Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave-irradiated rapid synthesis of antimicrobial pyrazine derivatives in reactive eutectic media†
Helen
Schneider
a,
Nabyl
Merbouh
*b,
Sandra
Keerthisinghe
b,
Markus
Antonietti
 a and
Svitlana
Filonenko
a and
Svitlana
Filonenko
 *a
*a
aMax Planck Institut für Kolloid- und Grenzflächenforschung, Am Mühlenberg 1, 14476 Potsdam, Germany. E-mail: svitlana.filonenko@mpikg.mpg.de
bSimon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6, Canada. E-mail: nabyl_merbouh@sfu.ca
First published on 16th November 2022
Abstract
Herein, we report the use of reactive eutectic media from ammonium formate and monosaccharides to synthesize poly(hydroxyalkyl)pyrazines via microwave irradiation. This enabled unprecedented fast rates as well as better atom economy compared to previous methods. We investigated the influence of water on the reaction yield as well as the physical properties of the eutectic media and could thereby drastically reduce the viscosity, while preserving high reaction yields. The results were consistent for different monosaccharides, using fructose, glucose, rhamnose and fucose as reactants. Furthermore, the major reaction products were separated, chemically analyzed and tested for their antimicrobial activity via high-throughput screening.
Introduction
For reasons of environmental concern, there are great efforts to modernize classical chemical procedures, making them cleaner, safer and easier to perform. The necessity for drastic changes towards green and sustainable chemistry and engineering is recognized by the UN, and is supported by its 17 sustainable development goals.1 One important approach in green chemistry is the aspiration to perform reactions under solvent-less conditions following the principle of minimizing the total amount of used solvents. In the particular case of solvent-less synthesis, this also improves the atom economy and energy efficiency of the reaction (no energy is required to heat or remove the solvent) and often provides good product yields in short reaction times due to the high concentration of reactants.2 Furthermore, many reactions rely on toxic or potentially explosive solvents, making solvent-less reactions additionally valuable.3 While solvent-less synthesis most often involves solid state reactions, they often have issues with homogenization and mixing.4 Hence, bringing the reaction mixture to a liquid state at the reaction temperature has obvious advantages. One way to perform such solvent-less reactions is the use of a eutectic melt between the reactants, where the reaction mixture melts at a lower temperature than any of its constituents. However, such melts often form at elevated temperatures and with high viscosities, making them unfavorable for industrial applications.Interestingly, the concept of eutectic systems has gained much attention in the last two decades due to the emergence of so-called deep eutectic solvents (DES).5 Such solvents are eutectic mixtures between Lewis acids and bases; thus hydrogen bond formation occurs and the resulting delocalization of the electron density is the driving force for their formation. By changing the nature and the ratio of constituents, DES are tunable in a broad range of physical and chemical properties and considered the so-called designer solvents. In this sense, they are similar to ionic liquids but in many cases cheaper, easy to prepare by simply mixing the components and biodegradable depending on the components. One of the main drawbacks of DES has been their high viscosity, but this has been tackled in recent years by the use of “solvent in DES”, meaning the introduction of water or other solvents as constituents of the DES but at much lower concentrations than those used in traditional synthesis. In this way viscosity can be drastically reduced while the characteristic properties of DES can still be retained.6–9
In this paper, we transferred the concept of DES dilution to eutectic melts for (almost) solvent-free or solvent-less reactions, one could also call them less-solvent reactions. We applied this concept to Maillard-type reactions for the synthesis of deoxyfructosazine (DOF) and fructosazine (FZ) from ammonium formate and reducing sugars, as shown in Scheme 1 with fructose. As reported previously by our group, ammonium formate and monosaccharides already form reactive eutectic mixtures by themselves10 but in the present work, water was added to the mixture as a third component.
