1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN)-based porous organic polymers for visible-light-driven organic transformations in water under aerobic oxidation†
Abstract
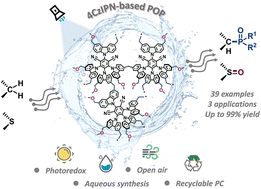
A series of hyper-crosslinked porous organic polymers (POPs) were constructed by a facile Friedel–Crafts alkylation reaction of the photoactive unit 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) with formaldehyde dimethyl acetal (FDA), which contains a certain quantity of hydrophilic ether group residues at the terminal of the polymetric networks to improve the dispersibility of POPs. Among them, POP-3 was demonstrated to be a robust heterogeneous photocatalyst for visible-light-promoted aqueous organic transformations, including the C(sp3)–P bond construction and selective oxidation of sulfides in water under mild conditions. Synthetic application of this protocol can be expediently extended to the phosphorylation of commercial drug molecules and the detoxification of the mustard gas simulant CEES. Moreover, the finely designed POP catalysts showed excellent stability, a high surface area and pore volume, and outstanding photoelectric response capability, along with good catalytic performance and reusability properties, which make these materials economical, sustainable, and eco-friendly photocatalysts.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...