Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Practical and sustainable preparation of pyrrolo[2,3-b]indoles by Cu/Fe catalyzed intramolecular C(sp2)–H amination†
Matteo
Corrieri
 ,
Lucia De
Crescentini
,
Lucia De
Crescentini
 ,
Fabio
Mantellini
,
Fabio
Mantellini
 ,
Giacomo
Mari
,
Giacomo
Mari
 ,
Stefania
Santeusanio
,
Stefania
Santeusanio
 and
Gianfranco
Favi
and
Gianfranco
Favi
 *
*
Department of Biomolecular Sciences, Section of Chemistry and Pharmaceutical Technologies, University of Urbino “Carlo Bo”, Via I Maggetti 24, 61029 Urbino, Italy. E-mail: gianfranco.favi@uniurb.it
First published on 6th September 2022
Abstract
A practical, robust and chemoselective approach toward the synthesis of pyrrolo[2,3-b]indoles via direct intramolecular C–H bond amination of α-indolylhydrazones has been achieved. This base- and oxidant-free chemoselective transformation relies on a Cu/Fe co-catalyst system that operates at 50 °C in air with water as the only reaction medium. The easy product isolation together with the recyclable catalyst aqueous system (reused at least five times, maintaining over 50% of its catalytic activity) can provide an effective environmentally benign approach to fused N-heterocycles of remarkable interest in pharmaceutical and medicinal chemistry. The ability of the hydrazone residue to act as a chelating/directing group as well as an aminating agent guarantees the success of this C–H functionalization.
The formation of carbon–nitrogen bonds for the preparation of nitrogen-containing molecules is a manifestly important transformation in organic chemistry. As a result, considerable efforts have focused on the discovery of sustainable, more efficient, and selective methods to access valuable N-heterocyclic frameworks. Until recently, conventional approaches for C–N bond construction, routinely used in academia and industry, focused on variations of metal-catalyzed Buchwald–Hartwig, Ullmann and Chan–Lam cross-coupling between aryl- or heteroaryl (pseudo)halides with amine nucleophiles.1–9 Despite the significant progress achieved in the field, including the contributions on the use of bio-based solvents and water,10,11 these methodologies are viable only on pre-functionalized substrates, such as aryl- or heteroaryl (pseudo)halides which entails extra steps making them inefficient and unattractive. Conversely, the direct C–H functionalization of non-preactivated substrates undoubtedly constitutes an appealing, cost-effective and atom economical C–N bond connection strategy12–18 (Fig. 1a). A number of amination reactions including the intramolecular variants,19–33 the first of which reported by pioneering work by Buchwald in 2005,19 has been successfully realized to date.
Within this context, noble catalysts (e.g., Pd, Rh, Ir and Ru) along with stoichiometric or excess amount of oxidant (e.g., Cu(OAc)2, AgOAc, PhI(OAc)2, CeSO4, and/or F+) and complex/specialized auxiliary ligands and/or additives have been employed predominantly. However, most of the metal catalysts operate at rather high temperature in toxic, polar, aprotic organic solvents (e.g., N,N-dimethylformamide (DMF), N-methylpyrrolidone (NMP), and N,N-dimethylacetamide (DMAc)) which meeting with limited success when used in water. Even with the enormous progress made over the past decade in the most emerging areas of photocatalytic34–36/electrochemical37–43/photoelectrocatalytic44 oxidative cross-coupling, however, there is still a necessity for the development of greener alternative and more efficient applicable methods which do not rely on noble metal catalysts45–48 (e.g., Cu, Fe, Zn, Mn, Co and Ni), avoid the use of toxic and/or hazardous organic solvents, and circumventing the need for external oxidants.49,50 Moreover, despite its conceptual simplicity, the intramolecular C–H bond amination of hydrazones51–56 possessing a “privileged” indole ring to afford value-added N-fused indoles remains elusive. Just recently, we reported a protocol for the synthesis of azacarbolines via PhIO2-promoted six-membered cycloamination-oxidation of α-indolylhydrazones57 (Fig. 1b, previous work). We envisioned that the pendant hydrazone residue in α-indolylhydrazones could be responsible for a five ring-closing C–H amination as result of the potential hydrazone–enamine tautomerization,58,59 thus providing a distinct approach for the C(2)–H functionalization of indoles and expedient synthesis of pyrroloindoles60 (Fig. 1b, this work). To accomplish this, the hydrazonic residue should serve both as a chelating/directing group61–63 and as an intramolecular nitrogen source51–57 under the action of the opportune metal.