DOF and FZ belong to the non-volatile poly(hydroxyalkyl)pyrazines and occur naturally in food products.11–13 They are produced in the reaction between reducing sugars with ammonium compounds – a Maillard type reaction which yields an elaborate series of products ranging from simple molecules to complex polymeric materials. Due to the pleasant smell and low odor threshold, the food as well as the Tabaco industry have made use of these reaction mixtures for years and they have also been used as food colorants.14 In recent years, isolated products, DOF and FZ have attracted attention as they were found to exhibit interesting biological activities. They showed antimicrobial activity for heat resisting E. coli15,16 as well as promising pharmaceutical activity for the treatment of diabetes,17 cancer18 and as immunomodulators.19
These findings spurred the development of synthesis routes for DOF. Wu et al. used cellobiose and inulin as biomass based starting materials, which were hydrolyzed to glucose and fructose respectively and then reacted with excess ammonium formate in aqueous solution. They optimized reaction conditions in the later step and obtained DOF in 33% yield from glucose and 28% yield from fructose at a temperature of 150 °C for 2 hours.20 Similarly long reaction times and high temperature conditions were reported in a pending patent by the Shanxi Institute of Coal Chemistry of CAS from 2019. The patent used different ammonium salts for the reaction with fructose, again with a large excess of the ammonium salt in aqueous solution. The highest yield of DOF of 60% was reported, using ammonium chloride and with reaction conditions of 120 °C for 2 hours.21 Another approach to synthesize DOF was via the self-condensation of glucosamine and fructosamine. The synthesis was performed in ionic liquids by Jia et al. and required reaction times of 3 hours.22 Faster rates were only reported when catalysts such as boric acid were used23 or by homogeneous catalysis with ionic liquids based on amino acids.22
In summary, the synthesis routes for DOF either suffer from long reaction times and excessive use of ammonium salts or require the use of much more expensive reagents and catalysts. In this paper, we aim to tackle these issues by using eutectic mixtures between monosaccharides, ammonium formate and water. For the monosaccharides, we will use fructose to optimize the reaction conditions and investigate the eutectic mixture. For the optimized conditions and water content, we will also look at the products from glucose and from two deoxy sugars, fucose and rhamnose, which have been much less investigated for this reaction. This diversity of the saccharides covers isomers and molecules with various functionalities and hydrophobicities.
Materials and methods
Materials
D-Fructose, D-glucose and L-rhamnose were purchased from Sigma-Aldrich (St Louis, MO, USA), L-fucose, ammonium formate and pyrazine were purchased from TCI (Tokyo, Japan).General reaction procedure
Eutectic mixtures were prepared by mixing 0.47 g of ammonium formate with different monosaccharides to obtain a molar ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Water was added in different amounts, using molar ratios ranging from 0 to 50. A listing of the educts in molar ratios as well as in weight percentages can be found in Table S1.† Samples were transferred into 10 ml quartz glass vials and after the addition of a magnetic stir bar, sealed with a Teflon-lined cap. Vials were heated under vigorous stirring with a laboratory microwave (Discover SP) with a power of 5 watts for mixtures without water. The power was increased with water addition to enable comparable heating rates. The temperature was monitored by infrared technology. The reactions were performed in the sealed reactors and any loss of components was avoided. Zero time was taken when the desired temperature was reached. Experiments were conducted at 80 °C, 100 °C and 120 °C and performed for 5 to 90 minutes. For comparison, the experiments were also performed with thermal heating in an oil bath, otherwise keeping the same reaction set-up (same reaction vessel and stirring bar).
1. Water was added in different amounts, using molar ratios ranging from 0 to 50. A listing of the educts in molar ratios as well as in weight percentages can be found in Table S1.† Samples were transferred into 10 ml quartz glass vials and after the addition of a magnetic stir bar, sealed with a Teflon-lined cap. Vials were heated under vigorous stirring with a laboratory microwave (Discover SP) with a power of 5 watts for mixtures without water. The power was increased with water addition to enable comparable heating rates. The temperature was monitored by infrared technology. The reactions were performed in the sealed reactors and any loss of components was avoided. Zero time was taken when the desired temperature was reached. Experiments were conducted at 80 °C, 100 °C and 120 °C and performed for 5 to 90 minutes. For comparison, the experiments were also performed with thermal heating in an oil bath, otherwise keeping the same reaction set-up (same reaction vessel and stirring bar).
