 Open Access Article
Open Access ArticleBenzaldehyde-mediated selective aerobic polyethylene functionalisation with isolated backbone ketones†
Jerald Y. Q.
Teo‡
a,
Celine W. S.
Yeung‡
 a,
Tristan T. Y.
Tan
a,
Tristan T. Y.
Tan
 a,
Wei Wei
Loh
a,
Xian Jun
Loh
a,
Wei Wei
Loh
a,
Xian Jun
Loh
 ab and
Jason Y. C.
Lim
ab and
Jason Y. C.
Lim
 *ab
*ab
aInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Singapore 138634. E-mail: jason_lim@imre.a-star.edu.sg
bDepartment of Materials Science and Engineering, National University of Singapore (NUS), 9 Engineering Drive 1, Singapore 117576
First published on 26th July 2022
Abstract
Post-synthetic functionalisation of commodity polymers such as polyethylene (PE) is an increasingly important strategy for giving waste plastics new ‘lease-of-lives’ as functional polymers. In particular, installing oxygenated functional groups such as alcohols and ketones is valuable due to their chemical versatility for further chemical modifications. However, this is especially challenging for PE due to its unreactive nature, and often requires hazardous and environmentally damaging chlorinated solvents owing to PE's excellent solvent resistance. Herein, we demonstrate a highly selective method to install isolated carbonyl groups on PE using only benzaldehyde and O2 as a terminal oxidant, without the need for stoichiometric organic oxidants or any hazardous organic solvents. This process relies on the aerobic auto-oxidation of benzaldehyde to benzoic acid, generating reactive radical intermediates in the process that are responsible for both C–H activation and exclusive carbonyl formation. Our method is compatible with copper(II) oxidation catalysts to enhance the extent of PE oxidation, and can also minimise the waste produced as the benzoic acid side product can be easily isolated and reused for different applications. The versatility of carbonyl-containing PEs for further chemical derivatisation offers many exciting options for transforming waste PE into diverse polymeric materials for a materials circular economy.
Introduction
Polyethylenes (PEs) are the single most widely-produced class of plastics today due to their low cost, versatility, durability and processibility. Highly popular for many domestic and industrial applications, particularly as packaging materials,1,2 more than 100 million tonnes of PE waste were produced in 2015 alone, with the vast majority disposed in landfills or incinerated.3 The PEs’ extremely linear and unsustainable cradle-to-grave life cycles are largely due to their chemical inertness towards hydrolysis and chemical degradation, as these polymers are exclusively made up of strong C–C and C–H bonds. This not only makes them highly environmentally persistent (taking up to 400 years to degrade),4 but it also severely hinders attempts to chemically recycle and valorise them into new functional products.5,6In recent years, post-synthetic chemical modification of PEs has emerged as a promising enabling technology for transforming these commodity polymers into functional materials for targeted applications.6–8 On top of leveraging on the existing high-volume industrial PE production capabilities, post-synthetic functionalisation also offers opportunities to mitigate the global plastic waste crisis. In particular, methods to install oxygenated functional groups (i.e. alcohols and ketones) on the inert PE polymer are attractive. These groups can imbue PEs with new properties, such as enhanced adhesiveness.9 Furthermore, the well-established and versatile reactivity of these oxygenated functionalities allow further modifications (e.g. ring opening polymerisation on alcohols and reductive amination on carbonyls) to access new applications such as polymer blend compatibilisation.10 The presence of in-chain carbonyls can also enhance the PEs’ photodegradability through Norrish-type photochemical chain-scission reactions.11,12 Installing these oxygenated groups, however, is challenging. Free radical chemistry often leads to excessive and uncontrollable chain cleavage, compromising on polymer strength and processibility.13 Although these undesirable side reactions can be elegantly circumvented by using organometallic catalysts,9,10 multiple functionalities (i.e. both OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are installed at the same time. Thus far, exclusive post-synthetic functionalisation of PE with carbonyls has yet to be achieved; such carbonyl-containing PEs were previously only accessible by catalytic copolymerisation of ethylene with carbon monoxide14–17 or metal carbonyls.12 However, these processes have the disadvantages of the need for high gas pressures of ethylene and/or hazardous carbon monoxide, relatively low polymer molecular weights, and poor selectivity for obtaining isolated carbonyls over alternating ones.
O) are installed at the same time. Thus far, exclusive post-synthetic functionalisation of PE with carbonyls has yet to be achieved; such carbonyl-containing PEs were previously only accessible by catalytic copolymerisation of ethylene with carbon monoxide14–17 or metal carbonyls.12 However, these processes have the disadvantages of the need for high gas pressures of ethylene and/or hazardous carbon monoxide, relatively low polymer molecular weights, and poor selectivity for obtaining isolated carbonyls over alternating ones.