In designing a complementary and convenient strategy to produce less saturated version of pyrrolo[2,3-b]indole molecules64–71via C–H bond functionalization, we herein report an unprecedented intramolecular C(sp2)–N bond amination strategy that utilizes a combination of more advantageous Cu72–75/Fe76–78 (the most abundant in the Earth's crust) catalyst, at 50 °C in the presence of air as terminal oxidant in aqueous system/medium (Fig. 1b, this work).
Besides remarkable biological activities exemplified by representative compounds such as pyrroindomycins (PYRs) Sirtuins inhibitor, and inibithor of growth of Bacillus subtilis (Fig. 1c), these 1,8-dihydro pyrrolo[2,3-b]indoles exhibit a broad spectrum of applications in optoelectronic materials and fluorescent probes.
We began our studies by testing the conversion of α-indolylhydrazone 1a to 1-amino pyrrolo[2,3-b]indole 2a using simple copper/iron salts (Table 1). After preliminary screening of a variety of conditions including nature of copper source, (co)catalyst loading, solvent, additive and temperature (see Table S1 of the ESI† for more details), we found that a combination79–87 of catalytic [commercially accessible] Cu(OAc)2·H2O (10 mol%) and FeCl3·6H2O (5 mol%) in an open flask at room temperature using water as the only reaction medium,88–91 was beneficial for this transformation, with the compound 2a being formed in 99% yield (entry 1). Reactions that employed other iron cocatalysts such as Fe2O3, Fe(NO3)3 9H2O, Fe(ClO4)3, Fe2(SO4)3·H2O or Fe(acac)3 led to inferior results in term of reactivity/efficiency (entries 2–6). Reducing the amount of catalyst/co-catalyst did not affect the yield albeit an extended reaction time was required for complete consumption of 1a (entry 7). On the other hand, we found that a mild heating (50 °C) significantly accelerated the reaction, and 1a was produced in almost quantitative yield within 3 h (entry 8).
| Entry | Catalyst [mol%] | Co-catalyst [mol%] | T [°C] | t [h] | Yield [%]b |
|---|---|---|---|---|---|
| a All reaction were performed on 0.2 mmol scale of 1a in 2 mL of solvent (0.1 M) under air atmospher for the indicate time. b Unless noted, yields are referred to the isolated product after column chromatography. c The unreacted starting material was recovered. d Without column chromatography. | |||||
| 1 | Cu(OAc)2·H2O (10) | FeCl3·6H2O (5) | r.t. | 24 | 99d |
| 2 | Cu(OAc)2·H2O (10) | Fe2O3 (5) | r.t. | 20 | 98d |
| 3 | Cu(OAc)2·H2O (10) | Fe(NO3)3 9H2O (5) | r.t. | 10 | 32 |
| 4 | Cu(OAc)2·H2O (10) | Fe(ClO4)3 (5) | r.t. | 48 | 73 |
| 5 | Cu(OAc)2·H2O (10) | Fe2(SO4)3·H2O (5) | r.t. | 48 | Tracec |
| 6 | Cu(OAc)2·H2O (10) | Fe(acac)3 (5) | r.t. | 40 | 99 |
| 7 | Cu(OAc)2·H2O (5) | FeCl3·6H2O (2.5) | r.t. | 40 | 99d |
| 8 | Cu(OAc)2·H2O (10) | FeCl3·6H2O (5) | 50 | 3 | 99d |
| 9 | Cu(OAc)2·H2O (10) | — | r.t. | 48 | Tracec |
| 10 | — | FeCl3·6H2O (10) | r.t. | 48 | Tracec |
Control experiments revealed that both Cu(OAc)2·H2O and FeCl3·6H2O were essential for this transformation as the omission of one of two catalysts resulted in only a trace amount of 2a (Table 1, entries 9 and 10). To our delight, the product 2a was purified simply by extraction with EtOAc, filtration through a plug of silica gel, concentration, and precipitation without the tedious column chromatography.