Analytical methods
Quantitative 1H NMR was applied to calculate the substrate conversion and yields of products using pyrazine as an internal standard as described in the ESI.†
Separation of DOF derivatives and testing of their antimicrobial activity
The separation of DOF derivatives was performed via column chromatography for the reaction mixtures from fructose and rhamnose. The following procedure was used: the reaction mixture was extracted several times with diethyl ether and dichloromethane until a clear extract was obtained. Afterwards the reaction mixture was reduced by vacuum evaporation at 45 °C and separated on a silica column (0.040–0.063 mm) eluted with ethyl acetate/2-propanol/water (4/2/1 by volume). Fractions containing the product were combined and concentrated under reduced pressure at 45 °C. The oil thus obtained was chromatographed again on a silica column (0.040–0.063 mm) eluted with 1-butanol/ethanol/water/ammonium hydroxide (25%) (8/2/2/1 by volume). Fractions containing the expected product were combined, concentrated under reduced pressure at 45 °C and freeze dried overnight. For fructose, the dried product was washed with ethanol, while for rhamnose the product was washed with acetone. After drying in a vacuum oven at 40 °C, the resulting white powder was identified to be the desired product as shown by 1H NMR. DOF from fructose and deoxyrhamnosazine (DOR) from rhamnose were both obtained with a purity of above 97%.In order to simplify the separation procedure, another method was developed, based on semi-preparative HPLC. The reaction mixtures from glucose and from fucose were separated as follows: the reaction mixtures were extracted with ethanol and concentrated under reduced pressure. The resulting oil was injected into an HPLC system, using semi-preparative C18 column (ACE 5, C18 250x21.2) with a flow rate of 15 mL min−1. The same method was used as described above for analytical HPLC. Fractions containing the desired product were combined. For glucose, the product was washed with ethanol, for fucose the product was washed with acetone. The resulting powder was identified to be DOF from glucose (>95% purity) and deoxyfucosazine (DOFu) from fucose (>93% purity).
Each compound was characterized by NMR as well as HR-MS. After at least 40 mg were prepared for each compound, they were tested on their antimicrobial activity. Test were performed with high-throughput screening on 19 different antimicrobial strains. Gram-negative bacteria include E. coli, K. aerogenes, K. pneumonia, S. enterica, A. baumanii, P. alcalifaciens, S. sonnei, V. cholera, P. aeruginosa, Y. pseudotuberculosis and O. anthropi. Gram-positive bacteria include B. subtilis, MSSA, MRSA, S. epidermidis, L. ivanovii, E. faecium, E. faecalis and S. pneumoniae. The test protocol can be found in the ESI.†
Results and discussion
Reaction conditions
The products from sugar-ammonia reactions, so-called ammonia-caramel, have been systematically studied since the 60s24,25 and the mechanism of formation of DOF and FZ has been elucidated in a number of papers by Komoto and co-workers.26–28 Therefore, separation and characterization of the main products by HPLC-MS and 1H NMR was straightforward and is shown in the ESI.†We chose fructose as the monosaccharide to optimize the reaction conditions due to its availability and because it was used in multiple studies by others and gave a good reference point. The influence of the molar ratio of reagents on the reaction yield has been investigated previously by our group.10 The present study, therefore, relied on a molar ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between ammonium formate and monosaccharide because we observed that excess ammonium formate only slightly increased the yields of the main products. Since we used microwave-heating in the present study, the reaction parameters with respect to temperature and time were optimized (Fig. 1). We observed relatively fast reaction rates, and the maximum yield was reached in less than 3 minutes at 120 °C, in 15 minutes at 100 °C and in 40 minutes at 80 °C. While the reaction at 80 °C seems to reach a plateau in the product yield, products at 100 °C and 120 °C underwent degradation under sustained reaction conditions. Interestingly, we could not observe any FZ, which is typically observed as a minor product for this reaction when performing the reaction in water.15,28 Presumably, the fast reaction rates that can be achieved in the eutectic mixtures and especially using the microwave approach solely favored the formation of DOF (ESI†).
1 between ammonium formate and monosaccharide because we observed that excess ammonium formate only slightly increased the yields of the main products. Since we used microwave-heating in the present study, the reaction parameters with respect to temperature and time were optimized (Fig. 1). We observed relatively fast reaction rates, and the maximum yield was reached in less than 3 minutes at 120 °C, in 15 minutes at 100 °C and in 40 minutes at 80 °C. While the reaction at 80 °C seems to reach a plateau in the product yield, products at 100 °C and 120 °C underwent degradation under sustained reaction conditions. Interestingly, we could not observe any FZ, which is typically observed as a minor product for this reaction when performing the reaction in water.15,28 Presumably, the fast reaction rates that can be achieved in the eutectic mixtures and especially using the microwave approach solely favored the formation of DOF (ESI†).
To our knowledge, such fast rates are unprecedented for this reaction and might be traced back to two explanations, which present the novelty of our approach: the use of eutectic mixtures or the use of microwave heating during the reaction.