Herein, we report a new approach to post-synthetically install carbonyls selectively onto PE that uses only O2 as the oxidant without non-renewable, hazardous chlorinated solvents and stoichiometric organic oxidants. As shown in Fig. 1, our approach capitalises on the well-known non-catalytic auto-oxidation of aldehydes to acids in O2,18,19 which forms a series of reactive radical intermediates that can also simultaneously oxidise the C–H bonds of PE. The use of O2 is attractive because it is abundant, inexpensive and non-polluting as it generates only water as the side product. Under our solvent-free conditions, benzaldehyde was chosen both as the reagent and bulk dispersant due to its low acute toxicity,20 low cost, miscibility with PE, and proven possibility for sustainable production from renewable biomass sources.21–23 Our method is unique for its possibility of low waste generation: thus far, PE's excellent solvent resistance24 has necessitated the use of hazardous petroleum-derived halogenated or aromatic solvents (e.g. 1,2-dichloroethane and xylenes). Therefore, eliminating them removes the single largest source of waste from any reaction, as they are almost never recovered for reuse.25 Furthermore, the avoidance of stoichiometric petroleum-derived organic reagents or oxidants eliminates any resulting organic by-products (e.g. 2,6-dichloropyridine from the 2,6-dichloropyridine N-oxide oxidant).9 Notably, the benzoic acid side product generated in our method is a commodity of immense industrial importance26 that can be easily isolated from the reaction in high purity and yield by recrystallisation with hot water.
 | ||
| Fig. 1 (A) Existing non-selective methods for oxidative PE post-synthetic functionalisation.9,10 (B) This work: halogenated-solvent-free aerobic functionalisation of PE with carbonyl groups exclusively. | ||
Results and discussion
Aerobic oxidation of PE facilitated by benzaldehyde
Oxidation of PE was performed by dissolving the polymer in benzaldehyde at 120 °C and stirring the reaction mixture under an O2 atmosphere for 24 hours. As the reaction progressed, increasing extents of benzaldehyde aerobic oxidation to benzoic acid were accompanied by a gradual appearance of a well-defined triplet 1H NMR signal (C2D2Cl4, 80 °C) at δ = 2.40 ppm (Fig. S1†), which is characteristic of isolated carbonyl groups on the polymer chain.9,27 After 24 hours, the oxidised PE was isolated in excellent yields (>90%) by simple precipitation in simple alcohols such as methanol or ethanol, which have been identified as environmentally preferrable.28 As shown in Fig. 2A, the 1H NMR spectrum of the isolated polymer showed no discernible signals at δ = 4.1 ppm [C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2–O–C(O)–], 3.6 ppm (C
2–O–C(O)–], 3.6 ppm (C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) –OH–) and 2.3 ppm [–O–C(O)–C
–OH–) and 2.3 ppm [–O–C(O)–C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2], clearly showing the selectivity of this reaction for C–H oxidation of PE to form in-chain carbonyls preferentially over esters and alcohols. Notably, the lack of signals at ∼2.7 ppm, corresponding to closely spaced carbonyl groups [e.g. –C(O)–C
2], clearly showing the selectivity of this reaction for C–H oxidation of PE to form in-chain carbonyls preferentially over esters and alcohols. Notably, the lack of signals at ∼2.7 ppm, corresponding to closely spaced carbonyl groups [e.g. –C(O)–C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2–CH2–C(O)–],12,27 shows that the carbonyls are separated by long methylene segments, and not clustered closely together. This is further corroborated by FTIR analysis (Fig. 2B), which showed the characteristic and prominent C
2–CH2–C(O)–],12,27 shows that the carbonyls are separated by long methylene segments, and not clustered closely together. This is further corroborated by FTIR analysis (Fig. 2B), which showed the characteristic and prominent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch for isolated carbonyls at 1720 cm−1,27 and a complete absence of absorption peaks at 1690 cm−1 and 3200 cm−1 for closely spaced carbonyl and hydroxyl groups, respectively. 13C NMR characterisation of the oxidised PE provided further evidence of carbonyl formation from the diagnostic C
O stretch for isolated carbonyls at 1720 cm−1,27 and a complete absence of absorption peaks at 1690 cm−1 and 3200 cm−1 for closely spaced carbonyl and hydroxyl groups, respectively. 13C NMR characterisation of the oxidised PE provided further evidence of carbonyl formation from the diagnostic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and α-methylene resonances at 211 and 43 ppm, respectively (Fig. S2†), which is consistent with that of PE containing isolated carbonyl groups previously reported.12 Using the relative integration of the carbonyl's α-methylene 1H NMR signals at 2.40 ppm to the signals of the unreacted methylene groups (0.8–1.6 ppm) (Fig. S3†), we obtained a total degree of PE functionalisation (TF) of 1.2% (Table 1, entry 1). This is notable for being the first known method to exclusively install carbonyl groups post-synthetically onto PE. Furthermore, benzoic acid could be easily isolated with excellent yields (>80%) and purity (Fig. S4†) by simple recrystallisation using hot water without any organic solvents.
O and α-methylene resonances at 211 and 43 ppm, respectively (Fig. S2†), which is consistent with that of PE containing isolated carbonyl groups previously reported.12 Using the relative integration of the carbonyl's α-methylene 1H NMR signals at 2.40 ppm to the signals of the unreacted methylene groups (0.8–1.6 ppm) (Fig. S3†), we obtained a total degree of PE functionalisation (TF) of 1.2% (Table 1, entry 1). This is notable for being the first known method to exclusively install carbonyl groups post-synthetically onto PE. Furthermore, benzoic acid could be easily isolated with excellent yields (>80%) and purity (Fig. S4†) by simple recrystallisation using hot water without any organic solvents.
| S/N | Dispersant | Gasb | T/°C | Molar ratio of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COOH COOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OHc OHc |
TF/%c |
|---|---|---|---|---|---|
a Reactions were performed under ambient lighting conditions for 24 hours (unless otherwise stated) under constant stirring, with 3 molar equivalents of benzaldehyde w.r.t. the number of PE repeating units present unless otherwise stated.
