer (Fig. 6C left).62 This fabrication method was used to create an assay for iron content in drinking water by drying colorimetric indicator into regions of the device (Fig. 6C middle and right). One significant advantage of this fabrication method is that it does not require the integration of separate porous substrates to create 3D channel networks.
Most recently, Achille et al. have used binder jetting 3D printing technology to create porous structures from poly(methyl methacrylate) (PMMA) powder plus a liquid binder.63 Specifically, a modified powder bed 3D printer (Z650 3DSystems) was used to alternately dispense the build powder and binder in a selective layer-by-layer fashion, enabling the printing of 3D porous channels with complex geometries. The authors note their rationale for using PMMA for the build powder included its hydrophilic surface properties, compatibility with many biologically-relevant molecules, and its off-the-shelf availability. The liquid binder (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of acetophenone and butanone) was chosen to have a viscosity and surface tension conducive to inkjet printing. The mixture produced a porous matrix (SEM image in Fig. 6D left) with a porosity of 41% and an average pore diameter of 25 μm (analyzed by X-ray computed tomography). Hydrophobic barriers in a layer were defined by dispensing a binder with alkyl succinic anhydride. Notably, the authors noted a wicking time coefficient of variation of less than 2.5% for fluid in their hydrophilic channels, approximately 10 times better than that reported for some lateral flow membranes. This fabrication method was used to implement an IgE assay (shown schematically in Fig. 6D right top), with response curve in buffer (Fig. 6D right bottom).
1 ratio of acetophenone and butanone) was chosen to have a viscosity and surface tension conducive to inkjet printing. The mixture produced a porous matrix (SEM image in Fig. 6D left) with a porosity of 41% and an average pore diameter of 25 μm (analyzed by X-ray computed tomography). Hydrophobic barriers in a layer were defined by dispensing a binder with alkyl succinic anhydride. Notably, the authors noted a wicking time coefficient of variation of less than 2.5% for fluid in their hydrophilic channels, approximately 10 times better than that reported for some lateral flow membranes. This fabrication method was used to implement an IgE assay (shown schematically in Fig. 6D right top), with response curve in buffer (Fig. 6D right bottom).
The work described in this section has focused on the use of printing technology to create porous matrices to serve as hydrophilic channels that support capillary flow. Noteworthy work in this category included the extrusion of a monolithic porous film containing multiple regions of tunable porosity and targeted for use in lateral flow assays,60 and the use of binder jetting to create a well-defined porous matrix to support capillary flow in a paper microfluidic device with coefficient of variation substantially less than that reported for commercial membranes used in lateral flow.63 Although this category of work is the least developed of those described in this review, there is great opportunity for the paper microfluidics community to expand on these promising demonstrations. Specifically, extension of this work to create well-characterized 3D printed networks of porous matrices with tunable pore diameters and surface chemistry, would substantially advance the development of paper microfluidic devices for a range of applications. The challenges associated with achieving this goal are discussed briefly below.
Future potential of 3D printing for paper microfluidics and summary
Moving forward, a focus on the direct 3D printing of porous materials with characteristics suitable for paper microfluidics applications would be of high value for the development of the next generation of paper microfluidic assays. Achieving this will require substantial effort to survey a wide range of materials, and then optimize printing hardware and parameters for the most promising candidates. Further, the promising candidates will need to be characterized for relevant properties, such as capillary flow time, surface adsorption properties for common capture reagents such as antibodies, and susceptibility to nonspecific adsorption, e.g., of key ‘representative’ proteins, in order to inform the assay development process. In addition, this detailed characterization would help to promote the adoption of promising new materials in the paper microfluidics community. Finally, a potential challenge to consider for 3D printing porous structures for larger scale use will be ensuring the reproducibility of the printed products (and thus, device performance) when different printing systems that include customizations are being used for the fabrication.Another high value area of focus that would advance the development of the next generation of paper microfluidic assays is using 3D printing to integrate multiple materials in the additive manufacturing process. Multi-material 3D printing has been used to embed electronic circuits within polymers64,65 and to integrate metal–organic frameworks (MOFs) for selective capture in 3D prints.66 Application of multi-material printing to paper microfluidic devices could enable fabrication of 3D geometries with integrated electronics for sensing and readout from a single print. A future area of focus within the theme of multi-material printing would be the co-printing of reagents as a part of the additive manufacturing process.67 Generally, the area of multi-material 3D printing has the potential to streamline paper microfluidic fabrication processes and reduce reliance on the pick and place assembly and sealing that is commonly characteristic of current paper microfluidic device fabrication. Consolidation of fabrication processes into a single automated print could also improve device robustness and reproducibility, as well as reduce overall fabrication time. One barrier to academic researchers is the higher cost of multi-materials printers compared to single material 3D printing tools, which are generally lower cost, but as the technology advances, accessibility would be expected to increase.