Regarding microwave heating, it has been observed that organic reactions performed in a microwave can exhibit unexpectedly high reaction rates. Such observations have been made since the mid-1980s, yet the underlying mechanism for the enhanced reactivity due to microwave irradiation is still a subject of an active debate. While the explanation of non-thermal effects is controversial (which are supposed to increase reaction rates not due to thermal effects but due to the polarizing field); it is generally agreed upon that microwave radiation can produce unusual heating effects such as hot-spots or selective heating of components.29–32 Therefore, we investigated the influence of the heating method on the reaction rate by repeating experiments under thermal heating, as shown in Fig. 1a and b. We could not observe any rate enhancement that would suggest a substantial influence of the heating method. Maximum yields were reached in 5 minutes at 120 °C and in 30 minutes at 100 °C. The slightly faster rates during microwave heating can easily be explained by different heating profiles. While microwaves can provide rapid heating of the bulk by direct energy transfer to the reaction mixture, conventional heating comprises a combination of conductive and convective heat transfer, which results in non-uniform heating via the reactor wall and lower heating rates.
Our results suggest that the effect of the heating method is only minor with respect to the reaction rate. That means that the eutectic mixture and the resulting high concentration of (both) reactants must be the reason for the faster rates observed.
Water addition to the reaction mixture
Subsequently, we investigated the effect of water as a third component in the eutectic mixture. Fig. 2 shows that small amounts of water did not decrease the overall reaction yield; however, at a water content above a molar ratio of 7, the product yield suffered from the presence of water. The interesting aspect of this finding is that the addition of water in such small amounts can already greatly reduce the viscosity of the medium, as shown previously for other eutectic systems.8,33 Indeed, we also measured a drastic decrease in viscosity due to water addition as shown in Fig. 3. In this regard, the decrease in viscosity after the reaction can be explained by water formation in the course of the Maillard reaction.A reduction in viscosity is highly beneficial for the use of this reaction on a larger scale, e.g. it would allow the reaction to be performed in a microwave-assisted continuous flow system which are one of the most promising approaches for the scale-up of microwave technology in organic synthesis. At the typical frequency of most microwave reactors of 2.45 GHz, the penetration depth is typically only on the order of centimeters, which makes batch reactions at production scale very difficult. Another advantage is that continuous flow systems benefit from the fast reaction rates of microwave irradiation.34
Another advantage of this approach is the efficient heating of the reaction mixture, compared to dilute systems. This is shown in Fig. 4A, which compares the heating rate of different mixtures by heating 0.5 g of sample for 60 seconds with a fixed 20 W applied power. Water is considered a moderate absorbing solvent to convert microwave energy into heat. Its performance increases with increasing salt concentration as ionic conduction is one of the two fundamental mechanism for absorbing microwave energy (the other one being dipole rotation). The results showed that the reaction mixtures are indeed very good absorbing solvents for microwave energy. Furthermore, samples with the highest concentration of reactants also showed the highest heating rates, which means that microwave energy for heating is used with maximum efficiency. In this way, most of the energy goes into the heating of reactants and less into the solvent molecules. This is visualized in Fig. 4B, which shows that samples with high water content barely reacted while the reactions of samples with the lowest water content (:3 and :5) were close to completion (considering the fast reaction rates at 120 °C in Fig. 1). Interestingly, samples :3 and :5 showed a different heating profile compared to more diluted samples, indicating that these mixtures were in a different dilution regime and did not form aqueous solutions but tertiary eutectic mixtures instead.