b Under 1 atmospheric pressure delivered using a balloon.
c Determined from the 1H NMR spectra of the oxidised PE polymers (C2D2Cl4, 80 °C) by relative integration of the peaks arising from C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) -OH (3.6 ppm), C -OH (3.6 ppm), C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2COOH (2.3 ppm), C 2COOH (2.3 ppm), C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2C(O)C 2C(O)C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2 (2.4 ppm) and unreacted C 2 (2.4 ppm) and unreacted C![[H with combining low line]](https://www.rsc.org/images/entities/b_i_char_0048_0332.gif) 2s (0.8–1.6 ppm). Spectra are shown in Fig. S3 to S16 in the ESI.†
d DCH = dicyclohexyl. 2s (0.8–1.6 ppm). Spectra are shown in Fig. S3 to S16 in the ESI.†
d DCH = dicyclohexyl.
|
|||||
| 1 | Benzaldehyde | O2 | 120 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.2 |
| 2 | Benzaldehyde | Ar | 120 | — | 0 |
| 3 | Benzaldehyde | Air | 120 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.7 |
| 4 | Benzaldehyde | O2 | 100 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.5 |
| 5 | Benzaldehyde | O2 | 140 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.3 |
| 6 | Benzaldehyde (1 eqv.) | O2 | 120 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.5 |
| 7 | 1,2,4-TCB | O2 | 120 | — | 0 |
| 8 | Benzaldehyde (in the dark) | O2 | 120 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.1 |
| 9 | Furfural | O2 | 130 | — | 0 |
| 10 | Benzaldehyde + benzyl alcohol (1 eqv) | O2 | 120 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.9 |
| 11 | Benzaldehyde + benzyl alcohol (6 eqv) | O2 | 120 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.3 |
| 12 | Benzaldehyde + [CuCl2·DCH18-crown-6] (1 mol%)d | O2 | 120 | 61.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.3 38.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
3.8 |
| 13 | Benzaldehyde + CuCl2 (1 mol%) | O2 | 120 | 61.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.3 29.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.7 9.7 |
1.8 |
A series of control experiments were then performed to better understand the requirements for PE oxidation. The necessity of O2 as an oxidant is evident from the lack of carbonyl formation under an argon atmosphere (entry 2), and a smaller extent of PE oxidation when air (21% O2) was used instead of pure O2 (entry 3). While a lower reaction temperature of 100 °C expectedly caused less oxidation (entry 4), raising the reaction temperature to 140 °C brought about increased PE oxidation (entry 5). The usage of less benzaldehyde (1 eqv. w.r.t. ethylene repeating units instead of 3) resulted in much lower TF, albeit also with exclusive carbonyl functionalisation (entry 6). Although PE can possess alkene defects that can result in the addition of hydroxyl, carbonyl and acid groups under aerobic oxidation,29,30 the lack of any functionalisation observed in O2 when benzaldehyde was replaced with 1,2,4-trichlorobenzene solvent (entry 7 and Fig. S10†) showed that the PE starting material by itself could not be oxidised under these reaction conditions. This was further corroborated when no oxidation was detected when molten PE was heated at 120 °C for 24 hours in the absence of benzaldehyde (Fig. S17†). In addition, to prove that benzaldehyde was able to facilitate the oxidation of the saturated C–H bonds of PE, we used identical reaction conditions to those in Table 1, entry 1 on n-icosane (linear C20H42), which afforded a similar TF of 1.3 with exclusive carbonyl functionalisation (Fig. S18†). Finally, to exclude the possibility of trace metal contamination on the surface defects of stirrer bars acting as the active oxidation catalyst,31 the PE oxidation reaction was repeated on a larger scale (2.5 g of PE) using a new, unused stirrer bar that was soaked overnight with the glassware in aqua regia to simultaneously investigate the effect of reaction scale-up. Our results showed that a comparable degree of PE oxidation (TF = 0.8) could also be achieved with exclusive carbonyl group formation (Fig. S19†), where by the slight diminution compared to that of Table 1, entry 1 is attributed to the less efficient O2 mixing with the liquid-phase reaction upon scale-up. These control experiments demonstrated that benzaldehyde was essential for aerobic C–H oxidation to carbonyls on PE.
The properties of carbonyl-containing PE (from Table 1, entry 1) were compared against the pre-oxidised PE to evaluate the influence of the benzaldehyde-mediated oxidation. Water contact angle measurements showed a slight decrease in the contact angle from (101.5 ± 0.1)° to (99.2 ± 0.2)° after oxidation (Fig. S20†), which is consistent with a slight increase in the surface hydrophilicity of the polymer due to the presence of carbonyl groups. Although high-temperature GPC analysis revealed that some C–C chain scission occurred during the PE oxidation process (Fig. S21†), thermogravimetric analysis (TGA) of both polymers showed comparable degradation temperatures (Td) (Fig. S22A†). Furthermore, differential scanning calorimetry (DSC) analysis revealed that the endothermic peak corresponding to the polymer melting temperature (Tm) did not shift considerably after oxidation (Fig. S22B†). These results indicated that the presence of carbonyl groups and changes in molecular weight distribution after oxidation did not adversely affect the stability of the PE towards thermal processing and melt properties of the oxidised polymer to a significant extent. With the versatile reactivity of carbonyls, there are ample opportunities for further chemical modifications of these PEs for transformation into functional polymers.