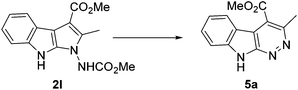
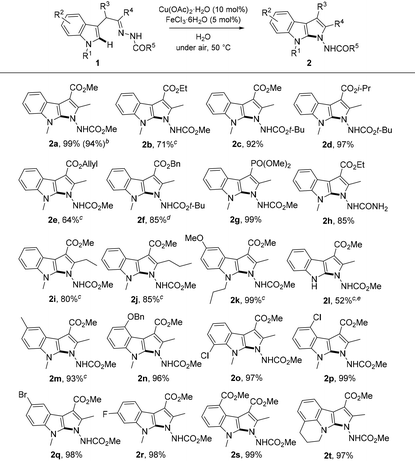
With these conditions in hand, the generality and scope of this novel intramolecular C–H amination process was explored (Table 2). First, the effect of different O-alkyl groups on the ester moiety of α-indolylhydrazone 1 was investigated. It was found that, in addition to methyl, ethyl, isopropyl, allyl or benzyl substituted substrates also worked well under the standard conditions, producing the desired products 2a–f in good to excellent yields. Incorporation of a phosphonate residue (R3 = PO(OMe)2) into the product (2g) was tolerated. Notably, the cyclization could also proceed successfully to deliver product 2h when a substrate with an amide N-protective group (R5 = NH2) was used. Compounds 2i,j, which vary in the substitution pattern at the R4, resulted in good yields. An evaluation of the substituents on indolic nitrogen revealed that N–H indole is most sensitive for this transformation. Specifically, for compound 2l, a spontaneous conversion to azacarboline 5a92 was registered. This observation imply that the substituent attached to the nitrogen of the indole nucleus is crucial in precluding ring expansion.
| a Reaction conditions: 1 (0.2 mmol), Cu(OAc)2·H2O (10 mol%) and FeCl3·6H2O (5 mol%) in H2O (2.0 mL) at 50 °C, 3–48 h. b 3.15 mmol scale reaction (0.933 g). c Isolated yields after column chromatography. d 50 °C for 36 h then 70 °C for 24 h. e A spontaneous conversion to azacarboline 5a (vide infra) was observed. |
|---|
|
Then, we examined the substituent (R2) effect on the indole ring. Delightedly, the reaction well tolerated either EDG or EWG groups at 4-, 5-, 6-, or 7-positions of the indole rings affording the corresponding products 2m–s in excellent yields. Finally, pyrrolo[2,3-b]indole 2t incorporating a ring system between the N and C7 atoms of the indole ring was successfully generated.
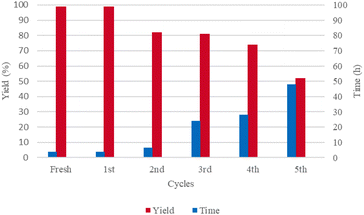
For the majority of the reactions, purification of products by column chromatography can be avoided. Also, the recovery and reutilization of the aqueous solution containing the Cu/Fe co-catalyst system was tested using the formation of compound 2a as model reaction (see (Fig. 2) and Table S2 of the ESI†). Thus, after the isolation of the latter compound, the recovered aqueous solution was used again to accomplish the same transformation up to five times. As shown in (Fig. 2), it is possible to use the water solution two times with no variation in both the yields and times of conversion of 1a to 2a. However, for the second recycling run, a clear decrease in the yield was observed. Comparable yield with a remarkable increase of the time of conversion resulted for the third run. While modest variations are observed for the fourth run, a more sensible change of both the yield and process time was registered for the subsequent run with the aqueous catalytic system that retains over 50% of its activity after five cycling runs. Interestingly, recycling involves not only the catalytic system but also the aqueous reaction medium itself, differently from most of the methods reported in the literature in which the metal species has to be prior separated from the reaction medium and often reactivated before its reuse.