Further characterization of the eutectic mixture
We performed DSC measurements for a better understanding of the influence of water content on the eutectic mixture (Fig. 5). Indeed, eutectic mixtures seem to have formed for water contents below a water ratio of 10. These samples lacked the typical melting and crystallization peaks of water but instead showed a glass transition temperature, typical of eutectic mixtures. The sample with a molar ratio of 10 displayed borderline behavior – it still exhibited a glass transition, which was fluctuating with each cycle, but was already showing signs of the melting peak of water. The two samples with the highest dilution did not behave as eutectic mixtures but rather like aqueous solutions and exhibited the typical melting and freezing peaks of water. The decrease of the freezing point from sample :50 to :20 can be explained by the well-known freezing-point depression due to increased solute concentration.We used 1H NMR to take a closer look at hydrogen bonding in our eutectic mixtures. By using DMSO-d6 as a standard but keeping the mixtures separated in a sealed capillary tube, we were able to investigate proton shifts as a function of water dilution (Fig. 6). A change in the chemical shift is a consequence of the change in the electron environment of the proton and thereby the strength of hydrogen bonding. A downfield shift indicates less shielding and thereby stronger hydrogen bonding, upfield shift indicates weaker hydrogen bonds. Under typical dilution (:50–:1000) we observed no change for water and very little change for the protons of fructose and formate ions, meaning that the chemical environment barely changed. However, when the water content decreased further (<:50), molecules started to see each other instead of being surrounded by a hydration shell. The chemical shift indicates that the liquid state could be maintained for reduced water content because the hydrogen bonds in water strongly increased, while hydrogen bonds of fructose and the formate ion weakened to a lesser extent. It seems likely that the increasing ionic strength of the solution enabled stronger polarization of the water molecule thereby enabling stronger hydrogen bonds. This explanation is also supported by the DSC results. As the strength of hydrogen bonding increased, water could not exhibit its typical melting/crystallization behavior but was incorporated into the eutectic mixture instead.
Changing the saccharide reactants
After the reaction conditions were optimized for the fructose reaction, we performed the same reaction with several other monosaccharides. Products and yields are shown below in Table 1, while their characterization using NMR and LC-MS is shown in the ESI.† It is in accordance with previous studies, that aldoses predominantly yield the 2,6-isomers while ketoses predominantly yield the 2,5-isomers. However, the respective other isomer is also observed in small amounts.28 Products from rhamnose and fucose have been less investigated but have been reported before by Bashiardes et al.17 These products are DOF derivatives that differ by the lack of hydroxyl groups on the terminal carbon. In the following we will call them deoxyrhamnosazine (DOR) and deoxyfucosazine (DOFu), following a scheme by Komoto.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monosaccharide
monosaccharide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water in a 1.5
water in a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratio for fructose and glucose and in a 1.5
7 ratio for fructose and glucose and in a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio for rhamnose and fucose. Experiments were performed at 100 °C for 15 minutes
3 ratio for rhamnose and fucose. Experiments were performed at 100 °C for 15 minutes
| Monosaccharide | Major product | Yield | Minor product | Yield |
|---|---|---|---|---|
| D-Fructose | 2,5-Deoxyfructosazine | 37.2% | 2,6-Isomer | 9.3% |
| D-Glucose | 2,6-Deoxyfructosazine | 26.1% | 2,5-Isomer | 5.9% |
| L-Rhamnose | 2,6-Deoxyrhamnosazine | 32.5% | 2,5-Isomer | 11.2% |
| L-Fucose | 2,6-Deoxyfucosazine | 23.9% | 2,5-Isomer | 6.0% |
Considering the effect of the increasing water content (Fig. 7) we have found that the reactions all followed a similar trend: for water dilution above a molar ratio of 7, yields dropped drastically for the chosen reaction conditions. A slightly earlier decrease of yields from rhamnose and fucose might be related to their terminal methyl groups, which increase the hydrophobic nature of the product.