Finally, although current 3D printing technology cannot match the scale, low material costs, and fabrication time of some established paper microfluidic manufacturing methods (such as for lateral flow assays), in the future, 3D printing manufacturing practices may be able to provide some degree of scaled-up production, as there has already been work to establish 3D printing farms (see ref. 68 for some examples). Further, even if 3D printing cannot match some fabrication technologies at the larger scales, it may enable the decentralized fabrication of diagnostics, e.g., on-demand in remote areas,13,69 or support local production when supply chains are disrupted.70
3D printing technology has been used to advance paper microfluidic device development in multiple ways. In this review article, we have described contributions to three categories of work; (I) the fabrication of housings or other accessory structures for paper microfluidic devices, (II) hydrophobic barrier definition in existing porous materials, and (III) the creation of porous matrices to serve as hydrophilic channels that support flow. This work has highlighted the multiple strengths of 3D printing for paper microfluidics: (i) 3D printing technology enables rapid prototyping processes with reasonable resolution for paper microfluidic device applications; (ii) 3D printing technology allows for the production of multilayer structures of 3D networks in an additive manner, thus reducing the need for post-printing fabrication processes such as alignment and sealing of layers that is common to current 3D paper microfluidic fabrication; and (iii) 3D printing technology enables the creation of novel porous matrices that support capillary flow as alternatives for commonly-used commercial membranes with customized characteristics, such as improved reproducibility.¶ In the future, further advances in 3D printing technology and its application to paper microfluidic device development could lead to the next generation of devices with 3D printed networks containing tunable porous materials and integrated components via additive manufacturing.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Corey Downs for useful feedback and Oregon State University for supporting this work.References
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Patterned paper as a platform for inexpensive, low-volume, portable bioassays, Angew. Chem., Int. Ed., 2007, 46(8), 1318–1320, DOI:10.1002/anie.200603817.
- A. W. Martinez, S. T. Phillips and G. M. Whitesides, Three-dimensional microfluidic devices fabricated in layered paper and tape, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(50), 19606–19611, DOI:10.1073/pnas.0810903105.
- S. Byrnes, G. Thiessen and E. Fu, Progress in the development of paper-based diagnostics for low-resource point-of-care settings, Bioanalysis, 2013, 5(22), 2821–2836, DOI:10.4155/bio.13.243.
- E. Fu and C. Downs, Progress in the development and integration of fluid flow control tools in paper microfluidics, Lab Chip, 2017, 17(4), 614–628, 10.1039/C6LC01451H.
- E. Fu, B. Lutz, P. Kauffman and P. Yager, Controlled reagent transport in disposable 2D paper networks, Lab Chip, 2010, 10(7), 918, 10.1039/b919614e.
- E. Fu, T. Liang, P. Spicar-Mihalic, J. Houghtaling, S. Ramachandran and P. Yager, Two-Dimensional Paper Network Format That Enables Simple Multistep Assays for Use in Low-Resource Settings in the Context of Malaria Antigen Detection, Anal. Chem., 2012, 84(10), 4574–4579, DOI:10.1021/ac300689s.
- A. V. Nielsen, M. J. Beauchamp, G. P. Nordin and A. T. Woolley, 3D Printed Microfluidics, Annu. Rev. Anal. Chem., 2020, 13(1), 45–65, DOI:10.1146/annurev-anchem-091619-102649.
- A. Naderi, N. Bhattacharjee and A. Folch, Digital Manufacturing for Microfluidics, Annu. Rev. Biomed. Eng., 2019, 21(1), 325–364, DOI:10.1146/annurev-bioeng-092618-020341.