In addition, this intramolecular C(sp2)–H amination was found to be scalable delivering product 2a in 94% yield (0.933 g) on 3.15 mmol scale.
To further explore the synthetic potential of the developed method, transformations of the thus formed pyrrolo[2,3-b]indoles were conducted (Scheme 1). Pyrroloindoles 3 and 4 can be easily obtained by N–N bond reductive cleavage (Magnus's protocol93) and removal of N-Boc protecting group, respectively. Also, azacarboline 5b can be prepared from compound 4 following our previously reported oxidation procedure.57 Alternatively, a Paal–Knorr pyrrole synthesis from 4 generated the N–N indole-pyrrole scaffold 6.94 The acetylenic dienophile DMAD also reacted in Diels–Alder reaction with 2 to give 9H-carbazole 7, after N-aminonitrene extrusion.95
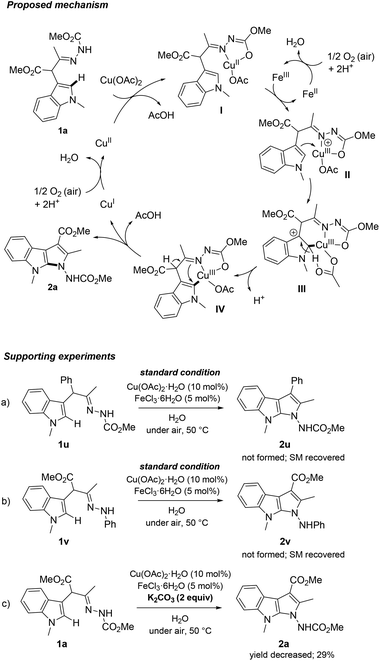
On the basis of literature information and our findings, a tentative mechanism for the transformation of 1a to 2a is illustrated in (Scheme 2). Initial N,O-bidentate coordination of the hydrazone moiety in 1a to the Cu(OAc)2 species occurs to form the metallacycle complex I with the release of AcOH. Oxidation of the Cu(II) adduct I to intermediate II by Fe(III) would then facilitate the subsequent electrophilic aromatic substitution. Proton abstraction at the 2-position of indole through a six-membered transition state III (or via acetate ligand-assisted concerted C–H activation pathway) may be followed to give endo-metallacycle96 intermediate IV. Finally, reductive elimination promoted by CH/NH tautomerization generates product 2a along with the Cu(I) specie. The catalytic cycle is completed by the regeneration of the active Cu(II) catalyst by air oxidation.
Some control experiments to support this mechanistic scenario were carried out. Under the reaction conditions, α-indolylhydrazone 1u bearing a phenyl substitution failed to produce 2u (Scheme 2a), which implies that the electron-withdrawing group (EWG) at the α-position of the hydrazone substrate results indispensable for the reaction to occur. Furthermore, no cycloamination was detected in the case of N-phenyl 1v because the copper complex via a bidentate coordination could not formed during the catalytic cycle, indicating the crucial role of N-carboxyalkyl moiety in directing/assisting the cyclization at 2-position of the indole ring. A pathway in which a hydrogen abstraction promotes the reductive elimination of metallacycle intermediate IV instead of a preliminary CH/NH tautomerization on 1a57 (not shown) is also supported by an experiment in which the addition of K2CO3 as a base resulted in the dramatically decrease of the product yield of 2a (29% yield).