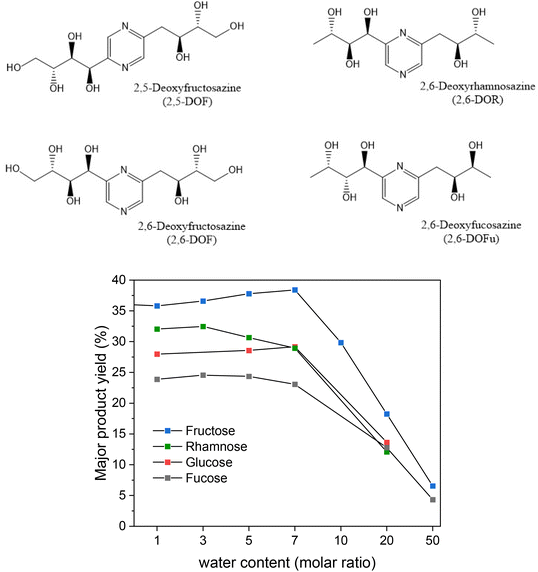 | ||
| Fig. 7 Yields of DOF derivatives from different monosaccharides with varying amounts of water in the reaction mixture. Experiments were performed at 100 °C for 15 minutes. | ||
Study on antimicrobial activity of DOF derivatives
After the separation of the major product from each reaction mixture, the respective compounds were thoroughly characterized, using NMR and HR-MS (ESI†). The antimicrobial properties of these DOF derivatives showed several hits in the high-throughput screening, as shown below in Fig. 8. 1,5-DOF for O. anthropi (TSB) as well as 1,6-DOR for S. pneumoniae (BHI) showed cumulative hits, with inhibition above 50%. They will therefore be investigated by additional screening, in order to quantify their antimicrobial effect on the respective strain.35,36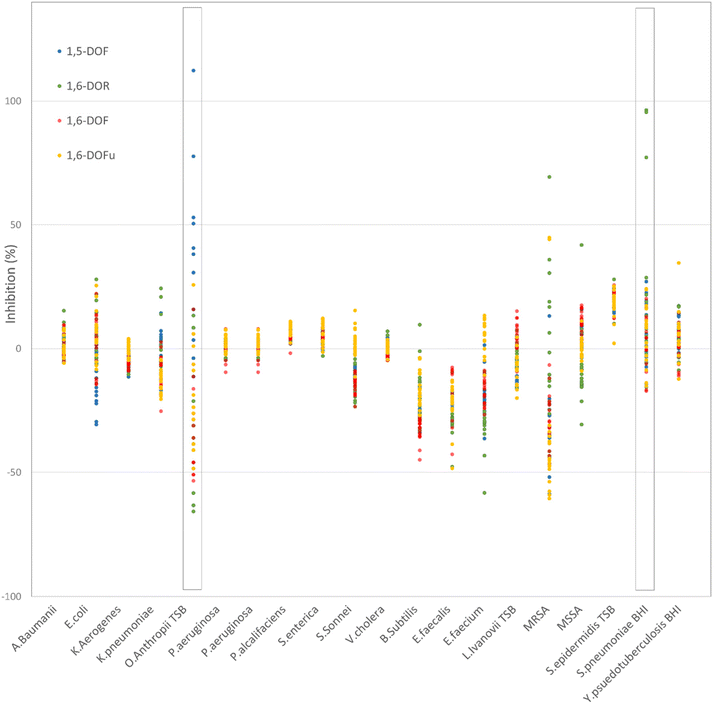 | ||
| Fig. 8 Graphical representation of the high-throughput screening for four different DOF derivative compounds. | ||
Conclusion
In this work, we showed that fructose and ammonium formate form a eutectic mixture where water can be incorporated as a third component. This approach resulted in a much better atom economical synthesis compared to previously reported synthetic routes. Reactants were used in an almost stoichiometric ratio (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of using one compound in great excess. The high concentration of reactants led to unprecedented fast reaction rates with yields up to 37.2% for 2,5-DOF and 9.3% for 2,6-DOF, while the incorporation of water also retained relatively low viscosities. Applying the microwave energy directly to the reaction mixture led to a high energy efficient heating method, making this approach attractive for microwave-assisted continuous flow systems.
1) instead of using one compound in great excess. The high concentration of reactants led to unprecedented fast reaction rates with yields up to 37.2% for 2,5-DOF and 9.3% for 2,6-DOF, while the incorporation of water also retained relatively low viscosities. Applying the microwave energy directly to the reaction mixture led to a high energy efficient heating method, making this approach attractive for microwave-assisted continuous flow systems.
In addition to fructose, other monosaccharides were successfully used as reactants, showing the same trend as fructose with respect to water addition. The respective reaction mixtures were successfully separated, by HPLC proving it to be a straightforward method for the purification of final compounds. The separated DOF derivative compounds were thoroughly characterized and successfully tested for their antimicrobial activity via high throughput screening. Promising candidates were identified and subjected to additional antibacterial screening: 5-DOF for O. anthropi as well as 1,6-DOR for S. pneumoniae.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors also thank Roger Linington (SFU) and the Centre for High-Throughput Chemical Biology (HTCB) for access to their core facilities. This work was financially supported by Max Planck Society. Open Access funding was provided by the Max Planck Society.References
- UN General Assembly, Transforming our world: the 2030 Agenda for Sustainable Development, United Nations, New York, NY, USA, 2015 Search PubMed.
- K. Tanaka, Solvent-free organic synthesis, John Wiley & Sons, 2009 Search PubMed.