Finally, to verify that our benzaldehyde-mediated aerobic PE oxidation process could also be applicable to actual PE plastic waste, we reacted 300 mg of a commercial LDPE plastic bag with benzaldehyde at 120 °C under O2. While the oxidised polymer product obtained after 24 hours was found to be similarly functionalised exclusively with carbonyl groups by 1H NMR spectroscopy (Fig. S23†), a considerably higher TF of 2.4 was obtained. This could be attributed to the high degree of branching in LDPE, whose many tertiary carbon atoms are more reactive to activation by H-abstraction (vide infra), leading to more extensive subsequent radical chain scission and carbonyl formation (vide infra).
Mechanism of PE oxidation
The mechanism of PE oxidation by benzaldehyde was further probed through a series of experimental and computational studies. Firstly, we ruled out the involvement of triplet carbonyls generated by the irradiation of benzaldehyde in hydrogen abstraction from PE,32,33 as well as the contributions of singlet oxygen (1O2) produced by photosensitisation in PE oxidation,34 as repeating the reaction in the dark did not decrease the resulting TF (Table 1, entry 8). Next, we determined that the auto-oxidation of benzaldehyde was responsible for PE oxidation by performing a negative control using furfural. Furfural undergoes auto-oxidation much more slowly compared to benzaldehyde, and it is known to proceed via a different mechanism which is not initiated by aldehyde H-abstraction35 (Fig. S12C†), unlike the case with benzaldehyde.19 Indeed, no observable PE oxidation in O2 was observed with furfural, even at a higher reaction temperature of 130 °C for 24 hours (Table 1, entry 9), despite some furfural oxidation occurring to form polyaromatics likely responsible for the dark colouration of the reaction mixture. Furthermore, when increasing quantities of benzyl alcohol—a known inhibitor of benzaldehyde auto-oxidation19—were added to benzaldehyde, the extent of PE oxidation was reduced considerably (Table 1, entries 10 and 11). Although the low TF of 0.3 (Table 1, entry 11) observed in the presence of 6 equivalents of benzyl alcohol could result from benzaldehyde dilution, we were able to rule out this effect by replacing the benzyl alcohol with a similar volume of 1,2,4-TCB solvent, which elicited a much higher TF of 0.9 (Fig. S24†). As the inhibitory effects of benzyl alcohol stem from intercepting the benzoylperoxy radical intermediates that play a key role in benzoic acid formation,19 these experiments suggested that these radicals, formed after initial formyl H-abstraction from benzaldehyde,36 are likely primarily responsible for PE C–H oxidation.Having gained some evidence that the oxidation was facilitated by radical species generated after the abstraction of the formyl hydrogen atom of benzaldehyde, we turned to computational modelling to determine which of such species were involved in the activation of PE. We also used modelling to gain insights into the exclusive carbonyl selectivity of the reaction, using n-pentane as a model compound for PE.§ All calculations were performed using ORCA 5.0.3.37–39
We first evaluated the reactivities of the various radical species known to be generated during benzaldehyde auto-oxidation,19 and their reactivities in hydrogen atom transfer (HAT) from both benzaldehyde and n-pentane (Fig. 3). The HAT from n-pentane by the benzoyl radical was found to proceed over a high activation barrier of 33.4 kcal mol−1 (TS1); hence this route is unlikely to be feasible for PE activation. Addition of O2 to the benzoyl radical yields the benzoylperoxy radical, which was found to react with both n-pentane and benzaldehyde with almost equal reaction barriers (TS2A 28.5 and TS3 28.1 kcal mol−1, respectively). This makes the benzoylperoxy radical a likely candidate for the active species in the PE activation, as it is consumed by both benzaldehyde and PE at similar rates.
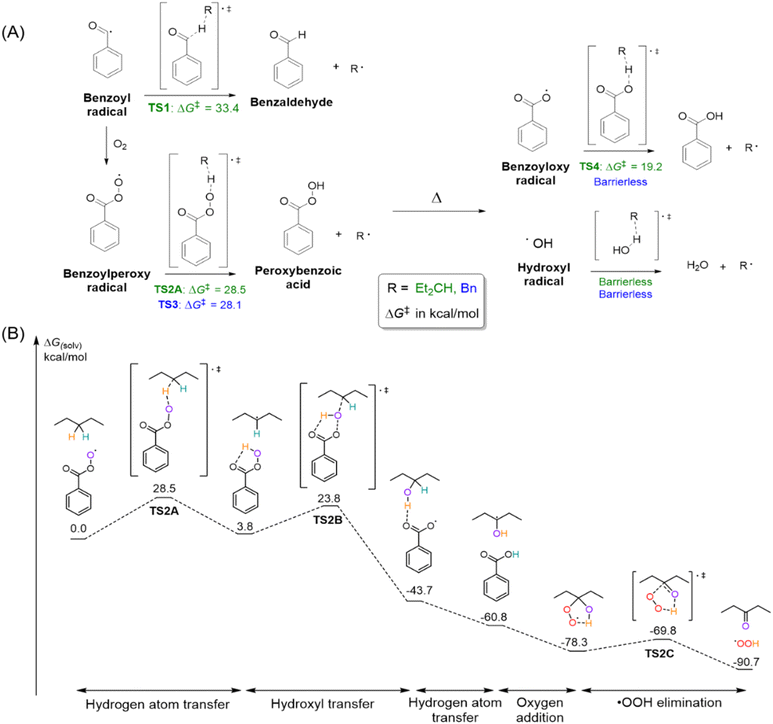 | ||
| Fig. 3 (A) Outline of radicals generated during benzaldehyde auto-oxidation, and the reaction barriers of the various radicals in their reaction with either n-pentane (green) or benzaldehyde (blue). Energies (in kcal mol−1) calculated at the DLPNO-CCSD(T)/def2-TZVP//B3LYP-D3/def2-TZVP level of theory.47,48 (B) Reaction profile between n-pentane and benzoylperoxy radicals. Reactive atoms are highlighted in colour to show their movement between molecules clearly. | ||
Peroxybenzoic acid, generated from the HAT by the benzoylperoxy radical, would yield a benzoyloxy radical and a hydroxyl radical upon O–O bond homolysis. While the benzoyloxy radical was found to react with n-pentane with a low barrier of 19.2 kcal mol−1 (TS4), the HAT from benzaldehyde to the benzoyloxy radical was found to be barrierless; hence the concentration of the benzoyloxy radical would have been too low to be an active species in PE oxidation. Finally, both benzaldehyde and n-pentane undergo HAT to the hydroxyl radical without any energetic barrier. Thus, the benzoylperoxy and hydroxyl radicals are most likely responsible for PE activation by the HAT.