Our new method is clearly distinguished from reported copper-catalyzed aerobic annulation of (hetero)aromatic hydrazones. With respect to Xiao and Xu protocol,56 this method furnishes 1,8-dihydro pyrrolo[2,3-b]indoles (instead of cinnolines) with excellent control of the chemoselectivity and the obvious benefit of providing more sustainable solution with water under mild reaction conditions.
Conclusions
In conclusion, we herein described the first biocompatible Cu/Fe-catalyzed chemoselective intramolecular C–H bond amination of α-indolylhydrazones. Under a cooperative action of iron(III) and copper(II) salts in the absence of an external oxidant or any other additive a variety of substituted pyrrolo[2,3-b]indoles (relevant bioactive pharmacophoric core) has been prepared in excellent yields. Easy products isolation, recyclability of the catalyst system, mild and clean conditions, use of aqueous medium are the main features that demonstrate the potential of this transformation for industrial application. Further new synthetic applications of this green and low-cost C(sp2)–H amination are currently underway in our laboratory.Author contributions
Matteo Corrieri: conceptualization, investigation, methodology, data curation, formal analysis; Lucia De Crescentini: data curation, formal analysis; Fabio Mantellini: data curation, resources, supervision; Giacomo Mari: investigation, methodology, and validation; Stefania Santeusanio: data curation, formal analysis; Gianfranco Favi: conceptualization, funding acquisition, supervision, project administration, writing – original draft and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the University of Urbino “Carlo Bo”, and the PhD Programs of Ministry of Education of Italy.References
- K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428–2439 CrossRef CAS.
- S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed.
- C. Sambiagio, S. P. Marsden, A. J. Blacker and P. C. McGowan, Chem. Soc. Rev., 2014, 43, 3525–3550 RSC.
- K. Okano, H. Tokuyama and T. Fukuyama, Chem. Commun., 2014, 50, 13650–13663 RSC.
- J. M. Honnanayakanavar, O. Obulesu; and S. Suresh, Org. Biomol. Chem., 2022, 20, 2993–3028 RSC.
- M. Carril, R. SanMartin and E. Domínguez, Chem. Soc. Rev., 2008, 37, 639–647 RSC.
- J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534–1544 CrossRef CAS PubMed.
- D. S. Surry and S. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338–6361 CrossRef CAS PubMed.
- C. Fischer and B. Koenig, Belstein J. Org. Chem., 2011, 7, 59–74 CrossRef CAS PubMed.
- A. F. Quivelli, P. Vitale, F. M. Perna and V. Capriati, Front. Chem., 2019, 7, 723 CrossRef CAS.
- L. Cicco, J. A. Hernández-Fernández, A. Salomone, P. Vitale, M. Ramos-Martín, J. González-Sabín, A. Presa Soto, F. M. Perna, V. Capriati and J. García-Álvarez, Org. Biomol. Chem., 2021, 19, 1773–1779 RSC.
- S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC.
- M.-L. Louillat and F. W. Patureau, Chem. Soc. Rev., 2014, 43, 901–910 RSC.
- V. S. Thirunavukkarasu, S. I. Kozhushkov and L. Ackerman, Chem. Commun., 2014, 50, 29–39 RSC.
- J. Jiao, K. Murakami and K. Itami, ACS Catal., 2016, 6, 610–633 CrossRef CAS.
- Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247–9301 CrossRef CAS PubMed.
- M. C. Henry, M. A. B. Mostafa and A. Sutherland, Synthesis, 2017, 4586–4598 CAS.
- K. Murakami, G. J. P. Perry and K. Itami, Org. Biomol. Chem., 2017, 15, 6071–6075 RSC.
- W. C. P. Tsang, N. Zheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560–11456 CrossRef CAS.
- W. C. P. Tsang, R. H. Munday, G. Brasche, N. Zheng and S. L. Buchwald, J. Org. Chem., 2008, 73, 7603–7610 CrossRef CAS PubMed.