- B. M. Sahoo and B. K. Banik, Solvent-less reactions: Green and sustainable approaches in medicinal chemistry, in Green Approaches in Medicinal Chemistry for Sustainable Drug Design, Elsevier, 2020, pp. 523–548 Search PubMed.
- G. Nagendrappa, Organic synthesis under solvent-free condition: An environmentally benign procedure—I, Resonance, 2002, 7(10), 59–68 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids, J. Am. Chem. Soc., 2004, 126(29), 9142–9147 CrossRef CAS PubMed.
- E. Posada, N. López-Salas, R. J. Riobóo, M. L. Ferrer, M. C. Gutiérrez and F. Del Monte, Reline aqueous solutions behaving as liquid mixtures of H-bonded co-solvents: microphase segregation and formation of co-continuous structures as indicated by Brillouin and 1 H NMR spectroscopies, Phys. Chem. Chem. Phys., 2017, 19(26), 17103–17110 RSC.
- N. López-Salas, J. Vicent-Luna, E. Posada, S. Imberti, R. Madero-Castro and S. Calero, et al. Further Extending the Dilution Range of the “Solvent-in-DES” Regime upon the Replacement of Water by an Organic Solvent with Hydrogen Bond Capabilities, ACS Sustainable Chem. Eng., 2020, 8(32), 12120–12131 CrossRef.
- N. López-Salas, J. M. Vicent-Luna, S. Imberti, E. Posada, R. MaJs and J. A. Anta, et al., Looking at the “water-in-deep-eutectic-solvent” system: a dilution range for high performance eutectics, ACS Sustainable Chem. Eng., 2019, 7(21), 17565–17573 CrossRef.
- P. J. Smith, C. B. Arroyo, F. Lopez Hernandez and J. C. Goeltz, Ternary deep eutectic solvent behavior of water and urea–choline chloride mixtures, J. Phys. Chem. B, 2019, 123(25), 5302–5306 CrossRef CAS PubMed.
- S. Filonenko, A. Voelkel and M. Antonietti, Valorization of monosaccharides towards fructopyrazines in a new sustainable and efficient eutectic medium, Green Chem., 2019, 21(19), 5256–5266 RSC.
- H. Tsuchida, M. Komoto and S. Mizuno, Isolation and identification of polyhydroxyalkylpyrazines in soy sauce, Nippon Shokuhin Kogyo Gakkaishi, 1990, 37(2), 154–161 CrossRef CAS.
- R. L. Magaletta and C.-T. Ho, Effect of roasting time and temperature on the generation of nonvolatile (polyhydroxyalkyl) pyrazine compounds in peanuts, as determined by high-performance liquid chromatography, J. Agric. Food Chem., 1996, 44(9), 2629–2635 CrossRef CAS.
- H. Tsuchida, K. Morinaka, S. Fujii, M. Komoto and S. Mizuno, Identification of novel non-volatile pyrazines in commercial caramel colors, Dev. Food Sci., 1986, 13, 85–94 CAS.
- H. E. Nursten, The Maillard reaction: chemistry, biochemistry, and implications, Royal Society of Chemistry, 2005 Search PubMed.
- A. Bhattacherjee, Y. Hrynets and M. Betti, Fructosazine, a polyhydroxyalkylpyrazine with antimicrobial activity: mechanism of inhibition against extremely heat resistant Escherichia coli, J. Agric. Food Chem., 2016, 64(45), 8530–8539 CrossRef CAS PubMed.
- Y. Hrynets, A. Bhattacherjee, M. Ndagijimana, D. J. Hincapie Martinez and M. Betti, Iron (Fe2+)-catalyzed glucosamine browning at 50 °C: identification and quantification of major flavor compounds for antibacterial activity, J. Agric. Food Chem., 2016, 64(16), 3266–3275 CrossRef CAS PubMed.
- G. Bashiardes, J.-C. Carry, M. Evers, B. Filoche and S. Mignani, Polyhydroxyalkylpyrazine derivatives, their preparation and medicaments comprising them, US6407110B2, 2002.
- V. Chesnokov, B. Gong, C. Sun and K. Itakura, Anti-cancer activity of glucosamine through inhibition of N-linked glycosylation, Cancer Cell Int., 2014, 14(1), 1–10 CrossRef PubMed.