- T. Birtchnell and W. Hoyle, What is 3D Printing? The definitive guide, 3D Hubs, 2014, Available: https://www.3dhubs.com/guides/3d-printing/#fdm Search PubMed.
- Dassault Systèmes, 3D-Printing Additive, 2021, Available: https://make.3dexperience.3ds.com/processes/3D-printing.
- 3D Printing Industry, Beginner's Guide to 3D Printing, 2017, Available: https://3dprintingindustry.com/3d-printing-basics-free-beginners-guide/#.
- N. P. Macdonald, J. M. Cabot, P. Smejkal, R. M. Guijt, B. Paull and M. C. Breadmore, Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms, Anal. Chem., 2017, 89(7), 3858–3866, DOI:10.1021/acs.analchem.7b00136.
- H. N. Chan, M. J. A. Tan and H. Wu, Point-of-care testing: applications of 3D printing, Lab Chip, 2017, 17(16), 2713–2739, 10.1039/C7LC00397H.
- J. Yang, et al., An integrative review on the applications of 3D printing in the field of in vitro diagnostics, Chin. Chem. Lett., 2021 DOI:10.1016/j.cclet.2021.08.105.
- A. Roda, E. Michelini, L. Cevenini, D. Calabria, M. M. Calabretta and P. Simoni, Integrating Biochemiluminescence Detection on Smartphones: Mobile Chemistry Platform for Point-of-Need Analysis, Anal. Chem., 2014, 86(15), 7299–7304, DOI:10.1021/ac502137s.
- M. L. Choobbari, M. B. Rad, A. Jahanshahi and H. Ghourchian, A sample volume independent paper microfluidic device for quantifying glucose in real human plasma, Microfluid. Nanofluid., 2020, 24(9), 1–12, DOI:10.1007/s10404-020-02382-y.
- S. J. Yeo, et al., Smartphone-based fluorescent diagnostic system for highly pathogenic H5N1 viruses, Theranostics, 2016, 6(2), 231–242, DOI:10.7150/thno.14023.
- N. Cheng, et al., Nanozyme-Mediated Dual Immunoassay Integrated with Smartphone for Use in Simultaneous Detection of Pathogens, ACS Appl. Mater. Interfaces, 2017, 9(46), 40671–40680, DOI:10.1021/acsami.7b12734.
- A. S. Paterson, et al., A low-cost smartphone-based platform for highly sensitive point-of-care testing with persistent luminescent phosphors, Lab Chip, 2017, 17(6), 1051–1059, 10.1039/C6LC01167E.
- A. N. Danthanarayana, E. Finley, B. Vu, K. Kourentzi, R. C. Willson and J. Brgoch, A multicolor multiplex lateral flow assay for high-sensitivity analyte detection using persistent luminescent nanophosphors, Anal. Methods, 2020, 12(3), 272–280, 10.1039/C9AY02247C.
- G.-R. Han, H. J. Koo, H. Ki and M.-G. Kim, Paper/Soluble Polymer Hybrid-Based Lateral Flow Biosensing Platform for High-Performance Point-of-Care Testing, ACS Appl. Mater. Interfaces, 2020, 12(31), 34564–34575, DOI:10.1021/acsami.0c07893.
- S. K. Biswas, et al., Smartphone-Enabled Paper-Based Hemoglobin Sensor for Extreme Point-of-Care Diagnostics, ACS Sens., 2021, 6(3), 1077–1085, DOI:10.1021/acssensors.0c02361.
- M. Mahmoud, C. Ruppert, S. Rentschler, S. Laufer and H.-P. Deigner, Combining Aptamers and Antibodies: Lateral Flow Quantification for Thrombin and Interleukin-6 with Smartphone Readout, Sens. Actuators, B, 2021, 333, 129246, DOI:10.1016/j.snb.2020.129246.
- H. J. Min, H. A. Mina, A. J. Deering and E. Bae, Development of a smartphone-based lateral-flow imaging system using machine-learning classifiers for detection of Salmonella spp, J. Microbiol. Methods, 2021, 188, 106288, DOI:10.1016/j.mimet.2021.106288.
- R. M. Dirkzwager, S. Liang and J. A. Tanner, Development of Aptamer-Based Point-of-Care Diagnostic Devices for Malaria Using Three-Dimensional Printing Rapid Prototyping, ACS Sens., 2016, 1(4), 420–426, DOI:10.1021/acssensors.5b00175.