As the reactions of saturated hydrocarbons with hydroxyl and hydroperoxyl radicals have already been well-studied,40–42 we probed the reaction between the benzoylperoxy radical and n-pentane in more detail (Fig. 3B), again using n-pentane as a model system for PE. As discussed earlier, the HAT between n-pentane and benzoylperoxy radicals is likely to proceed at 120 °C (TS2A, 28.5 kcal mol−1). The hydroxyl transfer between the peroxybenzoic acid and the generated carbon radical can proceed over a lower step barrier of 20.0 kcal mol−1 (TS2B). These two steps strongly resemble the oxygen rebound mechanism, which is an important pathway for the enzymatic hydroxylation of hydrocarbons. Similar metal-free rebound reactions have been previously studied in other peroxy acids36,43 and oxiranes.44 The electronic energy component of the reaction barrier TS2B is very low (ΔE‡el = 1.9 kcal mol−1, see ESI section 3.3 for details) and hence the rate of the hydroxyl transfer step can be considered diffusion-controlled. The reaction of the C-centred radical with 3O2 to give the alkyl peroxy radical is also diffusion-controlled, after which the reaction can propagate as previously reported,41 or stop via Russell termination45,46 (see the ESI† for details).
The transfer of the electrophilic hydroxyl group of the peracid to the nucleophilic C-centred radical is highly exergonic, with the free energy of the alcohol and benzoyloxy radical being −43.7 kcal mol−1 downhill of the starting material. Subsequent abstraction of the alcohol Cα–H by the benzoyloxy radical proceeds without a barrier (see Fig. 3B), and this high reactivity can be attributed to the weakening of the Cα–H bond due to hydrogen bonding between the alcohol and the benzoyloxy radical.49–52 The barrierless abstraction of the Cα–H bond corroborates the experimental observation that no alcohols were observed in the final oxidised polymers. Finally, addition of O2 to the carbon radical, followed by the elimination of a hydroperoxyl radical (TS2C), gives the product ketone. The reaction can then propagate through an attack of the hydroperoxyl radical on benzaldehyde.
Computational modelling also revealed useful insights into the lack of 1,2- or 1,3 diketones in the oxidised PE. Using 2-heptone as a model compound for a polyethylene chain bearing some ketones, we investigated the C–H activation step of the various positions along the 2-heptone chain by the benzoylperoxy radical (Scheme S1†). Although a HAT from the ketone's α-carbon to the benzoylperoxy radical leads to the most stable radical product due to carbonyl conjugation, the Cα–H activation barrier is high (30.9 kcal mol−1). Indeed, hydrogen abstraction barriers at all the positions (Cα-δ) are similar (28–30 kcal mol−1). This agrees with observations from prior studies, which report that the reactivity of electrophilic radicals with hydrogens near a ketone is kinetically disfavored.53–55 Thus, due to the absence of any specially activated methylene units on the carbonyl-containing PE towards HAT, methylene oxidation occurs at random along the PE chain.
Finally, we used computational modelling to investigate the reaction of peroxybenzoic acid with benzaldehyde, via a Baeyer–Villiger type mechanism. Baeyer–Villiger reactions can be acid-catalysed,56 and the benzoic acid produced in the reaction might well facilitate such a reaction, which would compete with the hydroxyl transfer reaction described earlier. However, we found that the reaction barriers associated with such a reaction are too high (56.7 kcal mol−1 uncatalysed, 55.6 and 41.1 kcal mol−1 catalysed); thus, the rate of consumption of peroxybenzoic acid by benzaldehyde can be considered insignificant (see Scheme S3† for details).
We then evaluated the influence of different benzaldehyde aromatic substituents on their efficacy for aerobic PE oxidation (see section S4†). Due to the high melting points of some of the resulting carboxylic acids beyond the reaction temperature, the reactions had to be performed in the presence of 1,1,2,2-tetrachloroethane (TCE) solvent to ensure PE solubility and effective stirring throughout their durations for access to O2.¶ The presence of TCE solvent resulted in less selective PE oxidation: even the reaction with benzaldehyde at a lower temperature of 100 °C resulted in additional chlorine functionalisation (3.9 ppm) in addition to isolated ketones. While isolated ketones remained the most abundant functional group in all cases, different benzaldehyde derivatives resulted in varying amounts of closely spaced carbonyls (δ ∼2.7 ppm), esters (4.1 and 2.3 ppm), carboxylic acids (2.3 ppm), alcohols (3.6 ppm) and chlorine (3.9 ppm) substituents (Table S3†). We attribute this to the possibility of hydrogen and chlorine-abstraction from the haloalkane by the various reactive radicals10,36 formed during the course of the reaction. It is notable that when the reaction was performed using benzaldehyde in the presence of 1,2,4-TCB as mentioned earlier (vide supra), less selective carbonyl functionalisation was also observed (Fig. S24†). These findings showed that PE oxidation in neat benzaldehyde without additional chlorinated solvents clearly afforded superior carbonyl selectivity.