- A. Zhu, J.-B. Huang, A. Clark, R. Romero and H. R. Petty, 2, 5-Deoxyfructosazine, a D-glucosamine derivative, inhibits T-cell interleukin-2 production better than D-glucosamine, Carbohydr. Res., 2007, 342(18), 2745–2749 CrossRef CAS PubMed.
- S. Wu, H. Fan, Q. Zhang, Y. Cheng, Q. Wang and G. Yang, et al. Conversions of cellobiose and inulin to deoxyfructosazine in aqueous solutions, Clean: Soil, Air, Water, 2011, 39(6), 572–576 CAS.
- Y. Wang, X. Hou, L. Jia, P. Liu and Y. Qiao, Inventor A method of 2,5- deoxy fructosazine is prepared using fructose, China, 2019 Search PubMed.
- L. Jia, Y. Wang, Y. Qiao, Y. Qi and X. Hou, Efficient one-pot synthesis of deoxyfructosazine and fructosazine from D-glucosamine hydrochloride using a basic ionic liquid as a dual solvent-catalyst, RSC Adv., 2014, 4(83), 44253–44260 RSC.
- L. Peng, L. Binbin, X. Jianping, Z. Yongli, H. Jun and R. Chengjie, Inventor Process for preparing deoxy fructosazine, China, 2006 Search PubMed.
- I. Ježo and I. Luzak, Aminolysis of sucrose. V. The reaction between sucrose and aqueous ethanolamine solution, Chem. Pap., 1964, 18(11), 837–851 Search PubMed.
- R. Kuhn, G. Krüger, H. J. Haas and A. Seeliger, Aminozucker–Synthesen, XXIV. Pyrazinbildung aus Aminozuckern, Justus Liebigs Ann. Chem., 1961, 644(1), 122–127 CrossRef CAS.
- H. Tsuchida, M. Kōmoto, H. Kato and M. Fujimaki, Isolation of deoxyfructosazine and its 6-isomer from the nondialyzable melanoidin hydrolyzate, Agric. Biol. Chem., 1975, 39(5), 1143–1148 CAS.
- H. Tsuchida, S. Tachibana and M. Komoto, Isolation and Identification of 2-(d-threo-Trihydroxypropyl)-5-(d-glycero-2′, 3′-dihydroxypropyl) pyrazine and Its 6-Isomer from the Browning Reaction between Xylose and Ammonium Formate, Agric. Biol. Chem., 1976, 40(6), 1241–1242 CAS.
- H. Tsuchida, S. Tachibana, K. Kitamura and M. Komoto, Formation of deoxyfructosazine and its 6-isomer by the browning reaction between fructose and ammonium formate, Agric. Biol. Chem., 1976, 40(5), 921–925 CAS.
- G. B. Dudley, R. Richert and A. Stiegman, On the existence of and mechanism for microwave-specific reaction rate enhancement, Chem. Sci., 2015, 6(4), 2144–2152 RSC.
- C. O. Kappe, Controlled microwave heating in modern organic synthesis, Angew. Chem., Int. Ed., 2004, 43(46), 6250–6284 CrossRef CAS PubMed.
- A. Loupy and R. S. Varma, Microwave effects in organic synthesis, Chim. Oggi, 2006, 24(3), 36 CAS.
- P. Priecel and J. A. Lopez-Sanchez, Advantages and limitations of microwave reactors: from chemical synthesis to the catalytic valorization of biobased chemicals, ACS Sustainable Chem. Eng., 2018, 7(1), 3–21 CrossRef.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Tailoring properties of natural deep eutectic solvents with water to facilitate their applications, Food Chem., 2015, 187, 14–19 CrossRef CAS PubMed.
- A. de la Hoz, A. Díaz-Ortiz and P. Prieto, Microwave-assisted green organic synthesis, 2016 Search PubMed.
- W. R. Wong, A. G. Oliver and R. G. Linington, Development of antibiotic activity profile screening for the classification and discovery of natural product antibiotics, Chem. Biol., 2012, 19(11), 1483–1495 CrossRef CAS PubMed.
- P. M. Hawkins, D. M. Hoi, C.-Y. Cheung, T. Wang, D. Quan and V. M. Sasi, et al., Potent Bactericidal Antimycobacterials Targeting the Chaperone ClpC1 Based on the Depsipeptide Natural Products Ecumicin and Ohmyungsamycin A, J. Med. Chem., 2022, 65(6), 4893–4908 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc03122a |
| This journal is © The Royal Society of Chemistry 2022 |