- J. A. Jordan-Hore, C. C. C. Johansson, M. Gulias, E. M. Beck and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 16184–16186 CrossRef CAS.
- B.-J. Li, S.-L. Tian, Z. Fang and Z.-J. Shi, Angew. Chem., Int. Ed., 2008, 47, 1115–1118 CrossRef CAS PubMed.
- S. W. Youn, J. H. Bihn and B. S. Kim, Org. Lett., 2011, 13, 3738–3741 CrossRef CAS PubMed.
- S. H. Cho, J. Yoon and S. Chang, J. Am. Chem. Soc., 2011, 133, 5996–6005 CrossRef CAS PubMed.
- K. Takamatsu, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2014, 16, 2892–2859 CrossRef CAS PubMed.
- G. Brasche and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 1932–1934 CrossRef CAS PubMed.
- Q. Xiao, W.-H. Wang, G. Liu, F.-K. Meng, J.-H. Chen, Z. Yang and Z.-J. Shi, Chem. – Eur. J., 2009, 15, 7292–7296 CrossRef CAS PubMed.
- H. Wang, Y. Wang, C. Peng, J. Zhang and Q. Zhu, J. Am. Chem. Soc., 2010, 132, 13217–13219 CrossRef CAS PubMed.
- K. Inamoto, T. Saito, M. Katsuno, T. Sakamoto and K. Hiroya, Org. Lett., 2007, 9, 2931–2934 CrossRef CAS PubMed.
- T.-S. Mei, X. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 10806–10807 CrossRef CAS PubMed.
- Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676–3677 CrossRef CAS PubMed.
- S. Yugandar, S. Konda and H. Ila, J. Org. Chem., 2016, 81, 2035–2052 CrossRef CAS.
- M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14058–14059 CrossRef CAS PubMed.
- L. Niu, H. Yi, S. Wang, T. Liu, J. Liu and A. Lei, Nat. Commun., 2017, 8, 1748 CrossRef.
- K. A. Mrgrey, A. Levens and D. Nicewicz, Angew. Chem., Int. Ed., 2017, 56, 15644–15648 CrossRef.
- A. Ruffoni, F. Juliá, T. D. Svejstrup, A. J. McMillan, J.- J. Douglas and D. Leonori, Nat. Chem., 2019, 11, 426–433 CrossRef CAS PubMed.
- T. Morofuji, A. Shimizu and J.-I. Yoshida, J. Am. Chem. Soc., 2013, 135, 5000–5003 CrossRef CAS PubMed.
- T. Morofuji, A. Shimizu and J.-I. Yoshida, J. Am. Chem. Soc., 2014, 136, 4496–4499 CrossRef CAS PubMed.
- T. Morofuji, A. Shimizu and J.-I. Yoshida, J. Am. Chem. Soc., 2015, 137, 9816–9819 CrossRef CAS PubMed.
- T. Morofuji, A. Shimizu and J.-I. Yoshida, Chem. – Eur. J., 2015, 21, 3211–3214 CrossRef CAS PubMed.
- Y. Zhang, Z. Lin and L. Ackermann, Chem. – Eur. J., 2021, 27, 242–246 CrossRef CAS PubMed.
- M. Puthanveedu, V. Khamraev, L. Brieger, C. Strohmann and A. P. Antonchick, Chem. – Eur. J., 2021, 27, 8008–8012 CrossRef CAS PubMed.
- N. Sauermann, T. H. Meyer, Y. Qiu and L. Ackermann, ACS Catal., 2018, 8, 7086–7103 CrossRef CAS.
- L. Zhang, L. Liarted, J. Luo, D. Ren, M. Grätzel and X. Gu, Nat. Catal., 2019, 2, 366–373 CrossRef CAS.
- P. Chirik and R. Morris, Acc. Chem. Res., 2015, 48, 2495–2495 CrossRef CAS PubMed.
- M. Beller, Chem. Rev., 2019, 119, 2089–2089 CrossRef CAS PubMed.