Generally, more electron-withdrawing substituents resulted in higher total degrees of PE functionalisation. As shown in Fig. 4, a good correlation was obtained when log10(TFR/TFH)—the ratio of the TF values obtained with substituent R to that of benzaldehyde (R = H)—was plotted as a function of the Hammett substituent constant (σR).57 While the electron-donating methoxy group of 4-anisaldehyde is expected to cause lower PE functionalisation, the exceptionally low TF observed on PE may be a result of competing oxidation of the methoxy groups of anisaldehyde. These findings further support our aforementioned mechanism, as more electron-withdrawing substituents are expected to increase the electrophilicity of the benzoylperoxy radical intermediates, making it more reactive for HAT from PE.58
Compatibility with copper-containing oxidation catalysts
Having demonstrated the feasibility of catalyst-free aerobic PE oxidation with benzaldehyde, we then investigated the compatibility of these reaction conditions with known oxidation catalysts. [CuCl2·dicyclohexyl (DCH)-18-crown-6] (Table 1, entry 12) was chosen due to its known efficacy for alkane oxidation in O2.59 The 1H NMR spectrum (Fig. S15†) of the resulting oxidised PE in the presence of this catalyst (1 mol% w.r.t. ethylene repeating units) revealed that other than carbonyls, a new prominent signal centred at δ = 2.30 ppm was present, which we attributed to COOH groups due to the lack of a corresponding ester signal at ∼4.1 ppm. FTIR spectroscopy (Fig. S25†) showed a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorbance peak centred at 1724 cm−1, which was considerably broadened compared with that of PE oxidised by benzaldehyde alone, as well as a prominent C–O absorption at 1248 cm−1.|| These indicated that the presence of the [CuCl2·DCH18-crown-6] complex could augment the degree of PE oxidation, also seen from the higher overall TF value obtained. Indeed, the likely presence of COOH groups also indicate that more extensive oxidative C–C chain cleavage occurred, which is further supported by high-temperature GPC analysis that showed considerable molecular weight reduction after oxidation (Fig. S21†). The efficacy of the crown ether complex for enhancing PE oxidation was evident when the reaction was repeated using anhydrous CuCl2 alone (Table 1, entry 13), which gave a lower TF of 1.8, albeit with detectable OH, C
O absorbance peak centred at 1724 cm−1, which was considerably broadened compared with that of PE oxidised by benzaldehyde alone, as well as a prominent C–O absorption at 1248 cm−1.|| These indicated that the presence of the [CuCl2·DCH18-crown-6] complex could augment the degree of PE oxidation, also seen from the higher overall TF value obtained. Indeed, the likely presence of COOH groups also indicate that more extensive oxidative C–C chain cleavage occurred, which is further supported by high-temperature GPC analysis that showed considerable molecular weight reduction after oxidation (Fig. S21†). The efficacy of the crown ether complex for enhancing PE oxidation was evident when the reaction was repeated using anhydrous CuCl2 alone (Table 1, entry 13), which gave a lower TF of 1.8, albeit with detectable OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH functionalisation (Fig. S16†). Like the aforementioned metal-free PE oxidation (Table 1, entry 1), high-purity benzoic acid could be recovered after the reaction by recrystallisation from hot water. The ability to modulate the outcome of the benzaldehyde-mediated PE oxidation using metal catalysts further suggests the possibility of exploiting this green oxidation approach to upcycle PE into long-chain carboxylic acids, without the need for highly corrosive concentrated nitric acid60 or nitrogen oxides61 as an oxidant. These long-chain carboxylic acids could find potential high-value applications as surfactants or chemical precursors for polymers and coatings.
O and COOH functionalisation (Fig. S16†). Like the aforementioned metal-free PE oxidation (Table 1, entry 1), high-purity benzoic acid could be recovered after the reaction by recrystallisation from hot water. The ability to modulate the outcome of the benzaldehyde-mediated PE oxidation using metal catalysts further suggests the possibility of exploiting this green oxidation approach to upcycle PE into long-chain carboxylic acids, without the need for highly corrosive concentrated nitric acid60 or nitrogen oxides61 as an oxidant. These long-chain carboxylic acids could find potential high-value applications as surfactants or chemical precursors for polymers and coatings.