- L. K. Lafleur, et al., A rapid, instrument-free, sample-to-result nucleic acid amplification test, Lab Chip, 2016, 16(19), 3777–3787, 10.1039/C6LC00677A.
- G. Scordo, D. Moscone, G. Palleschi and F. Arduini, A reagent-free paper-based sensor embedded in a 3D printing device for cholinesterase activity measurement in serum, Sens. Actuators, B, 2018, 258, 1015–1021, DOI:10.1016/j.snb.2017.11.134.
- L. F. de Castro, S. V. de Freitas, L. C. Duarte, J. A. C. de Souza, T. R. L. C. Paixão and W. K. T. Coltro, Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring, Anal. Bioanal. Chem., 2019, 411(19), 4919–4928, DOI:10.1007/s00216-019-01788-0.
- K. Yin, X. Ding, Z. Li, M. M. Sfeir, E. Ballesteros and C. Liu, Autonomous lab-on-paper for multiplexed, CRISPR-based diagnostics of SARS-CoV-2, Lab Chip, 2021, 21(14), 2730–2737, 10.1039/D1LC00293G.
- K. Plevniak, M. Campbell, T. Myers, A. Hodges and M. He, 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia, Biomicrofluidics, 2016, 10(5), 054113, DOI:10.1063/1.4964499.
- H. Asano and Y. Shiraishi, Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography, Anal. Chim. Acta, 2015, 883, 55–60, DOI:10.1016/j.aca.2015.04.014.
- E. Carrilho, A. W. Martinez and G. M. Whitesides, Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics, Anal. Chem., 2009, 81(16), 7091–7095, DOI:10.1021/AC901071P.
- K. Tenda, R. Ota, K. Yamada, T. Henares, K. Suzuki and D. Citterio, High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis, Micromachines, 2016, 7(5), 80, DOI:10.3390/mi7050080.
- Y. He, Q. Gao, W.-B. Wu, J. Nie and J.-Z. Fu, 3D Printed Paper-Based Microfluidic Analytical Devices, Micromachines, 2016, 7(7), 108, DOI:10.3390/mi7070108.
- G. I. Salentijn, P. E. Oomen, M. Grajewski and E. Verpoorte, Fused Deposition Modeling 3D Printing for (Bio)analytical Device Fabrication: Procedures, Materials, and Applications, Anal. Chem., 2017, 89(13), 7053–7061, DOI:10.1021/acs.analchem.7b00828.
- J. Nie, et al., 3D printed Lego®-like modular microfluidic devices based on capillary driving, Biofabrication, 2018, 10(3), 035001, DOI:10.1088/1758-5090/aaadd3.
- Y. Lu, W. Shi, L. Jiang, J. Qin and B. Lin, Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay, Electrophoresis, 2009, 30(9), 1497–1500, DOI:10.1002/elps.200800563.
- Y. Lu, B. Lin and J. Qin, Patterned Paper as a Low-Cost, Flexible Substrate for Rapid Prototyping of PDMS Microdevices via ‘Liquid Molding’, Anal. Chem., 2011, 83(5), 1830–1835, DOI:10.1021/AC102577N.
- A. To, C. Downs and E. Fu, Wax transfer printing to enable robust barrier definition in devices based on non-standard porous materials, J. Micromech. Microeng., 2017, 27(5), 057001, DOI:10.1088/1361-6439/AA65B2.
- W. Dungchai, O. Chailapakul and C. S. Henry, A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing, Analyst, 2010, 136(1), 77–82, 10.1039/C0AN00406E.
- J. M. Pearce, N. C. Anzalone and C. L. Heldt, Open-Source Wax RepRap 3-D Printer for Rapid Prototyping Paper-Based Microfluidics, J. Lab. Autom., 2016, 21(4), 510–516, DOI:10.1177/2211068215624408.
- C. K. Chiang, A. Kurniawan, C. Y. Kao and M. J. Wang, Single Step and Mask-Free 3D Wax Printing of Microfluidic Paper-Based Analytical Devices for Glucose and Nitrite Assays, Talanta, 2019, 194, 837–845, DOI:10.1016/j.talanta.2018.10.104.