- P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed.
- R. Singh, E. Sathish, A. K. Gupta and S. Goyal, Tetrahedron, 2021, 100, 132474 CrossRef CAS.
- J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740–4761 RSC.
- T. Dalton, T. Faber and F. Glorius, ACS Cent. Sci., 2021, 7, 245–261 CrossRef CAS PubMed.
- Z. Zheng, L. Tang, Y. Fan, X. Qi, Y. Du and D. Zhang-Negrerie, Org. Biomol. Chem., 2011, 9, 3714–3725 RSC.
- T. Zhang and W. Bao, J. Org. Chem., 2013, 78, 1317–1322 CrossRef CAS.
- M. Kashiwa, M. Sonoda and S. Tanimori, Eur. J. Org. Chem., 2014, 4720–4723 CrossRef CAS.
- D. Liang and Q. Zhu, Asian J. Org. Chem., 2015, 4, 42–45 CrossRef CAS.
- X.-Q. Hu, G. Feng, J.-R. Chen, D.-M. Yan, Q.-Q. Zhao, Q. Wei and W.-J. Xiao, Org. Biomol. Chem., 2015, 13, 3457–3461 RSC.
- C. Lan, Z. Tian, X. Liang, M. Gao, W. Liu, Y. An, W. Fu, G. Jiao, J. Xiao and B. Xu, Adv. Synth. Catal., 2017, 359, 3735–3740 CrossRef CAS.
- M. Corrieri, L. De Crescentini, F. Mantellini, G. Mari, S. Santeusanio and G. Favi, J. Org. Chem., 2021, 86, 17918–17929 CrossRef CAS PubMed.
- Y. Du, R. Liu, G. Linn and K. Zhao, Org. Lett., 2006, 8, 5919–5922 CrossRef CAS PubMed.
- S. Yugandar, S. Konda and H. Ila, J. Org. Chem., 2016, 81, 2035–2052 CrossRef CAS PubMed.
- Only one example of metal-catalyzed cycloamination of hydrazone was reported to obtain pyrrolo[2,3-b]indole scaffold; however a more than stoichiometric amount of FeBr3 as well as dichloroethane as solvent were employed. See ref. 49.
- C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603–6743 RSC.
- A. Ros, R. López-Rodríguez, B. Estepa, E. álvarez, R. Fernández and J. M. Lassaletta, J. Am. Chem. Soc., 2012, 134, 4573–4576 CrossRef CAS PubMed.
- Z. Huang, C. Wang and G. Dong, Angew. Chem., Int. Ed., 2016, 55, 5299–5303 CrossRef CAS PubMed.
- The pyrrolo[2,3-b]indole motif occurs in nature at the hexahydro level in alkaloids, but by contrast, the less saturated version such as 1,8-dihydro pyrrolo[2,3-b]indole is little known.
- B. Prasad, B. Y. Sreenivas, D. Rambabu, G. R. Krishna, C. M. Reddy, K. L. Kumar and M. Pal, Chem. Commun., 2013, 49, 3970–3972 RSC.
- S. P. Nikumbh, A. Raghunadh, V. N. Murthy, R. Jinkala, S. C. Joseph, Y. L. N. Murthy, B. Prasad and M. Pal, RSC Adv., 2015, 5, 74570–74574 RSC.
- S. Badigenchala, V. Rajeshkumar and G. Sekar, Org. Biomol. Chem., 2016, 14, 2297–2305 RSC.
- Z.-Y. Yang, T. Tian, Y.-F. Du, S.-Y. Li, C.-C. Chu, L.-Y. Chen, D. Li, J.-Y. Liu and B. Wang, Chem. Commun., 2017, 53, 8050–8053 RSC.
- W.-B. Shen, Q. Sun, L. Li, X. Liu, B. Zhou, J.-Z. Yan, X. Lu and L.-W. Ye, Nat. Commun., 2017, 8, 1748 CrossRef PubMed.