Conclusions
In conclusion, we demonstrate a new and exceptionally simple approach for aerobic C–H oxidation of PE to achieve exclusive functionalisation with isolated carbonyl groups for the first time. Using just benzaldehyde as the reagent and bulk dispersant, with readily available O2 as the stoichiometric oxidant, our approach bypasses the need for hazardous chlorinated solvents or stoichiometric organic oxidants typically required for PE post-synthetic chemistry. In fact, the presence of chlorinated solvents such as TCE was detrimental to achieving exclusive carbonyl selectivity. The potential of this method for minimal waste generation is evident from the easy isolation of the benzoic acid by-product in excellent yields and purity by recrystallisation from hot water for reuse in different applications. We also show that the extent and functional group selectivity of PE oxidation can be modulated with the use of additional oxidation catalysts. Considering the versatility of carbonyls for further chemical derivatisation, this solvent-free method can offer new avenues for valorising waste PEs into functional materials for various applications for a sustainable materials economy.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge Ms Xie Huiqing, Ms Tan Sze Yu and Dr Liu Qiping for help with TGA, DSC and high-temperature GPC analyses of the polymer samples, respectively. Computational modelling was supported by the A*STAR Computational Resource Centre through the use of its high performance computing facilities. The authors are grateful for the A*STAR Career Development Award (CDA) (Project ID No. C210112038) and the Central Research Fund (CRF) for generous financial support of this work.Notes and references
- B. Sethi, in Recycling of Polymers, 2016, pp. 55–114, DOI:10.1002/9783527689002.ch3.
- World Economic Forum, Ellen MacArthur Foundation and McKinsey & Company, The New Plastics Economy — Rethinking the future of plastics, 2016, http://www.ellenmacarthurfoundation.org/publications Search PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- L. Parker, A whopping 91% of plastic isn't recycled, https://www.nationalgeographic.com/science/article/plastic-produced-recycling-waste-ocean-trash-debris-environment.
- H. Zhou, C. M. Plummer, H. Li, H. Huang, P. Ma, L. Li, L. Liu and Y. Chen, Polym. Chem., 2019, 10, 619–626 RSC.
- C. W. S. Yeung, J. Y. Q. Teo, X. J. Loh and J. Y. C. Lim, ACS Mater. Lett., 2021, 3, 1660–1676 CrossRef CAS.
- N. K. Boaen and M. A. Hillmyer, Chem. Soc. Rev., 2005, 34, 267–275 RSC.
- J. B. Williamson, S. E. Lewis, R. R. Johnson III, I. M. Manning and F. A. Leibfarth, Angew. Chem., Int. Ed., 2019, 58, 8654–8668 CrossRef CAS PubMed.
- L. Chen, K. G. Malollari, A. Uliana, D. Sanchez, P. B. Messersmith and J. F. Hartwig, Chem, 2021, 7, 137–145 CAS.
- A. Bunescu, S. Lee, Q. Li and J. F. Hartwig, ACS Cent. Sci., 2017, 3, 895–903 CrossRef CAS PubMed.
- X. Wang, F. W. Seidel and K. Nozaki, Angew. Chem., Int. Ed, 2019, 58, 12955–12959 CrossRef CAS PubMed.
- S. Tang, F. W. Seidel and K. Nozaki, Angew. Chem., Int. Ed., 2021, 60, 26506–26510 CrossRef CAS PubMed.
- G. Moad, Prog. Polym. Sci., 1999, 24, 81–142 CrossRef CAS.
- E. Drent, R. van Dijk, R. van Ginkel, B. van Oort and R. I. Pugh, Chem. Commun., 2002, 964–965, 10.1039/B111629K.
- T. O. Morgen, M. Baur, I. Göttker-Schnetmann and S. Mecking, Nat. Commun., 2020, 11, 3693 CrossRef CAS PubMed.
- S.-Y. Chen, R.-C. Pan, M. Chen, Y. Liu, C. Chen and X.-B. Lu, J. Am. Chem. Soc., 2021, 143, 10743–10750 CrossRef CAS PubMed.
- M. Baur, F. Lin, T. O. Morgen, L. Odenwald and S. Mecking, Science, 2021, 374, 604–607 CrossRef CAS PubMed.
- L. Wang, Y. Zhang, R. Du, H. Yuan, Y. Wang, J. Yao and H. Li, ChemCatChem, 2019, 11, 2260–2264 CrossRef CAS.
- M. Sankar, E. Nowicka, E. Carter, D. M. Murphy, D. W. Knight, D. Bethell and G. J. Hutchings, Nat. Commun., 2014, 5, 3332 CrossRef PubMed.
- E. Gracia-Lor, J. V. Sancho, R. Serrano and F. Hernández, Chemosphere, 2012, 87, 453–462 CrossRef CAS PubMed.
- M. L. Passos and C. P. Ribeiro, Innovation in Food Engineering: New Techniques and Products, CRC Press, 2016 Search PubMed.
- X. Wu, L. Zhu, C. Zhu, C. Wang and Q. Li, Curr. Green Chem., 2019, 6, 135–146 CrossRef CAS.
- H. Li, Y. Meng, C. Shu, X. Li, A. A. Kiss and X. Gao, ACS Sustainable Chem. Eng., 2018, 6, 14114–14124 CrossRef CAS.
- K.-T. Chung, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2016, 34, 233–261 CrossRef CAS PubMed.
- E. A. Aboagye, J. D. Chea and K. M. Yenkie, iScience, 2021, 24, 103114 CrossRef CAS PubMed.
- Market volume of benzoic acid worldwide from 2015 to 2020, with a forecast for 2021 to 2026, https://www.statista.com/statistics/1245227/benzoic-acid-market-volume-worldwide/, (accessed 02 Feb 2022, 2022).
- S. S. Soomro, D. Cozzula, W. Leitner, H. Vogt and T. E. Müller, Polym. Chem., 2014, 5, 3831–3837 RSC.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- R. G. W. Norrish and C. H. Bamford, Nature, 1937, 140, 195–196 CrossRef CAS.