- S. B. Puneeth, M. Salve, R. Akshatha and G. Sanket, Realization of Microfluidic Paper-Based Analytical Devices Using a 3-D Printer: Characterization and Optimization, IEEE Trans. Device Mater. Reliab., 2019, 19(3), 529–536, DOI:10.1109/TDMR.2019.2927448.
- S. B. Puneeth and S. Goel, Novel 3D Printed Microfluidic Paper-Based Analytical Device With Integrated Screen-Printed Electrodes for Automated Viscosity Measurements, IEEE Trans. Electron Devices, 2019, 66(7), 3196–3201, DOI:10.1109/TED.2019.2913851.
- S. B. Puneeth, N. Munigela, S. A. Puranam and S. Goel, Automated Mini-Platform With 3-D Printed Paper Microstrips for Image Processing-Based Viscosity Measurement of Biological Samples, IEEE Trans. Electron Devices, 2020, 67(6), 2559–2565, DOI:10.1109/TED.2020.2989727.
- A. Zargaryan, N. Farhoudi, G. Haworth, J. F. Ashby and S. H. Au, Hybrid 3D printed-paper microfluidics, Sci. Rep., 2020, 10(1), 18379, DOI:10.1038/s41598-020-75489-5.
- Y. He, W. Wu and J. Fu, Rapid fabrication of paper-based microfluidic analytical devices with desktop stereolithography 3D printer, RSC Adv., 2015, 5(4), 2694–2701, 10.1039/C4RA12165A.
- X. Fu, B. Xia, B. Ji, S. Lei and Y. Zhou, Flow controllable three-dimensional paper-based microfluidic analytical devices fabricated by 3D printing technology, Anal. Chim. Acta, 2019, 1065, 64–70, DOI:10.1016/j.aca.2019.02.046.
- C. Park, H.-R. Kim, S.-K. Kim, I.-K. Jeong, J.-C. Pyun and S. Park, Three-Dimensional Paper-Based Microfluidic Analytical Devices Integrated with a Plasma Separation Membrane for the Detection of Biomarkers in Whole Blood, ACS Appl. Mater. Interfaces, 2019, 11(40), 36428–36434, DOI:10.1021/acsami.9b13644.
- C. Park, Y. D. Han, H. V. Kim, J. Lee, H. C. Yoon and S. Park, Double-sided 3D printing on paper towards mass production of three-dimensional paper-based microfluidic analytical devices (3D-μPADs), Lab Chip, 2018, 18(11), 1533–1538, 10.1039/c8lc00367j.
- S. H. Baek, C. Park, J. Jeon and S. Park, Three-Dimensional Paper-Based Microfluidic Analysis Device for Simultaneous Detection of Multiple Biomarkers with a Smartphone, Biosensors, 2020, 10(11), 187, DOI:10.3390/bios10110187.
- J. Jeon, C. Park, D. V. Ponnuvelu and S. Park, Enhanced Sensing Behavior of Three-Dimensional Microfluidic Paper-Based Analytical Devices (3D-μPADs) with Evaporation-Free Enclosed Channels for Point-of-Care Testing, Diagnostics, 2021, 11(6), 977, DOI:10.3390/diagnostics11060977.
- F. Li, N. P. Macdonald, R. M. Guijt and M. C. Breadmore, Increasing the functionalities of 3D printed microchemical devices by single material, multimaterial, and print-pause-print 3D printing, Lab Chip, 2019, 19(1), 35–49, 10.1039/C8LC00826D.
- Q. Wang, J. Sun, Q. Yao, C. Ji, J. Liu and Q. Zhu, 3D printing with cellulose materials, Cellulose, 2018, 25(8), 4275–4301, DOI:10.1007/s10570-018-1888-y.
- G. Chinga-Carrasco, Potential and Limitations of Nanocelluloses as Components in Biocomposite Inks for Three-Dimensional Bioprinting and for Biomedical Devices, Biomacromolecules, 2018, 19(3), 701–711, DOI:10.1021/acs.biomac.8b00053.
- D. Choudhury, S. Anand and M. W. Naing, The arrival of commercial bioprinters - Towards 3D bioprinting revolution!, Int. J. Bioprint., 2018, 4(2), 139, DOI:10.18063/ijb.v4i2.139.