- T. Liang, L. Gong, H. Zhao, H. Jiang and M. Zhang, Chem. Commun., 2020, 56, 2807–2810 RSC.
- Y. Dong, J. Yang, H. Zhang, X.-Y. Zhan, S. He, Z.-C. Shi, X.-M. Zhang and J.-Y. Wang, Org. Lett., 2020, 22, 5151–5156 CrossRef CAS.
- G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS PubMed.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- X. Guo, D. Gu, Z. Wu and W. Zhang, Chem. Rev., 2015, 115, 1622–1651 CrossRef CAS PubMed.
- M. A. Afsina, T. Aneeja, M. Neetha and G. Anilkumar, Eur. J. Org. Chem., 2021, 1776–1808 CrossRef.
- S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3317–3321 CrossRef CAS PubMed.
- C. Bolm, Nat. Chem., 2009, 1, 420–420 CrossRef CAS PubMed.
- Y. Li, T. Yo, T.-T. Wan and C.-M. Che, Tetrahedron, 2019, 75, 130607 CrossRef.
- M. Taillefer, N. Xia and A. Ouali, Angew. Chem., Int. Ed., 2007, 46, 934–936 CrossRef CAS.
- J. Mao, G. Xie, M. Wu, J. Guo and S. Jia, Adv. Synth. Catal., 2008, 350, 2477–2482 CrossRef CAS.
- S. Li, W. Jia and N. Jiao, Adv. Synth. Catal., 2009, 351, 569–575 CrossRef CAS.
- X. Liu and S. Zhang, Synlett, 2011, 268–272 CAS.
- F. Labre, Y. Gimbert, P. Bannwarth, S. Olivero, E. Duñach and P. Y. Chavant, Org. Lett., 2014, 16, 2366–2369 CrossRef CAS PubMed.
- N. Yi, R. Wang, H. Zou, W. He, W. Fu and W. He, J. Org. Chem., 2015, 80, 5023–5029 CrossRef CAS PubMed.
- C. Liu, Q. Zhang, H. Li, S. Guo, B. Xiao, W. Deng, L. Liu and W. He, Chem. – Eur. J., 2016, 22, 6208–6212 CrossRef CAS PubMed.
- Y. Li and X.-F. Wu, Commun Chem., 2018, 1, 1–8 CrossRef CAS.
- M. C. Henry, H. M. Senn and A. Sutherland, J. Org. Chem., 2019, 84, 346–364 CrossRef CAS.
- M. A. Ali, X. Yao, H. Sun and H. Lu, Org. Lett., 2015, 17, 1513–1516 CrossRef CAS PubMed.
- M. A. Ali, X. Yao, G. Li and H. Lu, Org. Lett., 2016, 18, 1386–1389 CrossRef CAS PubMed.
- L. Yang, H. Li, H. Zhang and H. Lu, Eur. J. Org. Chem., 2016, 5611–5615 CrossRef CAS.
- L. Shi and B. Wang, Org. Lett., 2016, 18, 2820–2823 CrossRef CAS PubMed.
- Although compound 2l was isolated in pure form, a conversion to 5a (see structure below) take places both in DMSO-d6 solution and in neat sample.
. - P. Magnus, N. Garizi, K. A. Seibert and A. Ornholt, Org. Lett., 2009, 11, 5646–5648 CrossRef CAS PubMed.
- K.-W. Chen, Z.-H. Chen, S. Yang, S.-F. Wu, Y.-C. Zhang and F. Shi, Angew. Chem., 2022, 61, e202116829 CAS.
- A. G. Schultz and M. Shen, Tetrahedron Lett., 1979, 20, 2969–2972 CrossRef.
- S. R. Sahoo, S. Dutta, S. A. Al-Thabaiti, M. Mokhtar and D. Maiti, Chem. Commun., 2021, 57, 11885–11903 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc02340g |
| This journal is © The Royal Society of Chemistry 2022 |