- M. Gardette, A. Perthue, J.-L. Gardette, T. Janecska, E. Földes, B. Pukánszky and S. Therias, Polym. Degrad. Stab., 2013, 98, 2383–2390 CrossRef CAS.
- E. O. Pentsak, D. B. Eremin, E. G. Gordeev and V. P. Ananikov, ACS Catal., 2019, 9, 3070–3081 CrossRef CAS.
- P. J. Wagner, in Triplet States III, Springer, Berlin, Heidelberg, 1st edn, 1976, vol. 66, pp. 1–52 Search PubMed.
- C. Walling and M. J. Gibian, J. Am. Chem. Soc., 1965, 87, 3361–3364 CrossRef CAS.
- J. Al-Nu'airat, I. Oluwoye, N. Zeinali, M. Altarawneh and B. Z. Dlugogorski, Chem. Rec., 2021, 21, 315–342 CrossRef PubMed.
- A. P. Dunlop, P. R. Stout and S. Swadesh, Ind. Eng. Chem., 1946, 38, 705–708 CrossRef.
- A. Bravo, H.-R. Bjorsvik, F. Fontana, F. Minisci and A. Serri, J. Org. Chem., 1996, 61, 9409–9416 CrossRef CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- I. Hermans, T. L. Nguyen, P. A. Jacobs and J. Peeters, ChemPhysChem, 2005, 6, 637–645 CrossRef CAS PubMed.
- Y. Ahn, X. Colin and G. Roma, Polymers, 2021, 13, 2143 CrossRef CAS PubMed.
- I. Hermans, J. Peeters and P. A. Jacobs, Top. Catal., 2008, 50, 124–132 CrossRef CAS.
- A. R. Groenhof, A. W. Ehlers and K. Lammertsma, J. Phys. Chem. A, 2008, 112, 12855–12861 CrossRef CAS PubMed.
- R. D. Bach, J. Phys. Chem. A, 2016, 120, 840–850 CrossRef CAS PubMed.
- R. Lee, G. Gryn'ova, K. U. Ingold and M. L. Coote, Phys. Chem. Chem. Phys., 2016, 18, 23673–23679 RSC.
- G. A. Russell, J. Am. Chem. Soc., 1957, 79, 3871–3877 CrossRef CAS.
- C. Riplinger and F. Neese, J. Chem. Phys., 2013, 138, 034106 CrossRef PubMed.
- C. Riplinger, B. Sandhoefer, A. Hansen and F. Neese, J. Chem. Phys., 2013, 139, 134101 CrossRef PubMed.
- E. Gawlita, M. Lantz, P. Paneth, A. F. Bell, P. J. Tonge and V. E. Anderson, J. Am. Chem. Soc., 2000, 122, 11660–11669 CrossRef CAS.
- I. Hermans, J.-F. Müller, T. L. Nguyen, P. A. Jacobs and J. Peeters, J. Phys. Chem. A, 2005, 109, 4303–4311 CrossRef CAS PubMed.
- U. Pal, S. Sen and N. C. Maiti, J. Phys. Chem. A, 2014, 118, 1024–1030 CrossRef CAS PubMed.
- Y. Tian and Z.-Q. Liu, Green Chem., 2017, 19, 5230–5235 RSC.
- F. De Vleeschouwer, V. Van Speybroeck, M. Waroquier, P. Geerlings and F. De Proft, Org. Lett., 2007, 9, 2721–2724 CrossRef CAS PubMed.
- S. Sarkar, K. P. S. Cheung and V. Gevorgyan, Chem. Sci., 2020, 11, 12974–12993 RSC.
- D. Ravelli, M. Fagnoni, T. Fukuyama, T. Nishikawa and I. Ryu, ACS Catal., 2018, 8, 701–713 CrossRef CAS.
- R. D. Bach, J. Org. Chem., 2012, 77, 6801–6815 CrossRef CAS PubMed.
- V. K. Yadav, in Steric and Stereoelectronic Effects in Organic Chemistry, ed. V. K. Yadav, Springer International Publishing, Cham, 2021, pp. 179–189, DOI:10.1007/978-3-030-75622-2_8.
- J. A. Howard and S. Korcek, Can. J. Chem., 1970, 48, 2165–2172 CrossRef CAS.
- N. Komiya, T. Naota, Y. Oda and S.-I. Murahashi, J. Mol. Catal. A: Chem., 1997, 117, 21–37 CrossRef CAS.
- E. Bäckström, K. Odelius and M. Hakkarainen, Ind. Eng. Chem. Res., 2017, 56, 14814–14821 CrossRef.
- A. Pifer and A. Sen, Angew. Chem., Int. Ed., 1998, 37, 3306–3308 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc02502g |
| ‡ These authors contributed equally to the manuscript. |
| § We note that these preliminary computational studies should be interpreted qualitatively, due to the various limitations of using a short chain alkane as a model system for polyethylene (see ESI† for details) |
| ¶ Performing these reactions in the absence of TCE solvent resulted in the reaction solidifying after some time, which prevented stirring for mixing with O2. A control experiment where PE was stirred in TCE at 100 °C without any benzaldehydes under an O2 atmosphere showed no discernible PE oxidative functionalisation. |
| || Lack of prominent OH stretch could be due to the small amount of COOH formed and broadened absorbance. |
| This journal is © The Royal Society of Chemistry 2022 |