- D. Fichou and G. E. Morlock, Open-Source-Based 3D Printing of Thin Silica Gel Layers in Planar Chromatography, Anal. Chem., 2017, 89(3), 2116–2122, DOI:10.1021/acs.analchem.6b04813.
- E. Jutila, R. Koivunen, I. Kiiski, R. Bollström, T. Sikanen and P. Gane, Microfluidic Lateral Flow Cytochrome P450 Assay on a Novel Printed Functionalized Calcium Carbonate-Based Platform for Rapid Screening of Human Xenobiotic Metabolism, Adv. Funct. Mater., 2018, 28(31), 1802793, DOI:10.1002/adfm.201802793.
- E. Jutila, R. Koivunen, R. Bollström and P. Gane, Wicking and chromatographic properties of highly porous functionalised calcium carbonate coatings custom-designed for microfluidic devices, J. Micromech. Microeng., 2019, 29(5), 055004, DOI:10.1088/1361-6439/ab0941.
- Y. Li, L. Tran, C. D. M. Filipe, J. D. Brennan and R. H. Pelton, Printed Thin Films with Controlled Porosity as Lateral Flow Media, Ind. Eng. Chem. Res., 2019, 58(46), 21014–21021, DOI:10.1021/acs.iecr.9b02177.
- H. Evard, H. Priks, I. Saar, H. Aavola, T. Tamm and I. Leito, A New Direction in Microfluidics: Printed Porous Materials, Micromachines, 2021, 12, 671, DOI:10.3390/MI12060671.
- X. Chen, D. Mo and M. Gong, A Flexible Method for Nanofiber-based 3D Microfluidic Device Fabrication for Water Quality Monitoring, Micromachines, 2020, 11(3), 276, DOI:10.3390/mi11030276.
- C. Achille, et al., 3D Printing of Monolithic Capillarity-Driven Microfluidic Devices for Diagnostics, Adv. Mater., 2021, 33(25), e2008712, DOI:10.1002/adma.202008712.
- E. Saleh, et al., 3D Inkjet Printing of Electronics Using UV Conversion, Adv. Mater. Technol., 2017, 2(10), 1700134, DOI:10.1002/admt.201700134.
- G. D. O'Neil, S. Ahmed, K. Halloran, J. N. Janusz, A. Rodríguez and I. M. Terrero Rodríguez, Single-step fabrication of electrochemical flow cells utilizing multi-material 3D printing, Electrochem. Commun., 2019, 99, 56–60, DOI:10.1016/j.elecom.2018.12.006.
- R. Li, et al., 3D Printing of Mixed Matrix Films Based on Metal–Organic Frameworks and Thermoplastic Polyamide 12 by Selective Laser Sintering for Water Applications, ACS Appl. Mater. Interfaces, 2019, 11(43), 40564–40574, DOI:10.1021/acsami.9b11840.
- C. A. Mandon, L. J. Blum and C. A. Marquette, Adding Biomolecular Recognition Capability to 3D Printed Objects, Anal. Chem., 2016, 88(21), 10767–10772, DOI:10.1021/acs.analchem.6b03426.
- L. Gregurić, 3D Printing Farm – 5 Great Showcases, All3DP, 2019, Available: https://all3dp.com/2/3d-printing-farm-great-showcases/.
- L. Corsini, C. B. Aranda-Jan and J. Moultrie, Using digital fabrication tools to provide humanitarian and development aid in low-resource settings, Technol. Soc., 2019, 58, 101117, DOI:10.1016/j.techsoc.2019.02.003.
- M. Z. Abbas, Industrial applications of 3D printing to scale-up production of COVID-19-related medical equipment, J. 3D Print. Med., 2021, 5(2), 97–110, DOI:10.2217/3dp-2021-0003.
Footnotes |
| † Authors contributed equally. |
| ‡ Note that choice of housing material or whether to include a housing is highly dependent on target application and user requirements including cost. |
| § Although it is certainly useful to know the range of channel widths and barrier widths achievable with a specific fabrication method, many applications of paper microfluidic devices do not require especially small feature sizes. |
| ¶ The latter was noted in the work of Achille et al.63 |
| This journal is © The Royal Society of Chemistry 2022 |